Risk of Depression and Suicidality among Diabetic Patients: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Experimental Section
2.1. Data Sources and Search Strategies
2.2. Eligibility Criteria and Study Selection
2.3. Data Extraction
2.4. Risk of Bias
2.5. Data Analysis
3. Results
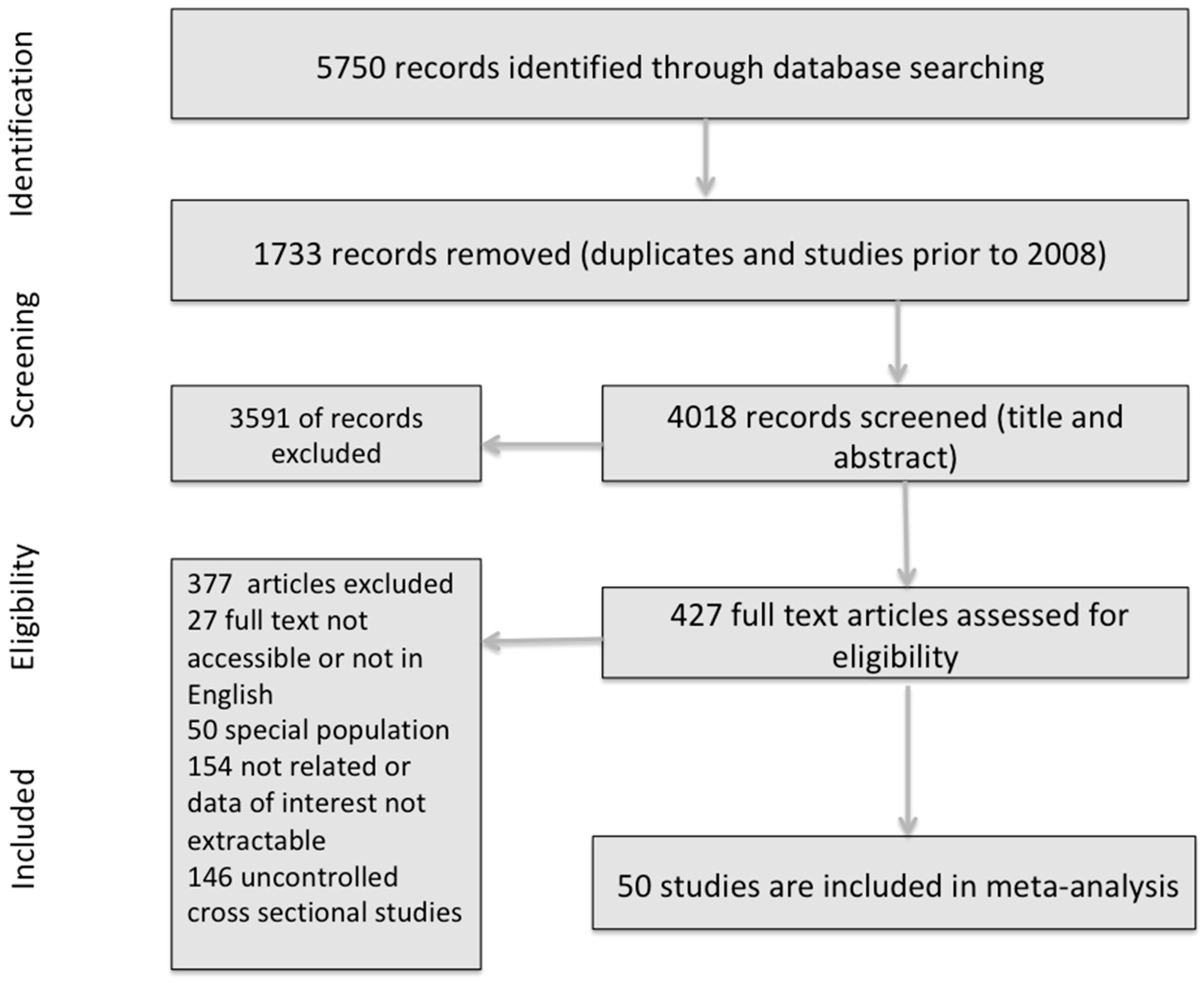
3.1. Study Selection
3.2. Study Characteristics
3.2.1. Depression
3.2.2. Suicidality
3.3. Main Meta-Analysis Results
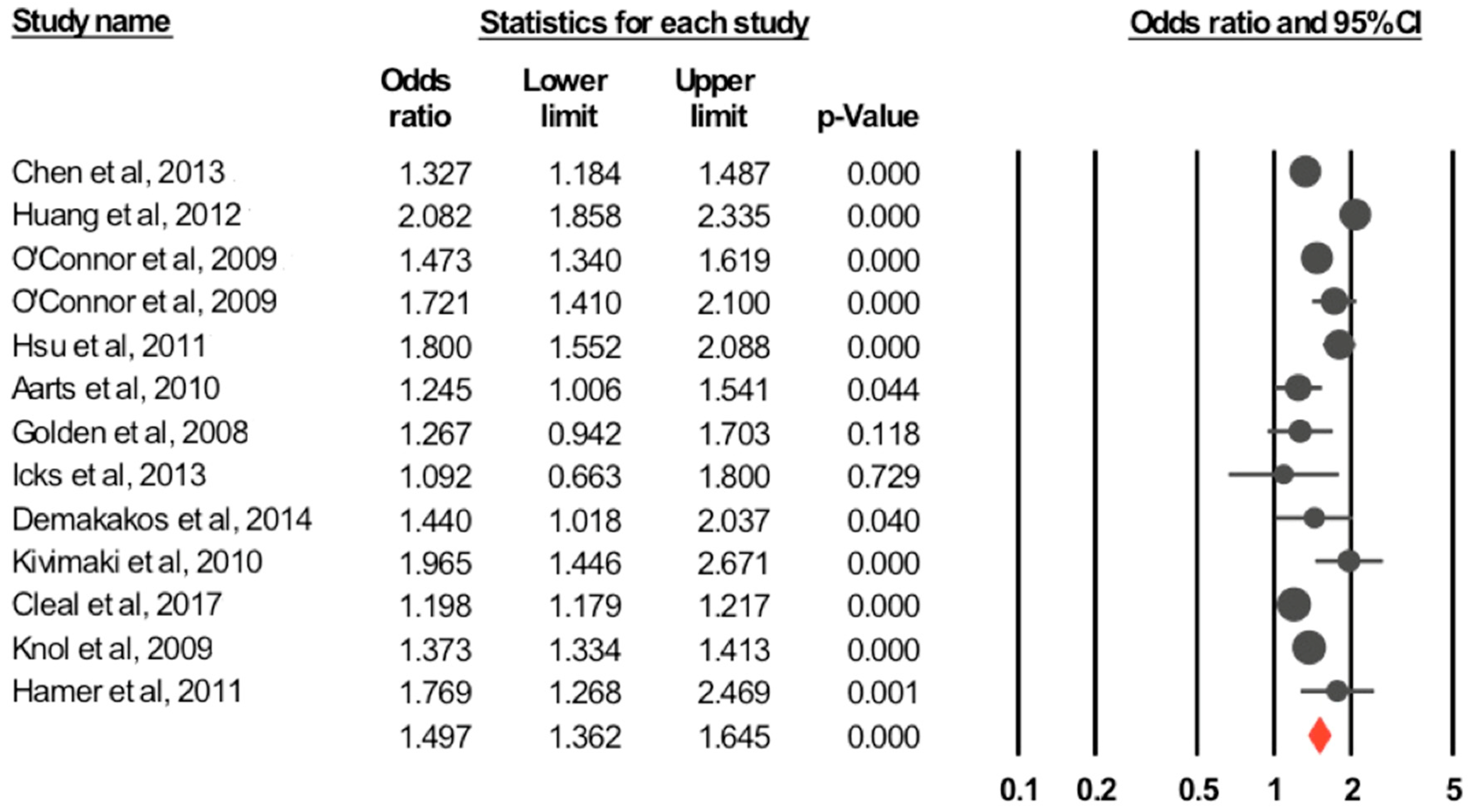
3.3.1. Depression
