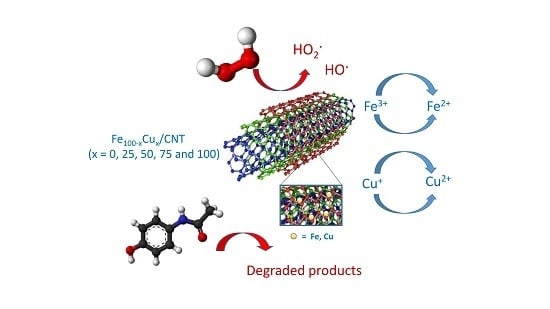
Fe-Cu Doped Multiwalled Carbon Nanotubes for Fenton-like Degradation of Paracetamol Under Mild Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Fe-Cu Doped Carbon Nanotubes
2.2. Characterization of Samples
2.3. Catalytic Activity
3. Results and Discussion
3.1. Characterization of Samples
3.2. Adsorption of Paracetamol
3.3. Decomposition of H2O2
3.4. Fenton-like Decomposition of Paracetamol
3.4.1. Leaching and Mineralization Degree
3.4.2. Influence of pH and Dosage of H2O2
3.4.3. Recyclability of Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Comninellis, C.; Kapalka, A.; Malato, S.; Parsons, S.A.; Poulios, I.; Mantzavinos, D. Advanced oxidation processes for water treatment: Advances and trends for R&D. J. Chem. Technol. Biotechnol. 2008, 83, 769–776. [Google Scholar]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment. I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment. II: Hybrid methods. Adv. Environ. Res. 2004, 8, 553–597. [Google Scholar] [CrossRef]
- Poyatos, J.M.; Munio, M.M.; Almecija, M.C.; Torres, J.C.; Hontoria, E.; Osorio, F. Advanced oxidation processes for wastewater treatment: State of the art. Water Air Soil Pollut. 2010, 205, 187–204. [Google Scholar] [CrossRef]
- Rodriguez, A.; Ovejero, G.; Sotelo, J.L.; Mestanza, M.; Garcia, J. Heterogeneous Fenton catalyst supports screening for mono azo dye degradation in contaminated wastewaters. Ind. Eng. Chem. Res. 2010, 49, 498–505. [Google Scholar] [CrossRef]
- Rahim Pouran, S.; Abdul Raman, A.A.; Wan Daud, W.M.A. Review on the application of modified iron oxides as heterogeneous catalysts in Fenton reactions. J. Cleaner Prod. 2014, 64, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Nidheesh, P.V. Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv. 2015, 5, 40552–40577. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation – A review. Appl. Catal. B Environ. 2015, 176–177, 249–265. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Yang, X.; Men, B.; Wang, D. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J. Environ. Sci. 2016, 39, 97–109. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Garrido-Ramirez, E.G.; Theng, B.K.G.; Mora, M.L. Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions—A review. Appl. Clay Sci. 2010, 47, 182–192. [Google Scholar] [CrossRef]
- Pereira, M.C.; Oliveira, L.C.A.; Murad, E. Iron oxide catalysts: Fenton and Fenton-like reactions—A review. Clay Miner. 2012, 47, 285–302. [Google Scholar] [CrossRef]
- Carrasco-Diaz, M.R.; Castillejos-Lopez, E.; Cerpa-Naranjo, A.; Rojas-Cervantes, M.L. Efficient removal of paracetamol using LaCu1−xMxO3 (M = Mn, Ti) perovskites as heterogeneous Fenton-like catalysts. Chem. Eng. J. 2016, 304, 408–418. [Google Scholar] [CrossRef]
- Kuznetsova, E.V.; Savinov, E.N.; Vostrikova, L.A.; Parmon, V.N. Heterogeneous catalysis in the Fenton-type system FeZSM-5/H2O2. Appl. Catal. B Environ. 2004, 51, 165–170. [Google Scholar] [CrossRef]
- Navalon, S.; Alvaro, M.; Garcia, H. Heterogeneous Fenton catalysts based on clays, silicas and zeolites. Appl. Catal. B Environ. 2010, 99, 1–26. [Google Scholar] [CrossRef]
