Laser-Assisted Fabrication for Metal Halide Perovskite-2D Nanoconjugates: Control on the Nanocrystal Density and Morphology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Perovskite–GO Nanoconjugate Colloid
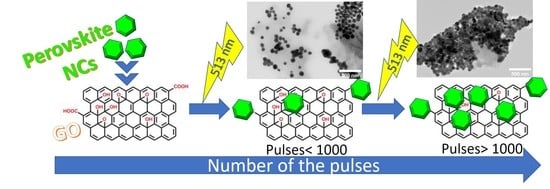
2.2. Photo-Induced Process for the Fabrication of the Perovskite–GO Nanoconjugates
2.3. Characterization of the Materials
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martín-García, B.; Polovitsyn, A.; Prato, M.; Moreels, I. Efficient charge transfer in solution-processed PbS quantum dot–reduced graphene oxide hybrid materials. J. Mater. Chem. C 2015, 3, 7088–7095. [Google Scholar] [CrossRef] [Green Version]
- Fei, H.; Ye, R.; Ye, G.; Gong, Y.; Peng, Z.; Fan, X.; Samuel, E.; Ajayan, P.M.; Tour, J.M. Boron- and Nitrogen-doped graphene quantum dots/graphene hybrid nanoplatelets as efficient electrocatalysts for oxygen reduction. ACS Nano 2014, 8, 10837–10843. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, Y.; Li, P.-J.; Fu, F.; Wang, Z.; Zhang, W. Self-assembled CoS2 nanoparticles wrapped by CoS2-quantum-dots-anchored graphene nanosheets as superior-capability anode for lithium-ion batteries. Electrochim. Acta 2015, 182, 424–429. [Google Scholar] [CrossRef]
- Gong, C.; Robertson, A.W.; He, K.; Ford, C.; Watt, A.A.R.; Warner, J.H. Interactions of Pb and Te atoms with graphene. Dalton Trans. 2014, 43, 7442–7448. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Zhang, J.; Xiao, H.; Hu, T.; Jia, J.; Wu, H.S. Facile in situ synthesis of a carbon quantum dot/graphene heterostructure as an efficient metal-free electrocatalyst for overall water splitting. Chem. Commun. 2019, 55, 1635–1638. [Google Scholar] [CrossRef]
- Sygletou, M.; Tzourmpakis, P.; Petridis, C.; Konios, D.; Fotakis, C.; Kymakis, E.; Stratakis, E. Laser induced nucleation of plasmonic nanoparticles on two-dimensional nanosheets for organic photovoltaics. J. Mater. Chem. A 2016, 4, 1020–1027. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Kiessling, L.; Stratakis, E.; Kymakis, E.; El-Sagheer, A.H.; Brown, T.; Kanaras, A. Programming the assembly of gold nanoparticles on graphene oxide sheets using DNA. J. Mater. Chem. C 2015, 3, 9379–9384. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Sygletou, M.; Petridis, C.; Kymakis, E.; Stratakis, E. Advanced photonic processes for photovoltaic and energy storage systems. Adv. Mater. 2017, 29, 1700335. [Google Scholar] [CrossRef]
- Busby, Y.; Agresti, A.; Pescetelli, S.; Di Carlo, A.; Noël, C.; Pireaux, J.-J.; Houssiau, L. Aging effects in interface-engineered perovskite solar cells with 2D nanomaterials: A depth profile analysis. Mater. Today Energy 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Palma, A.L.; Cinà, L.; Pescetelli, S.; Agresti, A.; Raggio, M.; Paolesse, R.; Bonaccorso, F.; Di Carlo, A. Reduced graphene oxide as efficient and stable hole transporting material in mesoscopic perovskite solar cells. Nano Energy 2016, 22, 349–360. [Google Scholar] [CrossRef]
- Attanzio, A.; Rosillo-Lopez, M.; Zampetti, A.; Ierides, I.; Cacialli, F.; Salzmann, C.G.; Palma, M. Assembly of graphene nanoflake–quantum dot hybrids in aqueous solution and their performance in light-harvesting applications. Nanoscale 2018, 10, 19678–19683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, A.J.; Dahod, N.S.; Tisdale, W.A. Ultrafast charge transfer at a quantum dot/2D materials interface probed by second harmonic generation. J. Phys. Chem. Lett. 2018, 9, 4227–4232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-S.; Doane, T.L.; Li, M.; Zang, H.; Maye, M.M.; Cotlet, M. 0D-2D and 1D-2D semiconductor hybrids composed of all inorganic perovskite nanocrystals and single-layer graphene with improved light harvesting. Part. Part. Syst. Charact. 2017, 35, 1700310. [Google Scholar] [CrossRef]