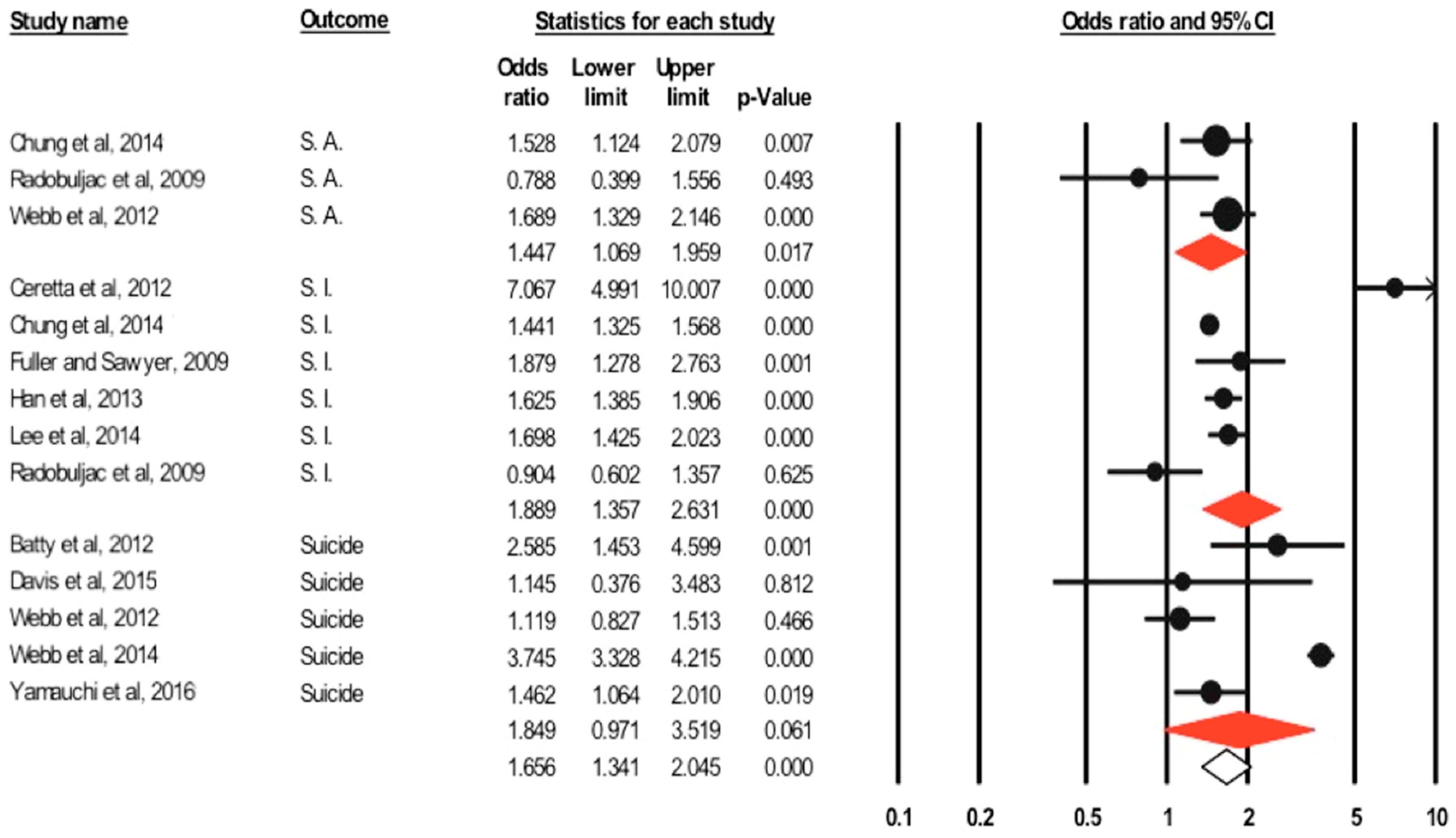
3.3.2. Suicidality
3.4. Subgroup Analysis and Meta-Regression
3.4.1. Depression
3.4.2. Suicidality
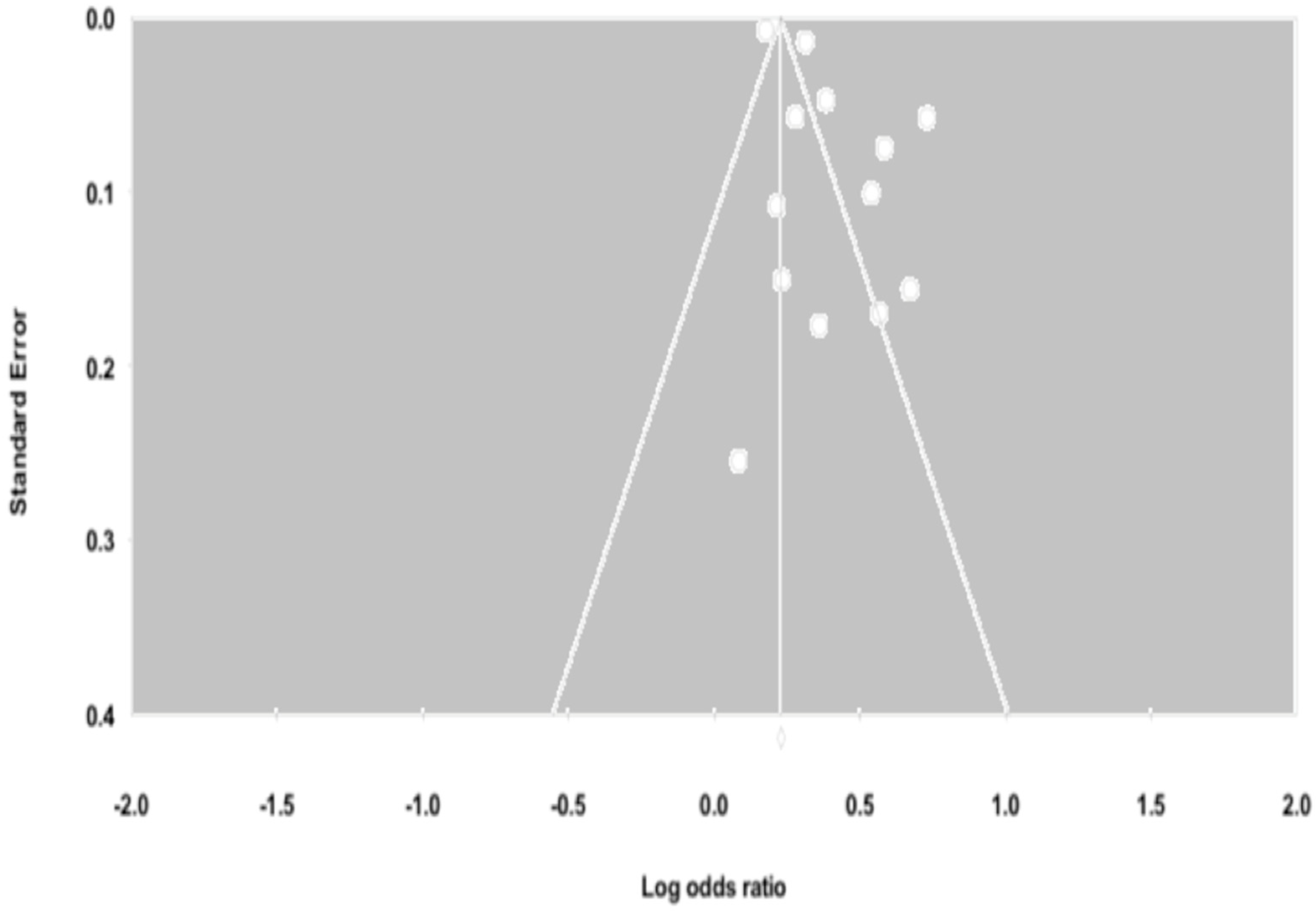
3.5. Influential Analysis and Publication Bias
4. Discussion
4.1. Strengths and Limitations
4.2. Implications for Future Research and Clinical Practice
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes, 7 Eds; International Diabetes Federation: Brussels, Belgium, 2015; Available online: http://www.diabetesatlas.org (accessed on 12 May 2018).
