Influence of the Pyrolysis Temperature and TiO2-Incorporation on the Properties of SiOC/SiC Composites for Efficient Wastewater Treatment Applications
Abstract
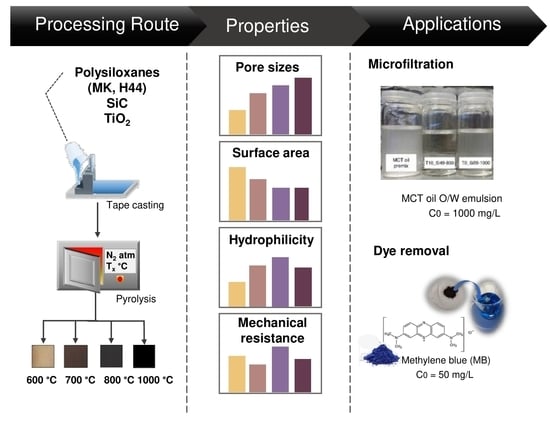
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Processing Route
2.3. Characterizations
3. Results and Discussions
3.1. Membrane Composition
3.2. Membrane Macrostructure
3.3. Microporosity and Surface Characteristics
3.4. Adsorption and Photocatalytic Activity
3.5. Mechanical Strength
3.6. Membrane Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Behroozi, A.H.; Ataabadi, M.R. Improvement in microfiltration process of oily wastewater: A comprehensive review over two decades. J. Environ. Chem. Eng. 2021, 9, 104981. [Google Scholar] [CrossRef]
- Otitoju, T.A.; Ahmad, A.L.; Ooi, B.S. Polyvinylidene fluoride (PVDF) membrane for oil rejection from oily wastewater: A performance review. J. Water Process Eng. 2016, 14, 41–59. [Google Scholar] [CrossRef]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Jamaly, S.; Giwa, A.; Hasan, S.W. Recent improvements in oily wastewater treatment: Progress, challenges, and future opportunities. J. Environ. Sci. 2015, 37, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Hakami, M.W.; Alkhudhiri, A.; Al-Batty, S.; Zacharof, M.P.; Maddy, J.; Hilal, N. Ceramic Microfiltration Membranes in Wastewater Treatment: Filtration Behavior, Fouling and Prevention. Membranes 2020, 10, 248. [Google Scholar] [CrossRef]
- Abadi, S.R.H.; Sebzari, M.R.; Hemati, M.; Rekabdar, F.; Mohammadi, T. Ceramic membrane performance in microfiltration of oily wastewater. Desalination 2011, 265, 222–228. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Gopal, S.; Nambikkattu, J.; Rambabu, K.; Aboulella, A.M.; Wickramasinghe, S.R.; Banat, F. Recent developments in porous ceramic membranes for wastewater treatment and desalination: A review. J. Environ. Manag. 2021, 293, 112925. [Google Scholar] [CrossRef]
- Zou, D.; Fan, Y. State-of-the-art developments in fabricating ceramic membranes with low energy consumption. Ceram. Int. 2021, 47, 14966–14987. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Viard, A.; Fonblanc, D.; Lopez-Ferber, D.; Schmidt, M.; Lale, A.; Durif, C.; Balestrat, M.; Rossignol, F.; Weinmann, M.; Riedel, R.; et al. Polymer Derived Si-B-C-N Ceramics: 30 Years of Research. Adv. Eng. Mater. 2018, 20, 1800360. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-Derived Ceramics: 40 Years of Research and Innovation in Advanced Ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Araldi Silva, B.; Belchior Ribeiro, L.F.; Gómez González, S.Y.; Hotza, D.; de Fátima Peralta Muniz Moreira, R.; De Noni Junior, A. SiOC and SiCN-based ceramic supports for catalysts and photocatalysts. Microporous Mesoporous Mater. 2021, 327, 111435. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, M.; Zhu, Y. Organosilicon polymer-derived ceramics: An overview. J. Adv. Ceram. 2019, 8, 457–478. [Google Scholar] [CrossRef] [Green Version]