- Xu, Y.-F.; Yang, M.-Z.; Chen, B.-X.; Wang, X.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. A CsPbBr3 perovskite quantum dot/graphene oxide composite for photocatalytic CO2 reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zu, Z.; Zang, Z.; Hu, Z.; Hu, W.; Yao, Z.; Chen, W.; Li, S.; Han, S.; Zhou, M. CsPbBr3/Reduced Graphene Oxide nanocomposites and their enhanced photoelectric detection application. Sensor Actuat B-Chem 2017, 245, 435–440. [Google Scholar] [CrossRef]
- Wang, Q.; Tao, L.; Jiang, X.; Wang, M.; Shen, Y. Graphene oxide wrapped CH3NH3PbBr3 perovskite quantum dots hybrid for photoelectrochemical CO2 reduction in organic solvents. Appl. Surf. Sci. 2019, 465, 607–613. [Google Scholar] [CrossRef]
- Muduli, S.K.; Pandey, P.; Devatha, G.; Babar, R.; M, T.; Kothari, D.; Kabir, M.; Pillai, P.; Ogale, S. Photoluminescence quenching in self-assembled CsPbBr3 quantum dots on few-layer black phosphorus sheets. Angew. Chem. Int. Ed. 2018, 57, 7682–7686. [Google Scholar] [CrossRef]
- Li, H.; Zheng, X.; Liu, Y.; Zhang, Z.; Jiang, T. Ultrafast interfacial energy transfer and interlayer excitons in the monolayer WS2/CsPbBr3 quantum dot heterostructure. Nanoscale 2018, 10, 1650–1659. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the optical properties of cesium lead halide perovskite nanocrystals by anion exchange reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef] [Green Version]
- Kostopoulou, A.; Brintakis, K.; Nasikas, N.K.; Stratakis, E. Perovskite nanocrystals for energy conversion and storage. Nanophotonics 2019, 8, 1607–1640. [Google Scholar] [CrossRef]
- Kostopoulou, A.; Kymakis, E.; Stratakis, E. Perovskite nanostructures for photovoltaic and energy storage devices. J. Mater. Chem. A 2018, 6, 9765–9798. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parobek, D.; Dong, Y.; Qiao, T.; Rossi, D.; Son, D.H. Photoinduced anion exchange in cesium lead halide perovskite nanocrystals. J. Am. Chem. Soc. 2017, 139, 4358–4361. [Google Scholar] [CrossRef] [PubMed]
- Sichert, J.A.; Tong, Y.; Mutz, N.; Vollmer, M.; Fischer, S.; Milowska, K.; Cortadella, R.G.; Nickel, B.; Cardenas-Daw, C.; Stolarczyk, J.; et al. Quantum size effect in organometal halide perovskite nanoplatelets. Nano Lett. 2015, 15, 6521–6527. [Google Scholar] [CrossRef] [PubMed]
- Bekenstein, Y.; Koscher, B.A.; Eaton, S.W.; Yang, P.; Alivisatos, A.P. Highly luminescent colloidal nanoplates of perovskite cesium lead halide and their oriented assemblies. J. Am. Chem. Soc. 2015, 137, 16008–16011. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Li, J.; Yi, Y.; Wang, J.; Kang, Y.; Chu, P.K.; Ong, H.C.; Ong, D.H.C.; Yu, X. In situ growth of all-inorganic perovskite nanocrystals on black phosphorus nanosheets. Chem. Commun. 2018, 54, 2365–2368. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, P.; Zhu, X.; Zhang, Q.; Wang, Z.; Liu, Y.; Zou, G.; Dai, Y.; Whangbo, M.-H.; Huang, B. Composite of CH3NH3PbI3 with reduced graphene oxide as a highly efficient and stable visible-light photocatalyst for hydrogen evolution in aqueous HI solution. Adv. Mater. 2018, 30, 1704342. [Google Scholar] [CrossRef]
- Kostopoulou, A.; Vernardou, D.; Savva, K.; Stratakis, E. All-inorganic lead halide perovskite nanohexagons for high performance air-stable lithium batteries. Nanoscale 2019, 11, 882–889. [Google Scholar] [CrossRef]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behavior of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, Y. Strong photoluminescence of Cs4PbBr6 crystals: a long mystery story. Sci. Bull. 2018, 63, 525–526. [Google Scholar] [CrossRef]
