1. Introduction
Abiotic stress, as excess or shortage of water, inadequate nutrients, and biotic stressors such as insects or fungi have the potential to reduce crop production significantly [
1]. Important biotic stressors are pest organisms like weeds and plant pathogenic fungi. The potential global loss in wheat due to weeds, fungal diseases and bacteria has been estimated to 38% [
2]. In conventional farming, fertilizers, herbicides and fungicides are applied to avoid nitrogen deficiency, weed competition and fungal diseases, respectively. However, the application of a uniform rate of these external inputs is rarely the best approach since the nitrogen demand and weed presence are generally heterogeneously distributed within fields (e.g., [
3,
4]). Hence, some parts will receive excess levels at the expense of others. Site-specific crop management, or precision agriculture, seeks to adjust the rates of external inputs to this spatial heterogeneity. To be cost-effective, precision farming requires sensors to measure this heterogeneity.
Sensing techniques for crop management have been suggested since the early 1980s but only a few have reached the market. The precision agriculture procedure that has been widely adopted, is precision fertilization. The development of a tailor-made sensor to estimate the nitrogen demand of the crop on-the-go has been a decisive factor. A series of optical sensors applicable for outdoor use exist, including spectrometers, fluorometers and optoelectronic sensors. Compared to imaging techniques, these can almost instantaneously provide simple measures like single bands, ratios and indices based on spectral reflectance. Hence, such measures are particularly suitable for automatic on-the-go field mapping for precision farming implements.
The applicability of spectrometers and fluorometers has been investigated for single stress factors like water shortage, nitrogen deficiency and weed identification in a series of crops especially wheat [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14].
Nitrogen deficiency can result in slower growth rate, smaller plants and reduced yield. Normalized Difference Vegetation Index (NDVI), which is one of the most widely used vegetation indices, has been used to determine nitrogen status, vegetation vigor or crop density in cereals [
8,
9]. Red Edge Inflection Point (REIP), NDVI, Modified Chlorophyll Absorption in Reflectance Index (MCARI), Greenness Index (G), Optimized Soil Adjusted Vegetation Index (OSAVI), an index from Zarco-Tejada and Miller (ZM), Photochemical Reflectance Index (PRI) and Normalized Phaeophytinization Index (NPQI) have been shown to correlate with nitrogen stress, including nitrogen deficiency, in maize and wheat [
10,
15]. Similar results are obtained by fluorescence indices (Table 2) [
13].
Drought stress in crops has been detected by various spectral bands and indices like REIP, NDVI, OSAVI, MCARI, G, ZM, PRI, a simple ratio proposed by Vogelmann
et al. [
16] (VOG1), and Plant Pigment Ratio (PPR) [
7,
9,
17,
18,
19,
20]. Fluorescence measurements with sensors like the Multiplex
® and Dualex
® sensor have also been correlated with water stress in wheat [
14].
Weed density is correlated with Leaf Area Index (LAI) and biomass. Different weed species may be discriminated based on their spectral reflectance curves [
21,
22]. Based on UV-induced fluorescence, Longchamps
et al. [
23] successfully classified maize and weeds into three plant groups (four maize hybrids, four dicotyledonous weed species and four monocotyledonous weed species). Tyystjärvi
et al. [
24] used chlorophyll fluorescence to classify six weed species, maize and barley into weeds and crop with a high correct classification rate (86.7%–96.1%).
Plant pathogenic fungi depend on their host plants for nutrients and carbon assimilates, therefore disturbing the plant growth. Rumpf
et al. [
25] demonstrated the potential of pre-symptomatic detection of plant diseases in sugar beet by use of spectral indices obtained by hyperspectral reflectance. Several indices like REIP, NDVI, MCARI, G, ZM, PRI, OSAVI, Red Edge Vegetation Stress Index (RVSI), Renormalized Difference Vegetation Index (RDVI), along with fluorescence indices have been correlated with fungal infection in various crops [
11,
26,
27,
28].