- Riedel, R.; Mera, G.; Hauser, R.; Klonczynski, A. Silicon-based-polymer-derived-ceramics: Synthesis propeties and applications—A review. J. Ceram. Soc. Jpn. 2006, 114, 425–444. [Google Scholar] [CrossRef] [Green Version]
- Colombo, P. Engineering porosity in polymer-derived ceramics. J. Eur. Ceram. Soc. 2008, 28, 1389–1395. [Google Scholar] [CrossRef]
- Greil, P. Polymer Derived Engineering. Adv. Eng. Mater. 2000, 2, 339–348. [Google Scholar] [CrossRef]
- Greil, P. Near Net Shape Manufacturing of PolymerDerived Ceramics. J. Eur. Ceram. Soc. 1998, 18, 1905–1914. [Google Scholar] [CrossRef]
- Scheffler, M.; Gambaryan-Roisman, T.; Takahashi, T.; Kaschta, J.; Muenstedt, H.; Buhler, P.; Greil, P. Pyrolytic decomposition of preceramic organo polysiloxanes. Ceram. Trans 2000, 115, 239–250. [Google Scholar]
- Wilhelm, M.; Soltmann, C.; Koch, D.; Grathwohl, G. Ceramers—Functional materials for adsorption techniques. J. Eur. Ceram. Soc. 2005, 25, 271–276. [Google Scholar] [CrossRef]
- Colombo, P.; Bernardo, E.; Parcianello, G. Multifunctional advanced ceramics from preceramic polymers and nano-sized active fillers. J. Eur. Ceram. Soc. 2013, 33, 453–469. [Google Scholar] [CrossRef]
- Mirkhalaf, M.; Yazdani Sarvestani, H.; Yang, Q.; Jakubinek, M.B.; Ashrafi, B. A comparative study of nano-fillers to improve toughness and modulus of polymer-derived ceramics. Sci. Rep. 2021, 11, 6951. [Google Scholar] [CrossRef] [PubMed]
- Greil, P. Active filler controlled pyrolysis of preceramic polymers. J. Am. Ceram. Soc. 1995, 78, 835–848. [Google Scholar] [CrossRef]
- Schmidt, H.; Koch, D.; Grathwohl, G.; Colombo, P. Micro-/Macroporous Ceramics from Preceramic Precursors. J. Am. Ceram. Soc. 2001, 84, 2252–2255. [Google Scholar] [CrossRef]
- Greil, P. Polymer-Filler Derived Ceramics with Hierarchial Microstructures. Key Eng. Mater. 1998, 159–160, 339–346. [Google Scholar] [CrossRef]
- Schumacher, D.; Wilhelm, M.; Rezwan, K. Modified solution based freeze casting process of polysiloxanes to adjust pore morphology and surface functions of SiOC monoliths. Mater. Des. 2018, 160, 1295–1304. [Google Scholar] [CrossRef]
- Steinau, M.; Travitzky, N.; Gegner, J.; Hofmann, J.; Greil, P. Polymer-Derived Ceramics for Advanced Bearing Applications. Adv. Eng. Mater. 2008, 10, 1141–1146. [Google Scholar] [CrossRef]
- Biasetto, L.; Francis, A.; Palade, P.; Principi, G.; Colombo, P. Polymer-derived microcellular SiOC foams with magnetic functionality. J. Mater. Sci. 2008, 43, 4119–4126. [Google Scholar] [CrossRef]
- Colombo, P.; Gambaryan-Roisman, T.; Scheffler, M.; Buhler, P.; Greil, P. Conductive Ceramic Foams from Preceramic Polymers. J. Am. Ceram. Soc. 2001, 84, 2265–2268. [Google Scholar] [CrossRef]
- Canuto de Almeida e Silva, T.; Mooste, M.; Kibena-Põldsepp, E.; Matisen, L.; Merisalu, M.; Kook, M.; Sammelselg, V.; Tammeveski, K.; Wilhelm, M.; Rezwan, K. Polymer-derived Co/Ni–SiOC(N) ceramic electrocatalysts for oxygen reduction reaction in fuel cells. Catal. Sci. Technol. 2019, 9, 854–866. [Google Scholar] [CrossRef]
- Wójcik-Bania, M.; Krowiak, A.; Strzezik, J.; Hasik, M. Pt supported on cross-linked poly(vinylsiloxanes) and SiCO ceramics—New materials for catalytic applications. Mater. Des. 2016, 96, 171–179. [Google Scholar] [CrossRef]