- World Health Organization. Global Report on Diabetes; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Robinson, D.J.; Luthra, M.; Vallis, M. Canadian diabetes association clinical practice guidelines expert committee. Canadian diabetes association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Can. J. Diabetes 2013, 37, S1–S212. [Google Scholar]
- Bădescu, S.V.; Tătaru, C.; Kobylinska, L.; Georgescu, E.L.; Zahiu, D.M.; Zăgrean, A.M.; Zăgrean, L. The association between diabetes mellitus and depression. J. Med. Life 2016, 9, 120. [Google Scholar] [PubMed]
- Katon, W.J. Epidemiology and treatment of depression in patients with chronic medical illness. Dialog. Clin. Neurosci. 2011, 13, 7. [Google Scholar]
- Anderson, R.J.; Freedland, K.E.; Clouse, R.E.; Lustman, P.J. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care 2001, 24, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Rotella, F.; Mannucci, E. Diabetes mellitus as a risk factor for depression. A meta-analysis of longitudinal studies. Diabetes Res. Clin. Pract. 2013, 99, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Nouwen, A.; Winkley, K.; Twisk, J.; Lloyd, C.E.; Peyrot, M.; Ismail, K.; Pouwer, F. European Depression in Diabetes (EDID) Research Consortium. Type 2 diabetes mellitus as a risk factor for the onset of depression: A systematic review and meta-analysis. Diabetologia 2010, 53, 2480–2486. [Google Scholar] [CrossRef] [PubMed]
- Samaan, Z.; Garasia, S.; Gerstein, H.C.; Engert, J.C.; Mohan, V.; Diaz, R.; Anand, S.S.; Meyre, D. Lack of association between type 2 diabetes and major depression: Epidemiologic and genetic evidence in a multiethnic population. Transl. Psychiatry 2015, 5, e618. [Google Scholar] [CrossRef] [PubMed]
- Agardh, E.; Allebeck, P.; Hallqvist, J.; Moradi, T.; Sidorchuk, A. Type 2 diabetes incidence and socio-economic position: A systematic review and meta-analysis. Int. J. Epidemiol. 2011, 40, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, I.; Tsigos, C. Stress hormones: Physiological stress and regulation of metabolism. Curr. Opin. Pharmacol. 2009, 9, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, N.C.; Kyratzi, E.; Lamprokostopoulou, A.; Chrousos, G.P.; Charmandari, E. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation 2015, 22, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Moulton, C.D.; Pickup, J.C.; Ismail, K. The link between depression and diabetes: The search for shared mechanisms. Lancet Diabetes Endocrinol. 2015, 3, 461–471. [Google Scholar] [CrossRef]
- Wang, X.; Bao, W.; Liu, J.; Ouyang, Y.Y.; Wang, D.; Rong, S.; Xiao, X.; Shan, Z.L.; Zhang, Y.; Yao, P.; et al. Inflammatory markers and risk of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2013, 36, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Egede, L.E.; Nietert, P.J.; Zheng, D. Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care 2005, 28, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Ciechanowski, P.S.; Katon, W.J.; Russo, J.E. Depression and diabetes: Impact of depressive symptoms on adherence, function, and costs. Arch. Int. Med. 2000, 160, 3278–3285. [Google Scholar] [CrossRef]
- Lin, E.H.; Katon, W.; Von Korff, M.; Rutter, C.; Simon, G.E.; Oliver, M.; Ciechanowski, P.; Ludman, E.J.; Bush, T.; Young, B. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care 2004, 27, 2154–2160. [Google Scholar] [CrossRef] [PubMed]
- Peyrot, M.; Rubin, R.R. Persistence of depressive symptoms in diabetic adults. Diabetes Care 1999, 22, 448–452. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Suicide. Available online: http://www.who.int/mediacentre/factsheets/fs398/en/ (accessed on 19 December 2017).
- Klonsky, E.D.; May, A.M.; Saffer, B.Y. Suicide, suicide attempts, and suicidal ideation. Ann. Rev. Clin. Psychol. 2016, 12, 307–330. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Kim, H.J.; Choi, Y.J.; Lee, K.W.; Kim, D.J. Increased risk of suicidal ideation in Korean adults with both diabetes and depression. Diabetes Res. Clin. Pract. 2013, 101, e14–e17. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.T.; Kontopantelis, E.; Doran, T.; Qin, P.; Creed, F.; Kapur, N. Suicide risk in primary care patients with major physical diseases: A case-control study. Arch. Gen. Psychiatry 2012, 69, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Radobuljac, M.D.; Bratina, N.U.; Battelino, T.; Tomori, M. Lifetime prevalence of suicidal and self-injurious behaviors in a representative cohort of Slovenian adolescents with type 1 diabetes. Pediatr. Diabetes 2009, 10, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2009; Available online: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 May 2018).
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis; Biostat Inc.: Englewood, NJ, USA, 2014. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, S.; Tweedie, R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar]
- Chung, J.H.; Moon, K.; Kim, D.H.; Min, J.W.; Kim, T.H.; Hwang, H.J. Suicidal ideation and suicide attempts among diabetes mellitus: The Korea National Health and Nutrition Examination Survey (KNHANES IV, V.) from 2007 to 2012. J. Psychosom. Res. 2014, 77, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Ceretta, L.B.; Réus, G.Z.; Abelaira, H.M.; Jornada, L.K.; Schwalm, M.T.; Hoepers, N.J.; Tomazzi, C.D.; Gulbis, K.G.; Ceretta, R.A.; Quevedo, J. Increased prevalence of mood disorders and suicidal ideation in type 2 diabetic patients. Acta Diabetol. 2012, 49, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Hahm, M.I.; Lee, S.G. Risk of suicidal ideation in diabetes varies by diabetes regimen, diabetes duration, and HbA1c level. J. Psychosom. Res. 2014, 76, 275–279. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.J.; Crain, A.L.; Rush, W.A.; Hanson, A.M.; Fischer, L.R.; Kluznik, J.C. Does diabetes double the risk of depression? Ann. Family Med. 2009, 7, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Tabák, A.G.; Lawlor, D.A.; Batty, G.D.; Singh-Manoux, A.; Jokela, M.; Virtanen, M.; Salo, P.; Oksanen, T.; Pentti, J.; et al. Antidepressant use before and after the diagnosis of type 2 diabetes: A longitudinal modeling study. Diabetes Care 2010, 33, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Cleal, B.; Panton, U.H.; Willaing, I.; Holt, R.I. Diabetes and depression in Denmark 1996–2010: National data stratified by occupational status and annual income. Diabet. Med. 2017, 34, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Knol, M.J.; Geerlings, M.I.; Grobbee, D.E.; Egberts, A.C.; Heerdink, E.R. Antidepressant use before and after initiation of diabetes mellitus treatment. Diabetologia 2009, 52, 425. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Chan, Y.T.; Chen, H.F.; Ko, M.C.; Li, C.Y. Population-based cohort analyses of the bidirectional relationship between type 2 diabetes and depression. Diabetes Care 2013, 36, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Wang, S.Y.; Lee, M.H.; Chiu, H.C. Prevalence and incidence of mental illness in diabetes: A national population-based cohort study. Diabetes Res. Clin. Pract. 2011, 93, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.M.; Su, L.T.; Chang, H.M.; Sung, F.C.; Lyu, S.Y.; Chen, P.C. Diabetes mellitus and risk of subsequent depression: A longitudinal study. Int. J. Nurs. Stud. 2012, 49, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Aarts, S.; Van den Akker, M.; van Boxtel, M.P.; Jolles, J.; Winkens, B.; Metsemakers, J.F. Diabetes mellitus type II as a risk factor for depression: A lower than expected risk in a general practice setting. Eur. J. Epidemiol. 2009, 24, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.H.; Lazo, M.; Carnethon, M.; Bertoni, A.G.; Schreiner, P.J.; Roux, A.V.; Lee, H.B.; Lyketsos, C. Examining a bidirectional association between depressive symptoms and diabetes. Jama 2008, 299, 2751–2759. [Google Scholar] [CrossRef] [PubMed]