Since multiple stressors often occur simultaneously in a field, it is of interest to develop a sensor-based method that is able to identify the type of stressors. Previous studies addressing concurrent, multiple stressors have only explored imaging technologies. For example, Karimi
et al. [
29] used hyperspectral imagery to identify combinations of various nitrogen application rates and weediness in maize. Backoulou
et al. [
12] used multispectral imagery to separate stress by a pest aphid from other concurrent stressors in wheat. Imaging technologies can be helpful in identifying weeds but not the rest of the stressors used in the current study. Our approach is to explore single spectral bands, simple ratios and indices measured by on-the-shelf non-imagery sensors. For this purpose we implemented both spectral and fluorescence parameters since the technologies by themselves are not able to indicate the nature of the stress factor. To our knowledge, such measures have not been tested to identify concurrent biotic and abiotic stressors in wheat until now.
The aim of this study was to determine whether simple sensor-based measures like single bands, ratios and indices obtained by on-the-shelf optical sensors can be used to detect abiotic and biotic stressors in spring wheat. The four stressors tested were water deficiency, nitrogen shortage, weed competition (Sinapis alba L.) and fungal infection (powdery mildew). We hypothesized that the selected sensors can be used to detect single stressors, even in the co-existence of other stress factors.
4. Discussion
The biomass and the shoot-to-root ratio measurements showed that the stressors affected plant development. Even though the stress factors were there, they were not causing extreme differences between stressed and non-stressed plants. The largest effect was measured for water content on dry matter production, where the dry matter yields in the water stressed pots averaged 29.5% less than that obtained in the non-water-stressed pots. Hence, the data set should be a good starting point for stress recognition.
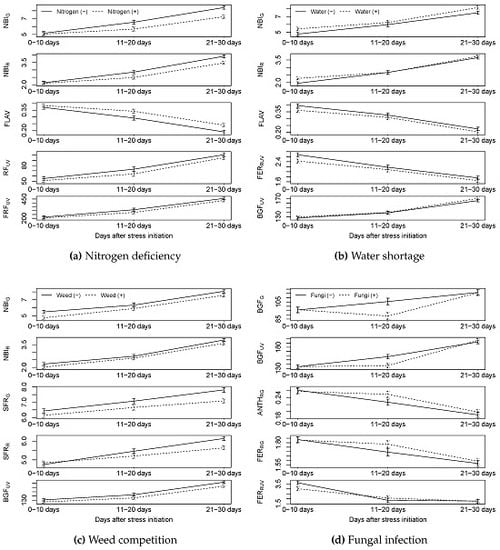
4.1. Could Nitrogen Deficiency Stress Be Detected by the Sensors?
When using the HandySpec indices REIP, ZM, VOG1, and GM1, we were able to discriminate between spring wheat grown with or without sufficient nitrogen supply. REIP was the only index enabling the detection of nitrogen deficiency as early as 11–20 days after onset of stress. It should be noted that all plants had the same nutrient conditions until BBCH 12 (ample resources). The delay in the differentiation between N-stressed and fertilized plants may be attributed to the early growth stages of the plants at time of fertilization, and their relatively limited N demand at this developing stage. As expected, REIP was lower for the stressed than the non-stressed plants. This agrees with the well documented red shift of the REIP due to higher concentrations of chlorophyll, which normally follows increased plant N availability. [
16,
49,
50,
51,
52,
53]. The indices VOG1, ZM and GM1 have also been proven to correlate with chlorophyll content [
16,
54,
55]. Taking into account that VOG and ZM are simple ratios centered around 730 nm (red), both can be considered as gross estimators of the REIP. In contrast, GM1 also involves reflection from the green area (dividing reflectance at 750 nm with that at 550 nm), and has been reported to correlate well with total chlorophyll along with nutrition and fertilization level [
8,
52,
53,
56].
Five of the twelve indices measured with the Multiplex
® sensor could be used to detect nitrogen deficiency as early as the 11–20 day period. The indices
and
clearly differentiated between
N-stressed and non-stressed plants, giving lower index values for the stressed than the non-stressed plants. Similar findings have also been reported for bermudagras and turfgrasses [
57,
58]. Longchamps and Khosla [
59] were able to distinguish between all their four
N-levels in an experiment with maize, using
and
. In our experiment, we also found out that an index related to flavonoids, FLAV, also contained information usable for nitrogen stress detection. In contrast with
and
, FLAV had higher, not lower values for the stressed plants. This agrees well with results from Agati
et al. [
58]. Several authors have reported that FLAV correlates with the flavonoid levels in fruits [
13,
60,
61,
62]. Cartelat
et al. [
13] found a negative correlation between flavonoid levels and chlorophyll content in wheat. All in all concerning nitrogen deficiency, both the spectrometer and the fluorometer were able to detect nitrogen deficiency. REIP, VOG1, ZM,
,
and FLAV can be used for this identification. On the other hand, the Isaria
® sensor did not provide significant data.