- Macedo, H.P.; Medeiros, R.L.B.A.; Ilsemann, J.; Melo, D.M.A.; Rezwan, K.; Wilhelm, M. Nickel-containing hybrid ceramics derived from polysiloxanes with hierarchical porosity for CO2 methanation. Microporous Mesoporous Mater. 2019, 278, 156–166. [Google Scholar] [CrossRef]
- Yan, X.; Su, D.; Han, S. Phase separation induced macroporous SiOC ceramics derived from polysiloxane. J. Eur. Ceram. Soc. 2015, 35, 443–450. [Google Scholar] [CrossRef]
- Mera, G.; Gallei, M.; Bernard, S.; Ionescu, E. Ceramic Nanocomposites from Tailor-Made Preceramic Polymers. Nanomaterials 2015, 5, 468–540. [Google Scholar] [CrossRef] [PubMed]
- Stabler, C.; Ionescu, E.; Graczyk-Zajac, M.; Gonzalo-Juan, I.; Riedel, R. Silicon oxycarbide glasses and glass-ceramics: “All-Rounder” materials for advanced structural and functional applications. J. Am. Ceram. Soc. 2018, 101, 4817–4856. [Google Scholar] [CrossRef]
- Huang, K.; Elsayed, H.; Franchin, G.; Colombo, P. Additive manufacturing of SiOC scaffolds with tunable structure-performance relationship. J. Eur. Ceram. Soc. 2021, 41, 7552–7559. [Google Scholar] [CrossRef]
- Lue, S.J.; Tsai, C.L.; Lee, D.-T.; Mahesh, K.P.O.; Hua, M.Y.; Hu, C.-C.; Jean, Y.C.; Lee, K.-R.; Lai, J.-Y. Sorption, diffusion, and perm-selectivity of toluene vapor/nitrogen mixtures through polydimethylsiloxane membranes with two cross-linker densities. J. Membr. Sci. 2010, 349, 321–332. [Google Scholar] [CrossRef]
- Miyazaki, T.; Nagasawa, H.; Tsuru, T.; Kanezashi, M. Design of a SiOC network structure with oxidation stability and application to hydrogen separation membranes at high temperatures. J. Membr. Sci. 2021, 625, 119147. [Google Scholar] [CrossRef]
- Icin, O.; Vakifahmetoglu, C. Dye removal by polymer derived ceramic nanobeads. Ceram. Int. 2021, 47, 27050–27057. [Google Scholar] [CrossRef]
- Mujib, S.B.; Cuccato, R.; Mukherjee, S.; Franchin, G.; Colombo, P.; Singh, G. Electrospun SiOC ceramic fiber mats as freestanding electrodes for electrochemical energy storage applications. Ceram. Int. 2020, 46, 3565–3573. [Google Scholar] [CrossRef]
- Xia, K.; Liu, X.; Liu, H.; Lu, Y.; Liu, Z.; Li, Y.; Duan, L.; Hou, Z.; Li, R.; Wang, D. Carbon-enriched SiOC ceramics with hierarchical porous structure as anodes for lithium storage. Electrochim. Acta 2021, 372, 137899. [Google Scholar] [CrossRef]
- Dong, B.-B.; Wang, F.-H.; Yang, M.-Y.; Yu, J.-L.; Hao, L.-Y.; Xu, X.; Wang, G.; Agathopoulos, S. Polymer-derived porous SiOC ceramic membranes for efficient oil-water separation and membrane distillation. J. Membr. Sci. 2019, 579, 111–119. [Google Scholar] [CrossRef]
- Zhang, Z.; Bao, Y.; Sun, X.; Chen, K.; Zhou, M.; He, L.; Huang, Q.; Huang, Z.; Chai, Z.; Song, Y. Mesoporous Polymer-Derived Ceramic Membranes for Water Purification via a Self-Sacrificed Template. ACS Omega 2020, 5, 11100–11105. [Google Scholar] [CrossRef]
- Canuto de Almeida e Silva, T.; Fernandes Kettermann, V.; Pereira, C.; Simões, M.; Wilhelm, M.; Rezwan, K. Novel tape-cast SiOC-based porous ceramic electrode materials for potential application in bioelectrochemical systems. J. Mater. Sci. 2019, 54, 6471–6487. [Google Scholar] [CrossRef]