- Icks, A.; Albers, B.; Haastert, B.; Pechlivanis, S.; Pundt, N.; Slomiany, U.; Erbel, R.; Jöckel, K.H.; Kruse, J.; Kulzer, B.; et al. Risk for high depressive symptoms in diagnosed and previously undetected diabetes: 5-year follow-up results of the Heinz Nixdorf Recall study. PLoS ONE. 2013, 8, e56300. [Google Scholar] [CrossRef] [PubMed]
- Demakakos, P.; Zaninotto, P.; Nouwen, A. Is the association between depressive symptoms and glucose metabolism bidirectional? Evidence from the English Longitudinal Study of Ageing (ELSA). Psychosom. Med. 2014, 76, 555. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Batty, G.D.; Kivimaki, M. Haemoglobin A1c, fasting glucose and future risk of elevated depressive symptoms over 2 years of follow-up in the English Longitudinal Study of Ageing. Psychol. Med. 2011, 41, 1889–1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, E.; Chamberlain, A.M.; Pendegraft, R.S.; Petterson, T.M.; Bobo, W.V.; Pathak, J. Quantifying the impact of chronic conditions on a diagnosis of major depressive disorder in adults: A cohort study using linked electronic medical records. BMC Psychiatry 2016, 16, 114. [Google Scholar] [CrossRef] [PubMed]
- James, B.O.; Omoaregba, J.O.; Eze, G.; Morakinyo, O. Depression among patients with diabetes mellitus in a Nigerian teaching hospital. S. Afr. J. Psychiatry 2010, 16, 61–64. [Google Scholar] [CrossRef]
- Lin, E.H.; Von Korff, M. Mental disorders among persons with diabetes—results from the World Mental Health Surveys. J. Psychosom. Res. 2008, 65, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Meurs, M.; Roest, A.M.; Wolffenbuttel, B.H.; Stolk, R.P.; de Jonge, P.; Rosmalen, J.G. Association of depressive and anxiety disorders with diagnosed versus undiagnosed diabetes: An epidemiological study of 90,686 participants. Psychosom. Med. 2016, 78, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.K.; Obsteter, J.; Hall, L.S.; Hayward, C.; Thomson, P.A.; Smith, B.H.; Padmanabhan, S.; Hocking, L.J.; Deary, I.J.; Porteous, D.J.; et al. Investigating shared aetiology between type 2 diabetes and major depressive disorder in a population based cohort. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 174, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Bessel, M.; Vigo, Á.; Poyastro, A.; Nunes, M.A.; Duncan, B.B.; Schmidt, M.I. Stages of hyperglycemia and common mental disorders in adults-The Brazilian Study of Adult Health (ELSA-Brasil). Sao Paulo Med. J. 2016, 134, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Inagaki, M.; Yonemoto, N.; Iwasaki, M.; Akechi, T.; Sawada, N.; Iso, H.; Noda, M.; Tsugane, S. History of diabetes and risk of suicide and accidental death in Japan: The Japan Public Health Centre-based Prospective Study, 1990–2012. Diabetes Metab. 2016, 42, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Berge, L.I.; Riise, T.; Fasmer, O.B.; Lund, A.; Oedegaard, K.J.; Hundal, Ø. Risk of depression in diabetes is highest for young persons using oral anti-diabetic agents. Diabet. Med. 2012, 29, 509–514. [Google Scholar] [CrossRef] [PubMed]
- van Dooren, F.E.; Denollet, J.; Verhey, F.R.; Stehouwer, C.D.; Sep, S.J.; Henry, R.M.; Kremers, S.P.; Dagnelie, P.C.; Schaper, N.C.; van der Kallen, C.J.; et al. Psychological and personality factors in type 2 diabetes mellitus, presenting the rationale and exploratory results from The Maastricht Study, a population-based cohort study. BMC Psychiatry 2016, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Icks, A.; Kruse, J.; Dragano, N.; Broecker-Preuss, M.; Slomiany, U.; Mann, K.; Jöckel, K.H.; Erbel, R.; Giani, G.; Moebus, S. Are symptoms of depression more common in diabetes? Results from the Heinz Nixdorf Recall study. Diabet. Med. 2008, 25, 1330–1336. [Google Scholar] [PubMed]
- Bruce, D.G.; Davis, W.A.; Hunter, M.L.; Peters, K.E.; Davis, T.M.; Starkstein, S.E. Lifetime depression history and depression risk in type 2 diabetes: A. case-control study. J. Diabetes Complicat. 2016, 30, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Foran, E.; Hannigan, A.; Glynn, L. Prevalence of depression in patients with type 2 diabetes mellitus in Irish primary care and the impact of depression on the control of diabetes. Ir. J. Med. Sci. 2015, 184, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Albertorio-Diaz, J.R.; Eberhardt, M.S.; Oquendo, M.; Mesa-Frias, M.; He, Y.; Jonas, B.; Kang, K. Depressive states among adults with diabetes: Findings from the National Health and Nutrition Examination Survey, 2007–2012. Diabetes Res. Clin. Pract. 2017, 127, 80–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäntyselkä, P.; Korniloff, K.; Saaristo, T.; Koponen, H.; Eriksson, J.; Puolijoki, H.; Timonen, M.; Sundvall, J.; Kautiainen, H.; Vanhala, M. Association of depressive symptoms with impaired glucose regulation, screen-detected, and previously known type 2 diabetes: Findings from the Finnish D2D Survey. Diabetes Care 2011, 34, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, V.; Adriaanse, M.C.; Van’t Riet, E.; Snoek, F.J.; Dekker, J.M.; Nijpels, G. Depression, anxiety and glucose metabolism in the general Dutch population: The new Hoorn study. PLoS ONE 2010, 5, e9971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Guo, X.; Jiang, H.; Sun, G.; Sun, Y.; Abraham, M.R. Diagnosed but Not Undiagnosed Diabetes Is Associated with Depression in Rural Areas. Int. J. Environ. Res. Public Health 2016, 13, 1136. [Google Scholar] [CrossRef] [PubMed]
- Saglam, Z.A.; Saler, T.; Erdem, T.Y.; Ataoglu, E.; Temiz, L.U.; Yenigun, M. The frequency of depression in Turkish patients with diabetes and diabetic complications. Endocrinologist 2010, 20, 19–22. [Google Scholar] [CrossRef]
- Kim, W.K.; Shin, D.; Song, W.O. Depression and its comorbid conditions more serious in women than in men in the United States. J. Women’s Health 2015, 24, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.; Ferrari, U.; Seissler, J.; Niessen, L.; Lechner, A. Association between depression and diabetes amongst adults in Bangladesh: A hospital based case–control study. J. Glob. Health 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wiltink, J.; Michal, M.; Wild, P.S.; Schneider, A.; König, J.; Blettner, M.; Münzel, T.; Schulz, A.; Weber, M.; Fottner, C.; et al. Associations between depression and diabetes in the community: Do symptom dimensions matter? Results from the Gutenberg Health Study. PLoS ONE 2014, 9, e105499. [Google Scholar] [CrossRef] [PubMed]