- Velichkova, F.; Delmas, H.; Julcour, C.; Koumanova, B. Heterogeneous fenton and photo-fenton oxidation for paracetamol removal using iron containing ZSM-5 zeolite as catalyst. AIChE J. 2017, 63, 669–679. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, J.H.; Costa, C.A.; Madeira, L.M.; Mata, G.; Vicente, M.A.; Rojas-Cervantes, M.L.; Lopez-Peinado, A.J.; Martin-Aranda, R.M. Fenton-like oxidation of Orange II solutions using heterogeneous catalysts based on saponite clay. Appl. Catal. B: Environ. 2007, 71, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Hassan, H.; Hameed, B.H. Iron-clay as effective heterogeneous Fenton catalyst for the decolorization of Reactive Blue 4. Chem. Eng. J. 2011, 171, 912–918. [Google Scholar] [CrossRef]
- Duarte, F.; Maldonado-Hodar, F.J.; Perez-Cadenas, A.F.; Madeira, L.M. Fenton-like degradation of azo-dye Orange II catalyzed by transition metals on carbon aerogels. Appl. Catal. B: Environ. 2009, 85, 139–147. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Y.; Zhang, Z.; Sun, L.; Lu, W.; Chen, W.; Chen, H. Activated carbon fibers as an excellent partner of Fenton catalyst for dyes decolorization by combination of adsorption and oxidation. Chem. Eng. J. 2014, 251, 348–354. [Google Scholar] [CrossRef]
- Carrasco-Diaz, M.R.; Castillejos-Lopez, E.; Cerpa-Naranjo, A.; Rojas-Cervantes, M.L. On the textural and crystalline properties of Fe-carbon xerogels. Application as Fenton-like catalysts in the oxidation of paracetamol by H2O2. Microporous Mesoporous Mater. 2017, 237, 282–293. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Zhao, G. Iron-copper bimetallic nanoparticles embedded within ordered mesoporous carbon as effective and stable heterogeneous Fenton catalyst for the degradation of organic contaminants. Appl. Catal. B Environ. 2015, 164, 396–406. [Google Scholar] [CrossRef]
- Gholami, P.; Dinpazhoh, L.; Khataee, A.; Hassani, A.; Bhatnagar, A. Facile hydrothermal synthesis of novel Fe-Cu layered double hydroxide/biochar nanocomposite with enhanced sonocatalytic activity for degradation of cefazolin sodium. J. Hazard. Mater. 2020, 381, 120742. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999–2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, M.J.; Martinez Bueno, M.J.; Lacorte, S.; Fernandez-Alba, A.R.; Agueera, A. Pilot survey monitoring pharmaceuticals and related compounds in a sewage treatment plant located on the Mediterranean coast. Chemosphere 2007, 66, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Serp, P.; Castillejos, E. Catalysis in Carbon Nanotubes. ChemCatChem 2010, 2, 41–47. [Google Scholar] [CrossRef]
- Melchionna, M.; Marchesan, S.; Prato, M.; Fornasiero, P. Carbon nanotubes and catalysis: The many facets of a successful marriage. Catal. Sci. Technol. 2015, 5, 3859–3875. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Liu, Y.; Chi, W.; Wang, Y.; Yue, X.; Huang, Q.; Yu, C. Catalytic Degradation of Phenol and p-Nitrophenol Using Fe3O4/MWCNT Nanocomposites as Heterogeneous Fenton-Like Catalyst. Water Air Soil Pollut. 2017, 228, 1–12. [Google Scholar] [CrossRef]
- Liao, Q.; Sun, J.; Gao, L. Degradation of phenol by heterogeneous Fenton reaction using multi-walled carbon nanotube supported Fe2O3 catalysts. Colloids Surf. A Physicochem. Eng. Asp. 2009, 345, 95–100. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, H.; Ji, L.; Shao, Y.; Li, Y. Fe3O4/MWCNT as a heterogeneous Fenton catalyst: Degradation pathways of tetrabromobisphenol A. RSC Adv. 2014, 4, 24900–24908. [Google Scholar] [CrossRef]
- Cleveland, V.; Bingham, J.-P.; Kan, E. Heterogeneous Fenton degradation of bisphenol A by carbon nanotube-supported Fe3O4. Sep. Purif. Technol. 2014, 133, 388–395. [Google Scholar] [CrossRef]