- Prenzel, T.; Wilhelm, M.; Rezwan, K. Pyrolyzed polysiloxane membranes with tailorable hydrophobicity, porosity and high specific surface area. Microporous Mesoporous Mater. 2013, 169, 160–167. [Google Scholar] [CrossRef]
- Dogrul, F.; Ozog, P.; Michalek, M.; Elsayed, H.; Galusek, D.; Liverani, L.; Boccaccini, A.R.; Bernardo, E. Polymer-Derived Biosilicate((R))-like Glass-Ceramics: Engineering of Formulations and Additive Manufacturing of Three-Dimensional Scaffolds. Materials 2021, 14, 5170. [Google Scholar] [CrossRef] [PubMed]
- Černý, M.; Chlup, Z.; Strachota, A.; Halasová, M.; Rýglová, Š.; Schweigstillová, J.; Svítilová, J.; Havelcová, M. Changes in structure and in mechanical properties during the pyrolysis conversion of crosslinked polymethylsiloxane and polymethylphenylsiloxane resins to silicon oxycarbide glass. Ceram. Int. 2015, 41, 6237–6247. [Google Scholar] [CrossRef]
- Kolář, F.; Machovič, V.; Svítilová, J.; Borecká, L. Structural characterization and thermal oxidation resistance of silicon oxycarbides produced by polysiloxane pyrolysis. Mater. Chem. Phys. 2004, 86, 88–98. [Google Scholar] [CrossRef]
- Brus, J.; Kolár, F.; Machovic, M.; Svítilová, J. Structure of silicon oxycarbide glasses derived from poly(methylsiloxane) and poly[methyl(phenyl)siloxane] precursors. J. Non-Cryst. Solids 2001, 289, 64–74. [Google Scholar] [CrossRef]
- Saha, A.; Raj, R. Crystallization Maps for SiCO Amorphous Ceramics. J. Am. Ceram. Soc. 2007, 90, 578–583. [Google Scholar] [CrossRef]
- Nishihora, R.K.; Quadri, M.G.N.; Hotza, D.; Rezwan, K.; Wilhelm, M. Tape casting of polysiloxane-derived ceramic with controlled porosity and surface properties. J. Eur. Ceram. Soc. 2018, 38, 4899–4905. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Hojamberdiev, M.; Prasad, R.M.; Morita, K.; Zhu, Y.; Schiavon, M.A.; Gurlo, A.; Riedel, R. Template-free synthesis of polymer-derived mesoporous SiOC/TiO2 and SiOC/N-doped TiO2 ceramic composites for application in the removal of organic dyes from contaminated water. Appl. Catal. B Environ. 2012, 115–116, 303–313. [Google Scholar] [CrossRef]
- Fontão, N.C.; Wilhelm, M.; Rezwan, K. Asymmetric polysiloxane-based SiOC membranes produced via phase inversion tape casting process. Mater. Des. 2021, 198, 109328. [Google Scholar] [CrossRef]
- Monash, P.; Pugazhenthi, G. Effect of TiO2 addition on the fabrication of ceramic membrane supports: A study on the separation of oil droplets and bovine serum albumin (BSA) from its solution. Desalination 2011, 279, 104–114. [Google Scholar] [CrossRef]
- Sotto, A.; Boromand, A.; Zhang, R.; Luis, P.; Arsuaga, J.M.; Kim, J.; Van der Bruggen, B. Effect of nanoparticle aggregation at low concentrations of TiO2 on the hydrophilicity, morphology, and fouling resistance of PES-TiO2 membranes. J. Colloid Interface Sci. 2011, 363, 540–550. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Das, P.R.; Ohl, C.; Wilker, V.; Kappa, M.; Scheffler, F.; Scheffler, M. Novel-Type Inorganic Foams from Preceramic Polymers with Embedded Titania Nanoparticles for Photo-Catalytic Applications. Adv. Eng. Mater. 2011, 13, 996–1001. [Google Scholar] [CrossRef]