- Adriaanse, M.C.; Dekker, J.M.; Heine, R.J.; Snoek, F.J.; Beekman, A.J.; Stehouwer, C.D.; Bouter, L.M.; Nijpels, G.; Pouwer, F. Symptoms of depression in people with impaired glucose metabolism or Type 2 diabetes mellitus: The Hoorn Study. Diabet. Med. 2008, 25, 843–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westra, S.; Simsek, S.; Rutters, F.; Krul-Poel, Y.M.; Stehouwer, C.D.; Dekker, J.M.; Pouwer, F. Low vitamin D levels are not a contributing factor to higher prevalence of depressive symptoms in people with Type 2 diabetes mellitus: The Hoorn study. Diabet. Med. 2017, 34, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Ross, J.; Seminog, O.; Hawton, K.; Goldacre, M.J. Risk of self-harm and suicide in people with specific psychiatric and physical disorders: Comparisons between disorders using English national record linkage. J. R. Soc. Med. 2014, 107, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.T.; Kontopantelis, E.; Doran, T.; Qin, P.; Creed, F.; Kapur, N. Risk of self-harm in physically ill patients in UK primary care. J. Psychosom. Res. 2012, 73, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.K.; Grannemann, B.D.; Lingvay, I.; Trivedi, M.H. Brief report: Depression and history of suicide attempts in adults with new-onset Type 2 Diabetes. Psychoneuroendocrinology 2013, 38, 2810–2814. [Google Scholar] [CrossRef] [PubMed]
- Igwe, M.N.; Uwakwe, R.; Ahanotu, C.A.; Onyeama, G.M.; Bakare, M.O.; Ndukuba, A.C. Factors associated with depression and suicide among patients with diabetes mellitus and essential hypertension in a Nigerian teaching hospital. Afr. Health Sci. 2013, 13, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Handley, T.E.; Ventura, A.D.; Browne, J.L.; Rich, J.; Attia, J.R.; Reddy, P.; Pouwer, F.; Speight, J. Suicidal ideation reported by adults with type 1 or type 2 diabetes: Results from Diabetes MILES—Australia. Diabet. Med. 2016, 33, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Sendela, J.; Zdunczyk, B.; Trippenbach-Dulska, H.; Szypowska, A. Prevalence of depressive symptoms in school aged children with type 1 diabetes–A questionnaire study. Psychiatr. Pol. 2015, 49, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Fuller-Thomson, E.; Sawyer, J.L. Lifetime prevalence of suicidal ideation in a representative sample of Canadians with type 1 diabetes. Diabet. Res. Clin. Pract. 2009, 83, e9–e11. [Google Scholar] [CrossRef] [PubMed]
- Batty, G.D.; Kivimaki, M.; Park, I.S.; Jee, S.H. Diabetes and raised blood glucose as risk factors for future suicide: Cohort study of 1 234 927 Korean men and women. J. Epidemiol. Community Health 2012, 66, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.T.; Lichtenstein, P.; Dahlin, M.; Kapur, N.; Ludvigsson, J.F.; Runeson, B. Unnatural deaths in a national cohort of people diagnosed with diabetes. Diabetes Care 2014, 37, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.A.; Starkstein, S.E.; Bruce, D.G.; Davis, T.M. Risk of suicide in Australian adults with diabetes: The Fremantle Diabetes Study. Int. Med. J. 2015, 45, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.S.; Mamun, A.A.; Clavarino, A.M.; Kairuz, T. Incidence and risk of depression associated with diabetes in adults: Evidence from longitudinal studies. Community Ment. Health J. 2015, 51, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Korczak, D.J.; Pereira, S.; Koulajian, K.; Matejcek, A.; Giacca, A. Type 1 diabetes mellitus and major depressive disorder: Evidence for a biological link. Diabetologia 2011, 54, 2483. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Van Harten, B.; de Leeuw, F.E.; Weinstein, H.C.; Scheltens, P.; Biessels, G.J. Brain imaging in patients with diabetes: A systematic review. Diabetes Care 2006, 29, 2539–2548. [Google Scholar] [CrossRef] [PubMed]
- Berge, L.I.; Riise, T.; Tell, G.S.; Iversen, M.M.; Østbye, T.; Lund, A.; Knudsen, A.K. Depression in persons with diabetes by age and antidiabetic treatment: A cross-sectional analysis with data from the Hordaland Health Study. PLoS ONE 2015, 10, e0127161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, R.A.; Tostes, M.; Queiroz, V.A.; Rodacki, M.; Zajdenverg, L. Insulin mediated improvement in glycemic control in elderly with type 2 diabetes mellitus can improve depressive symptoms and does not seem to impair health-related quality of life. Diabet. Metab. Syndr. 2015, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Renn, B.N.; Feliciano, L.; Segal, D.L. The bidirectional relationship of depression and diabetes: A systematic review. Clin. Psychol. Rev. 2011, 31, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Kammer, J.R.; Hosler, A.S.; Leckman-Westin, E.; DiRienzo, G.; Osborn, C.Y. The association between antidepressant use and glycemic control in the Southern Community Cohort Study (SCCS). J. Diabetes Complicat. 2016, 30, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roshanaei-Moghaddam, B.; Katon, W.J.; Russo, J. The longitudinal effects of depression on physical activity. Gen. Hosp. Psychiatry 2009, 31, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Nock, M.K.; Borges, G.; Bromet, E.J.; Alonso, J.; Angermeyer, M.; Beautrais, A.; Bruffaerts, R.; Chiu, W.T.; De Girolamo, G.; Gluzman, S.; et al. Cross-national prevalence and risk factors for suicidal ideation, plans and attempts. Br. J. Psychiatry 2008, 192, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimasu, K.; Kiyohara, C.; Miyashita, K. Suicidal risk factors and completed suicide: Meta-analyses based on psychological autopsy studies. Environ. Health Prev. Med. 2008, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- Mars, B.; Burrows, S.; Hjelmeland, H.; Gunnell, D. Suicidal behaviour across the African continent: A review of the literature. BMC Public Health 2014, 14, 606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Preventing Suicide: A Global Imperative; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Goldsmith, S.K. Reducing Suicide: A National Imperative; National Academy Press: Washington, DC, USA, 2002. [Google Scholar]
- Daigle, M. MMPI inmate profiles: Suicide completers, suicide attempters, and non-suicidal controls. Behav. Sci. Law 2004, 22, 833–842. [Google Scholar] [CrossRef] [PubMed]
- DeJong, T.M.; Overholser, J.C.; Stockmeier, C.A. Apples to oranges?: A direct comparison between suicide attempters and suicide completers. J. Affect. Disord. 2010, 124, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Author &Year | Country | Study Design | Setting | Patients N | Age | Female % | Type of Diabetes | Outcome Reported | Evaluation of Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Icks et al., 2013 [43] | Germany | Prospective cohort | Population based, mandatory residence in some cities | 3663 | 45–75 | 48.6 | Not specified, (included diagnosed, undiagnosed, and impaired glucose tolerance [IGT]) | Depression | Center for Epidemiologic Studies-Depression Scale (CES-D) (15 items) ≥17 |
| Cleal et al., 2017 [36] | Netherland | Prospective cohort | Population registers | 3,434,420 | 18–59 | 48.9 | Not specified Incident diabetes | Depression | Prescription of antidepressants |
| O’Connor et al., 2009 [34] | USA | Historical cohort 2 years | Patients enrolled in a health plan, services provided by primary care, family physicians and general internists | 2932 with incident diabetes and 14,144 with prevalent diabetes with equal number of matched controls | ≥40 Mean around 61 | 47.3% | Not specified but majority were type 2 International Classification of Diseases, ninth version (ICD-9) | Depression | ICD-9 ± antidepressants |
| Chen et al., 2013 [38] | Taiwan | Cohort | National health insurance claims and data linkage | Mean 60.1 ± 13.2 | 46.5 | Type 2 ICD-9 Clinical Modification (CM) | Depression | ICD-9 CM | |
| Hamer et al., 2011 [45] | UK | Prospective cohort | Community dwelling with older adults | 4338 | Mean 62.9 ± 9 | 45.2% | Not specified Self report physician diagnosis | Depression | CES-D (8 items) With a cut off ≥4 |
| Hsu et al., 2011 [40] | Taiwan | Cohort Median follow-up 6.5 years. | Claim data, national health insurance program | 14,048 diabetics and 55,608 control | ≥20 | Not specified (incident diabetes) ICD-9 CM | Depression | ICD-9 CM | |