4.2. Could Water Stress Be Detected by the Sensors?
Among the five indices presented for HandySpec
® capable in detecting shortage in water, PVR and G could detect it already 0–10 DAS. This was well before any visual symptoms of water deprivation occurred. PVR, MCARI and G showed relatively stable values from the 10th DAS and onwards. All the above indices utilize the reflection of 550 nm (green) in their formula. Lin
et al. [
17] pinpointed a shift of the region around 535–540 nm with the water content of
Cinnamomum camphora (Linn.) Seib. Kusnierek and Korsaeth [
63] identified the region 560–610 nm as one of three spectral regions in the range 400–950 nm containing significant information related to water status in spring wheat. Thenkabail
et al. [
8] associates the wavebands around 550 nm with total chlorophyll and biomass, therefore water stress measurements also derive indirectly from chlorophyll measurements. Wang
et al. [
64] also showed a robust correlation between PPR and chlorophyll concentration. In the current study, NDVI, SAVI, OSAVI, VOG1, LIC1, GM1 and ZM could also be used to differentiate between water deprived and non stressed plants 20–30 DAS. These indices have also previously been associated with water content in plants [
9,
17,
18,
19,
20]. The correlations between the indices NDVI, SAVI, OSAVI, VOG1, LIC1, GM1 and ZM at one side and plant water status at the other were probably an indirect result, as water deprivation affects other parameters of plant growth, like chlorophyll content and leaf area index, which these indices correlate better with.
Using the Multiplex
® device, four indices (
, FLAV,
, and
) enabled early detection of water shortage. Two of the indices, FLAV and NBI, have previously been associated with water tolerance in wheat [
14]. Concerning water stress for both the spectrometer and the fluorometer, the presented approach appears promising. It can be argued that the spectrometer performed better than the fluorometer in this task, since the basic recognition indices in the fluorometer are the same as for nitrogen. PVR and G from the spectrometer can be used for water identification. However, the robustness of all indices related with water should be confirmed by further studies.
4.3. Could Weed Competition Be Detected by the Sensors?
As expected, weed competition was correlated with many indices calculated from the HandySpec® data. As for water shortage, G and other indices correlating with the Leaf Area Index (LAI), like NDVI and OSAVI, could be used to identify weediness from the first days of the experiment and onwards. The stress factor weeds, differed markedly from the other stressors we imposed, as the weed plants were physically present from the first day of measurement. Since the pots occupy a predefined space, the plant biomass and the LAI were thus already higher at the beginning of the measurement period, compared with pots without any weeds planted.
Vegetation Indices like NDVI, REIP, OSAVI, ZM, RDVI and LIC1, contained not only the information needed to separate between pots with or without weeds, but they also showed an increase with time, reflecting the growth in biomass during the experiment (both treatments). Measurements were performed at the early growing stages of the plants (BBCH 12–40). Therefore, crop plants were also rapidly growing, resulting in a continuous increase in e.g., LAI, commonly shown to be positively correlated with NDVI. Indices like PVR and PPR could be used to identify weed presence from days 11–20 and beyond. Since the pot size and volume are finite, this result might be an indirect result from water stress, due to assumedly higher transpiration from pots with weed and wheat plants than from pots with wheat plants alone.
Weed treatments could be separated by means of the indices REIP and IBI as obtained by the active spectrometer Isaria
®. The REIP as calculated from the Isaria sensor gave higher values than the REIP calculated by the Handyspec
®-sensor. This agrees with the findings of Peteinatos
et al. [
65]. The Multiplex provided useful data, as four of its output-indices (
,
,
and
) could be used to separate the weed treatments.
