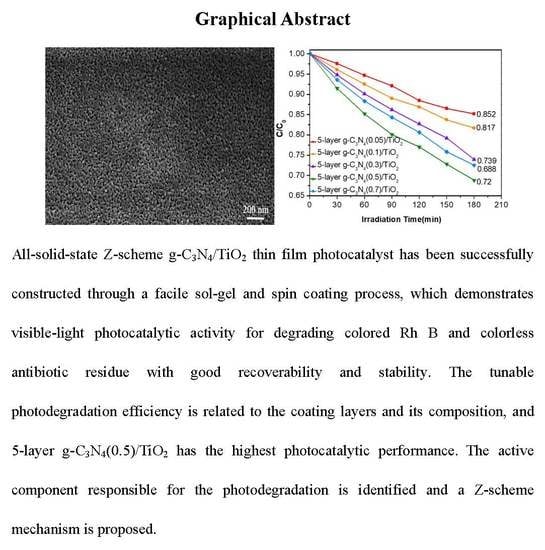
Facile Construction of All-Solid-State Z-Scheme g-C3N4/TiO2 Thin Film for the Efficient Visible-Light Degradation of Organic Pollutant
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation
2.3. Characterization
2.4. Photocatalytic Activity Measurement
2.5. Trapping Experiment
3. Results and Discussions
3.1. Structure, Composition, and Morphology
3.2. Optical Property
3.3. Photocatalytic Activity
3.3.1. Effects of Film Thickness on the Photocatalytic Performance
3.3.2. Effects of Film Composition on the Photocatalytic Performance
3.4. Photocatalytic Stability
3.5. Proposed Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- René, P.S.; Thomas, E.; Hofstetter, T.B.; Urs Von, G.; Bernhard, W. Global water pollution and human health. Annu. Rev. Energy 2010, 35, 109–136. [Google Scholar]
- Li, C.; Xu, Y.; Tu, W.; Chen, G.; Xu, R. Metal-free photocatalysts for various applications in energy conversion and environmental purification. Green Chem. 2017, 19, 882–899. [Google Scholar] [CrossRef]
- Frank, S.N.; Bard, A.J. Semiconductor electrodes. 12. Photoassisted oxidations and photoelectrosynthesis at polycrystalline titanium dioxide electrodes. J. Am. Chem. Soc. 1977, 99, 4667–4675. [Google Scholar] [CrossRef]
- Matthews, R.W. Photooxidation of organic impurities in water using thin films of titanium dioxide. J. Phys. Chem. 1987, 91, 3328–3333. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Z.; Gao, T.; Huang, Q.; Niu, F.; Qin, L.; Tang, P.; Huang, Y.; Sha, Z.; Wang, Y. Construction of hybrid Z-scheme Pt/CdS–TNTAs with enhanced visible-light photocatalytic performance. Appl. Catal. B 2015, 163, 16–22. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, Y.; Mølhave, K.; Sun, H. Engineering the surface/interface structures of titanium dioxide micro and nano architectures towards environmental and electrochemical applications. Nanomaterials 2017, 7, 382. [Google Scholar] [CrossRef] [Green Version]
- Nasr, M.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Recent progress on titanium dioxide nanomaterials for photocatalytic applications. ChemSusChem 2018, 11, 3023–3047. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Zhang, M.; Yuan, Q.; Dong, B. Ultrathin hexagonal SnS2 nanosheets coupled with g-C3N4 nanosheets as 2D/2D heterojunction photocatalysts toward high photocatalytic activity. Appl. Catal. B 2015, 163, 298–305. [Google Scholar] [CrossRef]
- Yan, S.C.; Lv, S.B.; Li, Z.S.; Zou, Z.G. Organic–inorganic composite photocatalyst of g-C3N4 and TaON with improved visible light photocatalytic activities. Dalton Trans. 2010, 39, 1488–1491. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Chen, Z.; Yang, Q.; Dong, X.; Zhang, J.; Qin, L. In-situ construction of all-solid-state Z-scheme g-C3N4/TiO2 nanotube arrays photocatalyst with enhanced visible-light-induced properties. Sol. Energy Mater. Sol. Cells 2016, 157, 399–405. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yu, J.; Jaroniec, M. All-solid-state Z-scheme photocatalytic systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef]
- Xu, Y.; Wen, W.; Wu, J.-M. Titania nanowires functionalized polyester fabrics with enhanced photocatalytic and antibacterial performances. J. Hazard. Mater. 2018, 343, 285–297. [Google Scholar] [CrossRef]
- Dong, F.; Wu, L.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Efficient synthesis of polymeric g-C3N4 layered materials as novel efficient visible light driven photocatalysts. J. Mater. Chem. 2011, 21, 15171–15174. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, Z.; Yang, Q.; Shen, C.; Tang, G.; Zhao, S.; Zhang, J.; Chen, D.; Wei, Q.; Dong, X. Facile construction of g-C3N4 nanosheets/TiO2 nanotube arrays as Z-scheme photocatalyst with enhanced visible-light performance. ChemCatChem 2016, 8, 3064–3073. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Fang, J.; Yang, Q.; Yang, X.; Zhao, W.; Zhou, D.; Qian, X.; Liu, C.; Shao, J. Facile synthesis of TiO2 film on glass for the photocatalytic removal of rhodamine B and tetracycline hydrochloride. Mater. Express 2019, 9, 437–443. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Fang, J.; Yang, Q.; Zhao, W.; Qian, X.; Zhou, D.; Liu, C.; Chen, M. Freestanding 3D MoS2 nanosheets/graphene aerogel heterostructure as a recyclable photocatalyst for efficiently degrading antibiotic residues. Mater. Lett. 2019, 252, 5–7. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, L.; Luo, Q.; Li, X.; An, J.; Duan, Y. Highly efficient visible light TiO2 photocatalyst prepared by sol–gel method at temperatures lower than 300 °C. J. Hazard. Mater. 2011, 192, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Wang, M.; Ali, R.; Nie, S.; Zeng, Q.; Yang, R.; Lau, W.-M.; He, L.; Tang, H.; Jian, X. Multi-layered porous hierarchical TiO2/g-C3N4 hybrid coating for enhanced visible light photocatalysis. Appl. Surf. Sci. 2019, 495, 143435. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Li, Q.; Yang, J. Enhanced visible light activity on direct contact Z-scheme g-C3N4-TiO2 photocatalyst. Appl. Surf. Sci. 2017, 391, 184–193. [Google Scholar] [CrossRef]
- Yan, H.; Yang, H. TiO2–g-C3N4 composite materials for photocatalytic H2 evolution under visible light irradiation. J. Alloy. Compd. 2011, 509, L26–L29. [Google Scholar] [CrossRef]
- Ye, L.; Liu, J.; Jiang, Z.; Peng, T.; Zan, L. Facets coupling of BiOBr-g-C3N4 composite photocatalyst for enhanced visible-light-driven photocatalytic activity. Appl. Catal. B 2013, 142, 1–7. [Google Scholar] [CrossRef]
- Alvarez, K.M.; Alvarado, J.; Soto, B.S.; Hernandez, M.A. Synthesis of TiO2 nanoparticles and TiO2-Zeolite composites and study of optical properties and structural characterization. Optik 2018, 169, 137–146. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, Z.; Zhang, H.; Li, Z.; Wu, X.; Zhang, X.; Zhang, Y.; Zhou, W. High thermostable ordered mesoporous SiO2–TiO2 coated circulating-bed biofilm reactor for unpredictable photocatalytic and biocatalytic performance. Appl. Catal. B 2016, 180, 521–529. [Google Scholar] [CrossRef]
- Guli, M.; Yao, J.; Zhao, J.; Rao, W.; Xiao, L.; Tian, H. Preparation and characterization of TiO2 anode film with spinodal phase separation structure in dye-sensitized solar cells. Opt. Mater. 2013, 35, 2175–2182. [Google Scholar] [CrossRef]
- Qian, X.; Chen, Z.; Yang, X.; Zhao, W.; Liu, C.; Sun, T.; Zhou, D.; Yang, Q.; Wei, G.; Fan, M. Perovskite cesium lead bromide quantum dots: A new efficient photocatalyst for degrading antibiotic residues in organic system. J. Clean. Prod. 2019, 249, 119335. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J. Photochem. Photobiol. C. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, S.; Zhao, W.; Liu, W. Study on the photocatalytic activity of TiN/TiO2 nanoparticle formed by ball milling. J. Nanopart. Res. 2009, 11, 931–938. [Google Scholar] [CrossRef]









| Sample Name | Energy Gap |
|---|---|
| 5-layer TiO2 | 3.7 eV |
| 3-layer g-C3N4(0.5)/TiO2 | 3.3 eV |
| 5-layer g-C3N4(0.5)/TiO2 | 3.25 eV |
| 7-layer g-C3N4(0.5)/TiO2 | 3.27 eV |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Yang, X.; Liu, C.; Qian, X.; Wen, Y.; Yang, Q.; Sun, T.; Chang, W.; Liu, X.; Chen, Z. Facile Construction of All-Solid-State Z-Scheme g-C3N4/TiO2 Thin Film for the Efficient Visible-Light Degradation of Organic Pollutant. Nanomaterials 2020, 10, 600. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10040600
Zhao W, Yang X, Liu C, Qian X, Wen Y, Yang Q, Sun T, Chang W, Liu X, Chen Z. Facile Construction of All-Solid-State Z-Scheme g-C3N4/TiO2 Thin Film for the Efficient Visible-Light Degradation of Organic Pollutant. Nanomaterials. 2020; 10(4):600. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10040600
Chicago/Turabian StyleZhao, Wan, Xiuru Yang, Chunxi Liu, Xiaoxiao Qian, Yanru Wen, Qian Yang, Tao Sun, Wenya Chang, Xin Liu, and Zhi Chen. 2020. "Facile Construction of All-Solid-State Z-Scheme g-C3N4/TiO2 Thin Film for the Efficient Visible-Light Degradation of Organic Pollutant" Nanomaterials 10, no. 4: 600. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10040600