| Golden et al., 2008 [42] | USA | Prospective cohort 3.1 years. | Part of multi ethnic study of atherosclerosis | 4847 | 45–84 | Type 2, fasting blood glucose (FBG) ≥126 or on oral hypoglycemic agent (OHA) or Insulin | Depression | CED-D ≥16 or use of antidepressant medications or both | |
| Huang et al., 2012 [39] | Taiwan | Prospective cohort 4 years. | Service claim records | 200,432 | Not specified ICD-9 CM | Depression | ICD-9 CM | ||
| Demakakos et al., 2014 [44] | UK | Prospective cohort | Community dwellings | 4238 | ≥50 | Not specified Self report physician diagnosis | Depression | CES-D (8 items) With a cut off ≥4 | |
| Knol et al., 2009 [37] | Netherland | Cohort | Pharmacy registry database | 49,593 diabetics and 154,441 non diabetics | >40 | Not specified Incident diabetes | Depression | Incident use of antidepressants | |
| Aarts et al., 2010 [41] | Netherland | Retrospective cohort 7.7–7.9 years. | General practice patients | 6140 diabetics and 18,416 control | >40–97 Mean 63.8 ± 11.2 | 51% among cases and 53% among control | Type 2 International Classification of Primary Care (ICPC) diagnosis based on FBG >124 | Depression | ICPC code through diagnostic interview |
| Kivimaki et al., 2010 [35] | Finland | Cohort | Employees Record linkage | 493 diabetics and 2450 control | 25–65 | 58% | Type 2 Incident diabetes, first diagnosed as eligible to treatment | Depression | Antidepressants |
| Ryu et al., 2016 [46] | USA | Nested case-control | Electronic health records of primary care patients | Cases with Major Depressive Disorders (MDD) = 11,375 and equal number of controls | Median age 43 | 65% | Not specified At least two diagnostic codes for the condition >30 days apart | Depression | ≥2 MDD-related (ICD-9-CM) diagnosis codes, ≥1 anti-depressant prescription, ≥1 mention of MDD diagnoses within inpatient or outpatient |
| Icks et al., 2008 [55] | Germany | Cross-sectional | Baseline data from German Heinz Nixdorf Recall study | 2090 diabetic and 4595 non diabetic | 45–75 | 50.2% | Not specified Self report physician diagnosis or medications, or FBG and random blood glucose (RBG) | Depression | CES-D short form ≥15 |
| James et al., 2010 [47] | Nigeria | Cross-sectional | Outpatient clinic in tertiary center | 200 cases and 200 control | 20–64 Mean 47.1 ± 9.6 | 54% | Not specified, diagnosed for >1 year Based on WHO criteria | Depression | Schedule for the Clinical Assessment in Neuropsychiatry (SCAN) and Beck Depression Inventory (BDI) (21 items) ≥10 |
| Bruce et al., 2016 [56] | Australia | Cross-sectional | Prior involvement in Brusselton Health Survey (community based study) | 184 cases and 184 paired controls | Mean 70.2 ± 10.1 | 50% | Type 2 Self report and FBG | Depression | Patient Health Questionnaire (PHQ-9) and Brief Lifetime Depression Scale (BLDS) according to Diagnostic and Statistical Manual for Mental Disorders (DSM-VI) criteria for major and minor depression |
| Lin et al., 2008 [48] | 17 countries | Cross-sectional | Household residing adults | 42,697 | Mean between 35.8–48.2 | Between 47.5%–55.1% | Not specified Self report physician diagnosis or medications | Depression | Composite International Diagnostic Interview (CIDI) |
| Van Doreen et al., 2016 [54] | Netherland | Cross-sectional | Baseline for a population based study | 862 | Mean 64 ± 7 | 30% in diabetics and 51% in non- diabetics | Type 2 on Insulin or Oral Glucose Tolerance Test (OGTT) | Depression | Mini-International Neuropsychiatric Interview (MINI) and PHQ-9 ≥10 |
| Foran et al., 2015 [57] | Ireland | Cross-sectional | Part of Cardiovascular Multi-morbidity in Primary Care study | 283 diabetic and 283 non diabetic | >50 Mean 68 ± 9.5 | 41% | Type 2 | Depression | Hospital Anxiety Depression Scale-Depression (HADS-D) |
| Chung et al., 2014 [31] | Korea | Cross-sectional | Korean National Health and Nutrition Examination Survey (KNHANES IV, V) | 34,056 | ≥20 | 57.1% | Not specified Self report diagnosis, FBG ≥126, current use of anti-diabetic medications | Depression | Composite International Diagnostic Interview-Short Form (CIDI-SF) |
| Albertorio-Diaz et al., 2017 [58] | USA | Cross-sectional | NHANES data 2007–2012 | 7717 | ≥20 | Type 1 & 2 Self report diagnosis and lab evaluation | Depression | PHQ-9, DSM-IV text revision (TR) diagnostic criteria | |
| Berg et al., 2012 [53] | Norway | Cross-sectional | Norwegian prescription database | 34,342,333 | ≥20 | 50.9% | Not specified On anti-diabetic treatment | Depression | Antidepressants |
| Meurs et al., 2016 [49] | Netherland | Cross-sectional | Lifeline cohort study population | 90,686 | 18–93 Mean 45 | 59% | Not specified Self-reported use of anti-diabetic medication or diagnosis of diabetes | Depression | MINI |
| Mantyselka et al., 2011 [59] | Finland | Cross-sectional | Based on population survey, subjects | 2712 | 45–74 | Type 2 Self report diagnosis | Depression | BDI ≥10 and ≥16 | |
| Clarke et al., 2016 [50] | UK | Cross-sectional | Scottish family health study | 23,690 | >18 | 51.2% | Type 2 Self report diagnosis and medication use | Depression | Structured Clinical Interview for DSM (SCID) |
| Bouwman et al., 2010 [60] | Netherland | Cross-sectional | 2667 | 40–65 | 46.4% | Type 2 FBG >7 mmol/L or 2hrPG 11.1 mmol/L | Depression | CES-D ≥16 | |
| Li et al., 2016 [61] | China | Cross-sectional | 11,531 | ≥35 | Not specified fasting plasma glucose (FPG) ≥7 mmol/L or previous diagnosis by a medical practitioner | Depression | PHQ-9 ≥10 | ||
| Saglam et al., 2010 [62] | Turkey | Cross- sectional | Outpatient diabetes clinic | 500 diabetic patients and 90 control | 35–65 | Type 1 & 2 Known diabetics for at least 1 yr. | Depression | BDI (21 items) >13 | |
| Kim et al., 2015 [63] | USA | Cross-sectional | NHANES 2007–2008 and 2009–2010 | 2266 | 20–79 | Not specified Self report diagnosis | Depression | PHQ-9 ≥10 | |
| Islam et al., 2015 [64] | Bangladesh | Cross-sectional | Tertiary hospital attendants | 591 cases and 591 control | 20–60 Mean 50.4 ± 11.4 | 57% | Not specified Attending physician diagnosis | Depression | PHQ-9 ≥10 |
| Wiltink et al., 2014 [65] | Germany | Cross-sectional | Gutenberg health study population | 15,010 | 35–74 Mean 55 | 50.4% | Not specified Self-reported diagnosis and FBG >126 or RBG >200 | Depression | PHQ-9 ≥10 |
| Bessel et al., 2016 [51] | Brazil | Cross-sectional | Civil servants active or retired | 14,447 | 35–74 | 54.1% | Not specified Self report diagnosis, medication use, HbA1c, OGTT | Depression | Clinical Interview Schedule-Revised (CIS-R) clinical interview criteria revised |
| Adriaanse et al., 2008 [66] | Netherland | Cross-sectional | The Hoorn study population | 550 | 69.5 ± 6.3 | 49.8% | Type 2, OGTT or on treatment | Depression | CES-D ≥16 |
| Westra et al., 2016 [67] | Netherland | Cross-sectional | 527 | 60–87 | Type 2 World Health Organization (WHO) criteria, known type 2 and using anti-diabetic medications or diet | Depression | CES-D ≥16 | ||
| Lee et al., 2014 [33] | South Korea | Cross-sectional | KNHANES dataset | 9159 | ≥40 | Not specified | Depression | Single question | |
| Ceretta et al., 2012 [32] | Brazil | Cross-sectional | Outpatients | 994 cases and 2145 controls | >18 | Type 2 >5 years. On insulin >1 year. | Depression and SI | MINI |
| Author & Year | Outcome | Total Number of Diabetic Patients | Number of Diabetic Events | Reported Estimate (95% CI) | Adjusted Estimate (95% CI) | Adjustments |
|---|---|---|---|---|---|---|