- Wang, S.; Boyjoo, Y.; Choueib, A. A comparative study of dye removal using fly ash treated by different methods. Chemosphere 2005, 60, 1401–1407. [Google Scholar] [CrossRef]
- Yener, J.; Kopac, T.; Dogu, G.; Dogu, T. Dynamic analysis of sorption of Methylene Blue dye on granular and powdered activated carbon. Chem. Eng. J. 2008, 144, 400–406. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Sharma, A. Kinetics and thermodynamics of Methylene Blue adsorption on Neem (Azadirachta indica) leaf powder. Dye. Pigment. 2005, 65, 51–59. [Google Scholar] [CrossRef]
- Ur Rehman, M.S.; Kim, I.; Han, J.I. Adsorption of methylene blue dye from aqueous solution by sugar extracted spent rice biomass. Carbohydr. Polym. 2012, 90, 1314–1322. [Google Scholar] [CrossRef]
- Mokhtar, M. Application of Synthetic Layered Sodium Silicate Magadiite Nanosheets for Environmental Remediation of Methylene Blue Dye in Water. Materials 2017, 10, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, M.; Wang, H.; Chen, J.; Su, L.; Wu, D.; Navrotsky, A. Structure and energetics of SiOC and SiOC-modified carbon-bonded carbon fiber composites. J. Am. Ceram. Soc. 2017, 100, 3693–3702. [Google Scholar] [CrossRef]
- Ray, M.; Bhattacharya, P.; Das, R.; Sondhi, K.; Ghosh, S.; Sarkar, S. Preparation and characterization of macroporous pure alumina capillary membrane using boehmite as binder for filtration application. J. Porous Mater. 2015, 22, 1043–1052. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, Y.; Guan, K.; Peng, C.; Qiu, W.; Wu, J. Integrated preparation of alumina microfiltration membrane with super permeability and high selectivity. J. Eur. Ceram. Soc. 2019, 39, 1316–1323. [Google Scholar] [CrossRef]
- Zou, D.; Qiu, M.; Chen, X.; Drioli, E.; Fan, Y. One step co-sintering process for low-cost fly ash based ceramic microfiltration membrane in oil-in-water emulsion treatment. Sep. Purif. Technol. 2019, 210, 511–520. [Google Scholar] [CrossRef]
- Elomari, H.; Achiou, B.; Ouammou, M.; Albizane, A.; Bennazha, J.; Alami Younssi, S.; Elamrani, I. Elaboration and characterization of flat membrane supports from Moroccan clays. Application for the treatment of wastewater. Desalination Water Treat. 2015, 57, 20298–20306. [Google Scholar] [CrossRef]
- Mouiya, M.; Abourriche, A.; Bouazizi, A.; Benhammou, A.; El Hafiane, Y.; Abouliatim, Y.; Nibou, L.; Oumam, M.; Ouammou, M.; Smith, A.; et al. Flat ceramic microfiltration membrane based on natural clay and Moroccan phosphate for desalination and industrial wastewater treatment. Desalination 2018, 427, 42–50. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, Z.; Zhang, Z.; Chu, Y.; Yuan, B.; Wei, Z. Development of highly porous mullite whisker ceramic membranes for oil-in-water separation and resource utilization of coal gangue. Sep. Purif. Technol. 2020, 237, 116483. [Google Scholar] [CrossRef]
- Rashad, M.; Logesh, G.; Sabu, U.; Balasubramanian, M. A novel monolithic mullite microfiltration membrane for oil-in-water emulsion separation. J. Membr. Sci. 2021, 620, 118857. [Google Scholar] [CrossRef]