All three sensors provided information for weed identification. HandySpec® and Isaria® provided bigger differences faster than the fluorometer. It should be noted that the classical method of detecting weeds is by combining high-density RGB-images and image analysis. Such an approach is, however, less useful for detecting the other stressors of interest in the current study. Moreover, image-based weed detection is normally performed with the weed plants at a very early development stage, mainly to reflect the timing of herbicide application in practice. In this study, we focused on the combined effect of more stressors, and thus selected sensors, which had a potential for identifying more than one stressor. Therefore, from our perspective, indices MCARI, G, NDVI and OSAVI were able to perform this task along with and .
4.4. Could Fungal Infection Be Detected by Sensors?
Two indices calculated from data obtained by the HandySpec
® could be used to identify infection by powdery mildew (
B. graminis) as soon as 0–10 DAS, NDVI and RVSI, the latter also 11–20 DAS. This clearly suggests that RVSI is suitable for early, pre-visual, detection of powdery mildew in wheat. Bauriegel
et al. [
11] and Bauriegel and Herppich [
27] identified the spectra around 550–560 nm, and 665–675 nm as important for identifying
Fusarium infection in wheat, but they could not find any significant results with NDVI, G and LIC1. Two of the above indices, MCARI and RDVI, use at least one of the above two spectral ranges in their calculations. Time-wise, the results showed an interesting point. A series of four indices could discriminate between infested and non-infested in the second time period. These could also provide indices which enabled a differentiation in the third period, but differences became smaller. Zhang
et al. [
26] made a similar observation when investigating yellow rust infection over 17 days (four measurements at 216 till 233 days after sowing) in wheat with NDVI, PRI, RVSI and MCARI. Their results also showed the highest correlations at the second measuring date (225 days after sowing), whereas five days later only PRI was correlated with yellow rust infection. We cannot explain this phenomenon based on the current data, but this should be investigated in more detail, since this may be a potential important issue related to pre-symptomatic fungi detection. Indices like PRI and MCARI that identified water stress and NDVI and MCARI that identified weed presence, were also able to identify fungi infection. Zhang
et al. [
26] reported similar results for PRI. The fact that all types of spectral indices correlated with presence of fungi can be attributed to the result that fungi infected plants had a reduced growth dynamic, a lower total biomass and LAI.
Multiplex
® could also differentiate between fungi infected and non-infected plants. Blue-Green fluorescence under green or ultraviolet excitation (
and
) and indices relative to Ferodoxines and Anthocyanins could be used to identify the fungal infection.
and
seemed sensitive only on fungal infection. This can make them an indicator for
B. graminis. Latouche
et al. [
66] used Multiplex
® to successfully identify
Plasmopara viticola in vineyards, yet the sensor setup was modified. Time-wise,
,
,
and
were able to identify the fungal infection in the second 10-day period. The result seems similar to the result of the HandySpec
® sensor. Only
was able to identify the fungal infection in the first 10-day period. However, it identified it only in the first 10-day period and the results, even if it is not statistically significant, were reversed in the second 10-day period. Therefore, more experimentation is needed to see if this index can be used as a tool to identify
B. graminis. Concerning fungal infection, the small time windows that it can be measured in, makes it challenging to be identified by both spectral and fluorescence data.
4.5. Could Combinations of Stressors Be Detected by Sensors?
A lot of indices both from the HandySpec
® and the Multiplex
® sensor showed correlations with more than one stress factor. In the combination of stress factors, if we take into account the different way that each index reacts to each stressor, the increase through time for most of the indexes and the different rate of increase per stressor, identifying combinations of stressors with the same index can be quite challenging. For example, REIP as presented in
Figure 3c in the first 10-day period can clearly differentiate weedy from weed free pots, but could not differentiate per weed group the nitrogen stress. As days passed by, the non-nitrogen stressed plants increased in value at a higher rate than the non-stressed plants. In the third 10-day period, there was a clear distinction, between the control and the stressed pots. The pots containing only one of the two stressors could not be differentiated from each other. Similar results can be noted for RDVI where, in the second 10-day period, it can clearly differentiate between all four water shortage and fungal infection combinations. On 21–30 DAS, only three groups are clearly distinguishable (control, combinations, one stressor). For weed presence and fungal infection, G presents the aforementioned three groups on the 10–20 DAS, but in the third 10-day period, fungal infection cannot be differentiated on the weed free pots. Weedy pots could clearly be differentiated from weed free and in their case fungal infection could also be pinpointed. On the other hand, VOG1 can only differentiate the control from the other three treatments in the third 10-day period. Zhang
et al. [
26] also pinpointed the difficulty of identifying more than one stress factor with the same index.