| Icks et al., 2013 [43] | Depression (diagnosed diabetics) | 255 | 18 | 1 (0.59–1.68) | age and sex, body mass index (BMI), myocardial infarction (MI), stroke, physical activity, education | |
| Cleal et al., 2017 [36] | Depression | 98,006 | 19,849 | |||
| O’Connor et al., 2009 [34] | Depression | Prevalent diabetes 14,144 | 1117 | For subjects with low physician visits OR = 1.46 (1.19–1.8 ) | Age, sex, number of primary care visits | |
| Incident diabetes 2932 | 276 | |||||
| Chen et al., 2013 [38] | Depression | 16,957 | 713 | Hazard Ratio (HR) = 1.43 (1.16–1.77) | Age, sex, geographic area, urbanization statuses, and various comorbidities | |
| Hamer et al., 2011 [45] | Depression | Odds ratio (OR) = 1.52 (1.01–2.3) | Age, baseline depressive symptoms, sex, smoking, alcohol intake, social status, C-reactive protein (CRP), Cholesterol, and BMI | |||
| Hsu et al., 2011 [40] | Depression | 14,048 | 258 | HR = 1.79 (1.54–2.07) | HR = 1.46 (1.24–1.71) | Age, sex, occupation and income and comorbidity including hypertension, stroke, hyperlipidemia and coronary artery disease |
| Golden et al., 2008 [42] | Depression | 417 | Incidence density 62/1000 for diabetic patients and 37/1000 non diabetics 60 developed depression | OR = 1.52 (1.09–2.12) | Race, ethnicity, exam site, BMI, Socio-economic status (SES), lifestyle factors, diabetes severity (dyslipidemia, hypertension (HTN), HTN medications microalbuminuria) | |
| Huang et al., 2012 [39] | Depression | 5685 | 331 (cumulative incidence) | Annual prevalence for diabetics 34/1000 for non-diabetics = 11/1000 Cumulative prevalence 92/1000 for diabetics and 41/1000 for non-diabetics | ||
| Demakakos et al., 2014 [44] | Depression | OR (52–64 years.) = 2.17 (1.33–3.56) OR (>65 years.) = 0.96 (0.59–1.57) | OR (52–64 years.) = 1.83 (1.06–3.18) OR (>65 years) = 0.81 (0.48–1.37) | Age, elevated depressive symptoms at baseline, sex, marital status, education, household wealth, cardio-vascular and non cardiovascular comorbidities, BMI health behavior smoking alcohol consumption frequency and physical activity | ||
| Knol et al., 2009 [37] | Depression | 49,593 | 7631 | Relative risk (RR) = 1.71 (1.36–2.13) | Age, sex, chronic disease | |
| Aarts et al., 2010 [41] | Depression | 6140 | 122 | HR = 1.32 (1.19–1.48) | HR = 1.26 (1.12–1.42) | Age, practice identification code and depression preceding diabetes |
| Kivimaki et al., 2010 [35] | Depression | 493 | 36 | OR = 2 (1.57–2.55) | Matching was based on 6 variables: age group, sex, socioeconomic position, type of employment, type of employer, and geographic area workplace | |
| Ryu et al., 2016 [46] | Depression | 237 | 205 | OR = 2.8 (1.9–4.1) | Educational level and obesity | |
| Icks et al., 2008 [43] | Depression | 352 | 47 | OR (male) = 0.5 (0.27–0.91) OR (female) = 1.14 (0.73–1.76) | Age, co-morbidity, depression induced medications, smoking, activity level, living without a partner, and education | |
| James et al., 2010 [47] | Depression | 200 | 60 | |||
| Bruce et al., 2016 [56] | Depression | 184 | 23 | |||
| Lin et al., 2008 [48] | Depression | OR = 1.38 (1.15–1.66) | Age and gender | |||
| Van Doreen et al., 2016 [54] | Depression | 253 | 22 | OR = 1.73 (1.38–3.6) | Age, sex and education level | |
| Foran et al., 2015 [57] | Depression | 283 | 62 | |||
| Chung et al., 2014 [31] | Depression | 3846 | 678 | OR = 1.376 (1.258–1.504) | OR = 1.178 (1.07–1.297) | Age, sex, smoking, alcohol, education, income, physical activity, number of chronic diseases, presence of major cancer |
| Albertorio-Diaz et al., 2017 [58] | Depression | OR (minor) = 2.38 (1.78–3.19) OR (major) = 2.81 (1.92–4.11) | OR (minor) = 1.95 (1.39–2.74) OR (major) = 2.28 (1.45–3.57) | Effects of age, sex, race and ethnicity, education, body mass index, and poverty | ||
| Berg et al., 2012 [53] | 121,392 | 15,511 | OR = 1.53 (1.5–1.56) | Age and gender | ||
| Meurs et al., 2016 [49] | Depression | 1811 | 90 | OR = 1.39 (1.1–1.76) | Age, sex, added comorbidity and anxiety disorders | |
| Mantyselka et al., 2011 [59] | Depression | OR (>10) = 1.35 (0.84–2.15) OR (>16) = 1.56 (0.65–3.5) | Demographic, lifestyle, and biological factors | |||
| Clarke et al., 2016 [50] | Depression | 913 | 130 | |||
| Bouwman et al., 2010 [60] | Depression | 181 | 38 | OR = 1.86 (1.27–2.72) | OR = 1.77 (1.13–2.78) | Age, education, family history of diabetes, triglycerides, high density lipoproteins (HDL) cholesterol, total Cholesterol, hypertension, smoking and waist circumference |
| Li et al., 2016 [61] | Depression | 529 | 40 | OR = 1.7 (1.25–2.31) | Age, sex, and race, education level, family income, marital status, and family history of diabetes body mass index, diet score, sleep duration, current smoking, drinking status, and physical activity history of chronic disease, and any medication | |
| Seglam et al., 2010 [62] | Depression | 500 | 169 | |||
| Kim et al., 2015 [63] | Depression | 175 | 41 | OR = 2.24 (1.43–3.51) | OR = 1.65 (0.93–2.92) | Age, education, race/ethnicity, marital status, ratio of family income to poverty, physical activity, BMI, and waist circumference were controlled. |
| Islam et al., 2015 [64] | Depression | 591 | 100 | OR = 6.4 (3.4–12.3) | Education, age occupation, marital status, BMI, HTN, no of complications | |
| Wiltink et al., 2014 [65] | Depression | 1074 | 107 | |||
| Bessel et al., 2016 [51] | Depression | 1096 | 63 | (Prevalence ratio) PR = 1.31 (0.97–1.78) | Sex, age, race, marital status and smoking, physical activity, body mass index and waist-hip ratio. | |
| Adriaanse et al., 2008 [66] | Depression | 126 | 22 | OR (male) = 2.04 (0.76–5.49) OR (female) = 3.18 (1.31–7.74) | OR (male) = 1.52 (0.47–4.94) OR (female) = 2.76 (1.01–7.5) | Age, low education and diabetes symptoms (hyperglycemic, cardiovascular, neuropathic pain, sensibility and ophthalmological) |
| Westra et al., 2016 [67] | Depression | OR = 3.04 (1.57–5.88) | OR = 1.98 (0.95–4.12) | Age, total body fat percentage, physical activity, education level, time of blood/CES-D collection, serum 25-hydroxyvitamin D, sex | ||
| Lee et al., 2014 [33] | Depression | 811 | 152 | |||
| Ceretta et al., 2012 [32] | Depression | 996 | 664 | OR = 6.5 (5.4–7.5) | OR = 1.8 (1.7–2) |
| Author & Year | Country | Study Design | Setting | Patients N | Age | Female % | Type of Diabetes | Outcome Reported | Evaluation of Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Singhal et al., 2014 [68] | England | Retrospective Cohort | Hospital day cases or inpatients | 2,230,207 diabetic patient | ≥10 | -- | Not specified (Hospital records) | Self-Harm & Suicide | Record linkage/ICD-10 |
| Webb et al., 2012 [69] | UK | Nested Case Control | General practice research database | 48,426 | 17–87 | 45.4 | Not specified (ICD-9) | Self-Harm | ICD-9 |
| Myers et al., 2013 [70] | USA | Cross Sectional | Outpatients | 145 | 18–75 | 59.3 | Type 2 (self- reported) | Suicide attempt | Self- reported |
| Radobuljac et al., 2009[25] | Slovenia | Cross Sectional | 625 | 14–19 | 59 | Type 1 (record data) | Suicidal ideation & attempt | Self- reported | |
| Lee et al., 2014 [33] | Korea | Cross Sectional | KNHANES data V | 8322 | ≥40 | -- | Not specified (self- reported physician diagnosis) | Suicidal ideation | Self-reported |
| Chung et al., 2014 [31] | Korea | Cross Sectional | KNHANES data IV, V | 34,056 | ≥20 | 57 | Not specified (self- reported physician diagnosis) | Suicidal ideation & attempt | CIDI-SF |