- Eom, J.-H.; Yeom, H.-J.; Kim, Y.-W.; Song, I.-H. Ceramic Membranes Prepared from a Silicate and Clay-mineral Mixture for Treatment of Oily Wastewater. Clays Clay Miner. 2015, 63, 222–234. [Google Scholar] [CrossRef]
- Li, L.; Gao, E.Z.; Abadikhah, H.; Wang, J.W.; Hao, L.Y.; Xu, X.; Agathopoulos, S. Preparation of a Porous, Sintered and Reaction-Bonded Si3N4 (SRBSN) Planar Membrane for Filtration of an Oil-in-Water Emulsion with High Flux Performance. Materials 2018, 11, 990. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Material | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|
| Fly ash | 13.4 | [57] |
| Granular active carbon | 21.5 | [58] |
| Neem leaf | 8.8/19.6 | [59] |
| Rice biomass8 | 8.1 | [60] |
| SNCM * | 20.0 | [61] |
| T10_Si49-700 | 9.3 (24 h) ** 15.2 (96 h) *** | This study |
| T10_Si49-600 | 6.6 (24 h) ** 12.8 (96 h) *** | This study |
| Membrane Material | Sintering Temp. (°C) | Porosity (%) | Pore Size (µm) | Thickness (mm) | Pressure (bar) | Flexural Strength (MPa) | Oil Conc. (mg/L) | Oil Rej. (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | 1350 | 47 | 0.4 | 5 | 1 | 15 | 284 | 99.9 | [63] |
| Al2O3 | 1300 | 37 | 0.5 | 3 | 2 | 22 | 200 | 99 | [64] |
| Al2O3 + fly ash-mullite | 1050 | 34.5 | 0.1 | 3 | 0.5 | 30 | - | - | [65] |
| Moroccan clay | 950 | 31–40 | 1.5–2.8 | 1.5 | 0–0.12 | 14–16 | - | - | [66] |
| Moroccan clay/phosphate. | 1100 | 28 | 2.5 | 1.6 | 0.12 | 17.5 | - | - | [67] |
| Mullite whisker (MoO3) | 1400 | 47 | 0.19 | 1.5 | 0.5–2 | 34 ± 4 | 250 | 97 | [68] |
| Monolithic mullite | 1400 | 64 | 0.3 | - | 2 | 42 ± 5 | 200–1000 | 96 | [69] |
| Silicate/clay-mineral | 1000 1050 1100 | 32 33 34 | 0.29 0.37 0.67 | 3 | 3 | 32 ± 3 30 ± 5.5 28 ± 5 | 600 | 86 | [70] |
| SiOC | 1200 | 42 | 0.59 | 0.65 | 0.5–2.0 | 23 ± 2 | 1000 | 94.6 | [41] |
| TiO2/clay/quartz/feldspar | 950 | 37–52 | 0.8–1.0 | 2 | 0.7–3.5 | 28–33 | 50–200 | 70–99 | [54] |
| Si3N4 | 1650 | 46–56 | 0.61 | - | 1–2 | 51–105 | 1000 | 83–88 | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontão, N.C.; Ferrari, L.N.; Sapatieri, J.C.; Rezwan, K.; Wilhelm, M. Influence of the Pyrolysis Temperature and TiO2-Incorporation on the Properties of SiOC/SiC Composites for Efficient Wastewater Treatment Applications. Membranes 2022, 12, 175. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes12020175
Fontão NC, Ferrari LN, Sapatieri JC, Rezwan K, Wilhelm M. Influence of the Pyrolysis Temperature and TiO2-Incorporation on the Properties of SiOC/SiC Composites for Efficient Wastewater Treatment Applications. Membranes. 2022; 12(2):175. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes12020175
Chicago/Turabian StyleFontão, Natália C., Lucas N. Ferrari, Joice C. Sapatieri, Kurosch Rezwan, and Michaela Wilhelm. 2022. "Influence of the Pyrolysis Temperature and TiO2-Incorporation on the Properties of SiOC/SiC Composites for Efficient Wastewater Treatment Applications" Membranes 12, no. 2: 175. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes12020175