- De Weerd, C.; Lin, J.; Gomez, L.; Fujiwara, Y.; Suenaga, K.; Gregorkiewicz, T. Hybridization of single nanocrystals of Cs4PbBr6 and CsPbBr3. J. Phys. Chem. C 2017, 121, 19490–19496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Yang, H.; Song, K.; El-Zohry, A.M.; Han, Y.; Bakr, O.M.; Bredas, J.-L.; Mohammed, O.F. Point defects and green emission in zero-dimensional perovskites. J. Phys. Chem. Lett. 2018, 9, 5490–5495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkerman, Q.A.; Park, S.; Radicchi, E.; Nunzi, F.; Mosconi, E.; De Angelis, F.; Brescia, R.; Rastogi, P.; Prato, M.; Manna, L. Nearly Monodisperse insulator Cs4PbX6 (X = Cl, Br, I) nanocrystals, their mixed halide compositions and their transformation into CsPbX3 nanocrystals. Nano Lett. 2017, 17, 1924–1930. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Maggioni, D.; Baranov, D.; Dang, Z.; Prato, M.; Akkerman, Q.A.; Goldoni, L.; Caneva, E.; Manna, L.; Abdelhady, A.L. Green-emitting powders of zero-dimensional Cs4PbBr6: Delineating the intricacies of the synthesis and the origin of photoluminescence. Chem. Mater. 2019, 31, 7761–7769. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Abdelhady, A.L.; Manna, L. Zero-dimensional cesium lead halides: History, properties and challenges. J. Phys. Chem. Lett. 2018, 9, 2326–2337. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Li, N.; Wang, C.; Yao, W.; Wang, H.; Huang, K. Room-temperature synthesis of pure perovskite-related Cs4PbBr6 nanocrystals and their ligand-mediated evolution into highly luminescent CsPbBr3 nanosheets. J. Nanoparticle Res. 2017, 19, 258. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Zhou, Y.; Zhao, Q.; Zhang, J.-Y.; Ma, J.-P.; Xuan, T.-T.; Guo, S.-Q.; Yong, Z.-J.; Wang, J.; Kuroiwa, Y.; et al. Cs4PbBr6/CsPbBr3 perovskite composites with near-unity luminescence quantum yield: Large-scale synthesis, luminescence and formation mechanism and white light-emitting diode application. Acs Appl Mater. Interfaces 2018, 10, 15905–15912. [Google Scholar] [CrossRef]
- Liu, Z.; Bekenstein, Y.; Ye, X.; Nguyen, S.C.; Swabeck, J.; Zhang, D.; Lee, S.-T.; Yang, P.; Ma, W.; Alivisatos, A.P. Ligand mediated transformation of cesium lead bromide perovskite nanocrystals to lead depleted Cs4PbBr6 nanocrystals. J. Am. Chem. Soc. 2017, 139, 5309–5312. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Eaton, S.W.; Yu, Y.; Dou, L.; Yang, P. Solution-phase synthesis of cesium lead halide perovskite nanowires. J. Am. Chem. Soc. 2015, 137, 9230–9233. [Google Scholar] [CrossRef]
- Kostopoulou, A.; Sygletou, M.; Brintakis, K.; Lappas, A.; Stratakis, E. Low-temperature benchtop-synthesis of all-inorganic perovskite nanowires. Nanoscale 2017, 9, 18202–18207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sygletou, M.; Kyriazi, M.-E.; Kanaras, A.; Stratakis, E. Anion exchange in inorganic perovskite nanocrystal polymer composites. Chem. Sci. 2018, 9, 8121–8126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexaki, K.; Kostopoulou, A.; Sygletou, M.; Kenanakis, G.; Stratakis, E. Unveiling the structure of MoSx nanocrystals produced upon laser fragmentation of MoS2 platelets. ACS Omega 2018, 3, 16728–16734. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.; Dhanabalan, B.; Castelli, A.; Dhall, R.; Bustillo, K.C.; Marchelli, D.; Spirito, D.; Petralanda, U.; Shamsi, J.; Manna, L.; et al. Temperature-driven transformation of CsPbBr3 nanoplatelets into mosaic nanotiles in solution through self-assembly. Nano Lett. 2020, 20, 1808–1818. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostopoulou, A.; Brintakis, K.; Serpetzoglou, E.; Stratakis, E. Laser-Assisted Fabrication for Metal Halide Perovskite-2D Nanoconjugates: Control on the Nanocrystal Density and Morphology. Nanomaterials 2020, 10, 747. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10040747
Kostopoulou A, Brintakis K, Serpetzoglou E, Stratakis E. Laser-Assisted Fabrication for Metal Halide Perovskite-2D Nanoconjugates: Control on the Nanocrystal Density and Morphology. Nanomaterials. 2020; 10(4):747. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10040747
Chicago/Turabian StyleKostopoulou, Athanasia, Konstantinos Brintakis, Efthymis Serpetzoglou, and Emmanuel Stratakis. 2020. "Laser-Assisted Fabrication for Metal Halide Perovskite-2D Nanoconjugates: Control on the Nanocrystal Density and Morphology" Nanomaterials 10, no. 4: 747. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10040747