| Han et al., 2013 [23] | Korea | Cross Sectional | KNHANES data IV | 17,065 | ≥20 | 57.6 | Not specified (self- reported physician diagnosis) | Suicidal ideation | Self-reported |
| Igwe et al., 2013 [71] | Nigeria | Cross Sectional | Outpatient endocrinology clinic | 270 | 18–64 mean: 51 ± 10.1 | 64.3 | Type 1 & Type 2 at least one year after diagnosis (consultant diagnosis) | Suicidal ideation | MINI |
| Handley et al., 2016 [72] | Australia | Cross Sectional | Diabetes MILES national survey | 3338 | 18–70 Mean: 51.7 (13.8) | 53.8 | Type 1 & Type 2 (the National Diabetes Services Scheme Register) | Suicidal ideation | PHQ-9 (item 9) |
| Ceretta et al., 2012 [32] | Brazil | Cross Sectional | Outpatients public health facility | 994 cases and 2145 control | >18 | 56.6–59.2 | Type 2 (self-reported) | Suicidal ideation | MINI |
| Sendela et al., 2015 [73] | Poland | Cross Sectional | Outpatients | 477 | 7–18 Mean: 13.1 ± 2.7 | 51.3 | Type 1 | Suicidal ideation | CDI (Item 9) |
| Fuller and Sawyer, 2009 [74] | Canada | Cross Sectional | Canadian Community Health Survey (CCHS) | 82,675 | ≥12 | -- | Type 1 (self-reported diagnosis and Insulin within one month of diagnosis) | Suicidal ideation | Self- reported |
| Batty et al., 2012 [75] | Korea | Prospective Cohort | Cancer prevention study participants | 1,234,927 | 30–95 | -- | Not specified (self report physician diagnosis or medication, study detected diabetes if FBG ≥126 with no history of diabetes) | Suicide | Death Certificates |
| Yamauchi et al., 2016 [52] | Japan | Prospective Cohort | 105,408 | 51.2 ± 7.9 | -- | Not specified (self-report of physician diagnosis or medication usage) | Suicide | Death Certificates/ICD-10 | |
| Webb et al., 2014 [76] | Sweden | Cohort | Data records | 252,191 cases and 1,260,214 controls | Median 69.3 Inter quartile range (IQR) = (59.2–78.7) | 44.5 | Type 1 & Type 2 (diabetes register) | Suicide | Death Register |
| Davis et al., 2015 [77] | Australia | Cohort | Fremantle diabetes study | 1413 + 5660 | 18–89.7 Mean: 62.3 ± 12.7 | 50.2 | Not specified | Suicide | Death Certificate or coroner’s determination |
| Webb et al., 2012 [24] | Nested Case Control | Primary care longitudinal database | 473 cases 17,460 controls | 17–87 Median: 38 | -- | Not specified (ICD-9) | Suicide | ICD-10/ data linkage |
| Author & Year | Outcome | Total Number of Diabetic Patients | Number of Diabetic Events | Reported Estimate (95% CI) | Adjusted Estimate (95% CI) | Adjustments |
|---|---|---|---|---|---|---|
| Singhal et al., 2014 [68] | Suicidal Attempt (SA) | 2,230,207 | 12,433 | Rate ratio (RR) = 1.6 (1.5–1.6) | -- | -- |
| Suicide | 2,230,207 | 626 | RR = 1 (0.9–1.1) | -- | -- | |
| Webb et al., 2012 [69] | Self-Harm | 81 | Odds ratio (OR) = 1.62 (1.28–2.06) | OR = 1.28 (1–1.64) | Clinical depression | |
| Myers et al., 2013 [70] | S A | 145 | 14 | -- | -- | -- |
| Radobuljc et al., 2009 [25] | S A | 126 | 11 | -- | -- | -- |
| Self-Harm | 126 | 16 | -- | - | -- | |
| Suicidal Ideation (SI) | 126 | 45 | -- | -- | -- | |
| Lee et al., 2014 [33] | SI | 811 | 187 | OR = 1.24 (0.95–1.61) | Age, sex, marital status, educational level, co-morbidities, depressive symptoms, stress | |
| Chung et al., 2014 [31] | S A | 3846 | 49 | OR = 1.562 (1.48–2.13) | OR = 1.413 (1.02–1.96) | Age, sex, smoking, alcohol, education, income, physical activity, number of chronic diseases and presence of major cancer |
| SI | 3846 | 796 | OR = 1.481 (1.36–1.61) | 1.15 (1.05–1.26) | Age, sex, smoking, alcohol, education, income, physical activity, number of chronic diseases and presence of major cancer | |
| Han et al., 2013 [23] | SI | 1110 | 206 | OR = 1.24 (1.02–1.51) | Age, sex, body mass index, household income, educational level, marital status, smoking, alcohol, and other chronic | |
| Igwe et al., 2013 [71] | SI | 270 | 17 | |||
| Handley et al., 2016 [72] | SI | 3338 | 477 | |||
| Ceretta et al., 2012 [32] | SI | 996 | 131 | OR = 7.1 (5–10) | OR = 2 (1.6–2.3) | |
| Sendela et al., 2015 [73] | SI | 477 | 47 | |||
| Fuller and Sawyer, 2009 [74] | SI | 190 | 31 | OR = 1.61 (1.08–2.42) | Age and sex | |
| Batty et al., 2012 [75] | Suicide | 13,452 | 12 | Hazard ratio (HR) (male) = 2.55 (1.3–5), HR (female) = 3.64 (1.12–11.86) | Exercise, smoking status, alcohol consumption, body mass index, height, blood pressure and blood cholesterol. | |
| Yamauchi et al., 2016 [52] | Suicide | 4898 | 41 | OR (male) = 1.2 (0.9–1.8) OR (female) = 1.5 (0.7–3) | Age at study entry, public health center area, smoking status, alcohol-drinking habits, body mass index cohabitation, employment status, hours of sleep, frequency of physical exercise, stress level and history of major physical illnesses | |
| Webb et al., 2014 [76] | Suicide | 252,191 | 482 | RR = 3.36 (2.99–3.79) | Age, sex and country of birth | |
| Davis et al., 2015 [77] | Suicide | 1413 | 4 | OR = 1.16 (0.38–3.51) | Age and sex | |
| Webb et al., 2012 [24] | Suicide | 892 | 47 | OR = 1.18 (0.85–1.62) | OR = 0.9 (0.65–1.26) | Sex and age by the case-control matching with added adjustment for clinical depression. |
| Reference Group | Category | Coefficient | 95% CI | p-Value | R2 | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Depression Evaluation | Depressive Symptoms | Anti-depressants | −0.026 | −0.283 | 0.230 | 0.218 | 0.34 |
| Disorders | 0.126 | −0.111 | 0.363 | ||||
| Level of Adjustment * | No | Full (>5) | 0. 299 | −0.126 | 0.726 | 0.386 | 0.1 |
| Partial (<5) | 0.024 | −0.162 | 0.212 | ||||
| Female Percent | 0.023 | −0.002 | 0.048 | 0.07 | 0 | ||
| Diabetes | Prevalent Diabetes | Incident Diabetes | −0.014 | −0.191 | 0.162 | 0.874 | 0.18 |
| Geographical location | North America | Asia | 0.083 | −0.155 | 0.032 | 0.111 | 0.36 |
| Scandinavian | −0.137 | −0.375 | 0.101 | ||||
| Europe | −0.147 | −0.403 | 0.108 | ||||
| Subgroup | N | Odds Ratio | 95% CI | p-Value for Group | p-Value for between Groups | p-Value for Heterogeneity | I2 for Heterogeneity | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Completed Suicide | ||||||||
| Sex (unadjusted) | 0.552 | |||||||
| Male | 2 | 1.536 | 0.78 | 3.027 | 0.215 | 0.068 | 69.96 | |
| Female | 2 | 2.097 | 0.971 | 4.528 | 0.059 | 0.232 | 29.89 | |
| Sex (adjusted) | 0.696 | |||||||
| Male | 2 | 1.651 | 0.796 | 3.427 | 0.178 | 0.51 | 73.71 | |
| Female | 2 | 2.059 | 0.895 | 4.733 | 0.089 | 0.21 | 36.34 | |
| Suicidal ideation | ||||||||
| Type of diabetes | 0.211 | |||||||
| Type 1 | 2 | 1.306 | 0.637 | 2.678 | 0.464 | 0.01 | 84.75 | |
| Type 2 & not specified | 4 | 2.212 | 1.473 | 3.32 | <0.001 | 0.032 | 70.82 | |
| Risk of bias | 0.35 | |||||||
| High | 1 | 1.625 | 1.385 | 1.906 | <0.001 | 1 | 0 | |
| Low | 2 | 3.435 | 0.849 | 13.898 | 0.084 | <0.001 | 98.06 | |
| Moderate | 3 | 1.371 | 1.005 | 1.872 | 0.047 | 0.032 | 70.83 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elamoshy, R.; Bird, Y.; Thorpe, L.; Moraros, J. Risk of Depression and Suicidality among Diabetic Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2018, 7, 445. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm7110445
Elamoshy R, Bird Y, Thorpe L, Moraros J. Risk of Depression and Suicidality among Diabetic Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2018; 7(11):445. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm7110445
Chicago/Turabian StyleElamoshy, Rasha, Yelena Bird, Lilian Thorpe, and John Moraros. 2018. "Risk of Depression and Suicidality among Diabetic Patients: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 7, no. 11: 445. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm7110445