In cases where the results of the two stressors are heading in the opposite direction, identifying the existence of one stressor or the other can be easy. We can see this in PPR, NDVI and MCARI for separating water shortage and weed presence and in PVR for the identification of weed presence and fungal infection from 10–20 DAS onwards. On the other hand, identification between the control and the combinations of the two stressors is harder. The ability to identify the control from the combination of two stressors relies only on the condition, if the results on the index for one stressor are higher than the other. NDVI is not able to perform this, while the rest of the aforementioned indices can perform this separation in the third 10-day period.
Combination of more than one stressor with the aid of the same index seems harder for the Multiplex® sensor. can separate nitrogen deficiency in the combination with water shortage from the second 10-day period onwards. can do the same for the combination of nitrogen deficiency and weed presence. For both indices, only the pots having nitrogen deficiency could differentiate the second stressor (water deficiency and weed presence) in the third 10-day period. and could differentiate fungal infection in the second 10-day period in the combination of water shortage and weed presence, respectively. In the third 10-day period, could separate only water shortage and only weeds. Identification of both stressors was not performed simultaneously but in different time periods. That pinpoints the constraints of creating a robust stress identification based on sensor values. Depending on the circumstances, a similar result could be attributed to one or more different stressors. A similar example can be which can differentiate water stress in weed free pots in the first 10-day period. However, from the 11–20 DAS onwards, the differentiation performed is only weedy and weed free pots regardless of the water stress. Concerning interaction of more than two factors, no significant results were identified.
5. Conclusions
Water deficiency and fungal infection could be detected pre-symptomatic, i.e., 0–10 days after stress induction (DAS), with one or more of the sensors tested. The presence or absence of weeds could also be identified using the same sensors (present during the entire experiment).
Nitrogen shortage could be detected 10–20 DAS by the index REIP (Red Edge Inflection Point) measured with HandySpec® and several indices based on Multiplex. The lack in detection of nitrogen shortage earlier could be attributed to the early growth stage (BBCH 12–14) and hence the limited nitrogen needs. Water deprivation in spring wheat could be detected as early as 0–10 days after stress induction (DAS) by the Photosynthetic Vigor Ratio (PVR; 550 nm − 650 nm/550 nm + 650 nm), the Plant Pigment Ratio (PPR; 550 nm − 450 nm/550 nm + 450 nm) and Greenness (G; 554 nm/677 nm) from the HandySpec® spectrometer and several indices based on the Multiplex® fluorometer. Weediness (Sinapis alba) could be detected from 0–10 DAS and onwards by indices measured by all three sensors, including REIP and biomass index (IBI) from the Isaria sensor. Fungal infection in spring wheat (Blumeria graminis f. sp. tritici) could be detected from 0–10 DAS by Red-edge Vegetation Stress Index (RVSI; (714 nm + 752 nm/2) − 733), Photochemical Reflectance Index (PRI; 531 nm − 570 nm/531 nm + 570 nm) and Normalized Phaeophytinization Index (NPQI; 415 nm − 435 nm/415 nm + 435 nm) measured with HandySpec® and by the ratio (Fluorescence Excitation Ratio; Red/Green excitiation) measured with Multiplex®. One of the main findings of this study was that the results both from spectral and from fluorescence data varied more due to the plant development rather than the presence or absence of a stress factor. In a lot of cases, where values were attributed to stressed plants at the early stages, ten or twenty days later the same values are then attributed to the non stressed plants. The study shows that it is possible to distinguish between a stressed plant and an unstressed control plant at the same growth stage, but we cannot pinpoint a general threshold value to use for discrimination regardless of the plant development stage. This hinders the stress identification process. More work needs to be done in order to model how the spectral indices change with the growth stage of the plants. In some cases, interactions of more than one stress factor could be viewed with the aid of the same index, yet these results are more complicated to interpret. Interactions with more than two stress factors could not present any significant results. None of the indices showed a significant three-way interaction.