- Yu, L.; Yang, X.; Ye, Y.; Wang, D. Efficient removal of atrazine in water with a Fe3O4/MWCNTs nanocomposite as a heterogeneous Fenton-like catalyst. RSC Adv. 2015, 5, 46059–46066. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J. Fe3O4-MWCNT Magnetic Nanocomposites as Efficient Fenton-Like Catalysts for Degradation of Sulfamethazine in Aqueous Solution. Chem. Sel. 2017, 2, 10727–10735. [Google Scholar]
- Deng, J.; Wen, X.; Li, J. Degradation of methylene blue by heterogeneous Fenton-like reaction using Fe3O4/carbon nanotube composites. Acta Sci. Circumstantiae 2014, 34, 1436–1442. [Google Scholar]
- Zhang, L.; Nie, Y.; Hu, C.; Qu, J. Enhanced Fenton degradation of Rhodamine B over nanoscaled Cu-doped LaTiO3 perovskite. Appl. Catal. B Environ. 2012, 125, 418–424. [Google Scholar] [CrossRef]
- Delgado-Gomez, F.J.; Calvino-Casilda, V.; Cerpa-Naranjo, A.; Rojas-Cervantes, M.L. Alkaline-doped multiwall carbon nanotubes as efficient catalysts for the Knoevenagel condensation. Mol. Catal. 2017, 443, 101–109. [Google Scholar] [CrossRef]
- Sendel, E.B. Colorimetric Determination of Trases of Metals; IntersciencePublishers, Inc.: Hoboken, NJ, USA, 1959. [Google Scholar]
- Velichkova, F.; Julcour-Lebigue, C.; Koumanova, B.; Delmas, H. Heterogeneous Fenton oxidation of paracetamol using iron oxide (nano)particles. J. Environ. Chem. Eng. 2013, 1, 1214–1222. [Google Scholar] [CrossRef] [Green Version]
- Franz, M.; Arafat, H.A.; Pinto, N.G. Effect of chemical surface heterogeneity on the adsorption mechanism of dissolved aromatics on activated carbon. Carbon 2000, 38, 1807–1819. [Google Scholar] [CrossRef]
- Kong, S.-H.; Watts, R.J.; Choi, J.-H. Treatment of petroleum-contaminated soils using iron-mineral-catalyzed hydrogen peroxide. Chemosphere 1998, 37, 1473–1482. [Google Scholar] [CrossRef]
- Huang, H.H.; Lu, M.C.; Chen, J.N. Catalytic Decomposition of Hydrogen Peroxide and 2-chlorophenol with iron oxides. Water Res. 2001, 35, 2291–2299. [Google Scholar] [CrossRef]
- Li, K.; Zhao, Y.; Janik, M.J.; Song, C.; Guo, X. Magnetic mesoporous Fe3O4/C/Cu composite as Fenton-like catalysts. Appl. Surf. Sci. 2017, 396, 1383–1392. [Google Scholar] [CrossRef]
- Costa, R.C.C.; Lelis, M.d.F.F.; Oliveira, L.C.A.; Fabris, J.D.; Ardisson, J.D.; Rios, R.R.V.A.; Silva, C.N.; Lago, R.M. Remarkable effect of Co and Mn on the activity of Fe3−xMxO4 promoted oxidation of organic contaminants in aqueous medium with H2O2. Catal. Commun. 2003, 4, 525–529. [Google Scholar] [CrossRef]
- Deng, J.; Jiang, J.; Zhang, Y.; Lin, X.; Du, C.; Xiong, Y. FeVO4 as a highly active heterogeneous Fenton-like catalyst towards the degradation of Orange II. Appl. Catal. B Environ. 2008, 84, 468–473. [Google Scholar] [CrossRef]
- Sires, I.; Garrido, J.A.; Rodriguez, R.M.; Cabot, P.l.l.; Centellas, F.; Arias, C.; Brillas, E. Electrochemical Degradation of Paracetamol from Water by Catalytic Action of Fe2+, Cu2+, and UVA Light on Electrogenerated Hydrogen Peroxide. J. Electrochem. Soc. 2006, 153, D1–D9. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.E.; Ray, M.B. Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res. 2008, 42, 3480–3488. [Google Scholar] [CrossRef]
- Pereira, M.F.R.; Soares, S.F.; Orfao, J.J.M.; Figueiredo, J.L. Adsorption of dyes on activated carbons: Influence of surface chemical groups. Carbon 2003, 41, 811–821. [Google Scholar] [CrossRef]










| Catalyst | Fe3O4 | Cu | Cu2O |
|---|---|---|---|
| CNTO | - | - | - |
| Fe100/CNT | 2.7 | - | - |
| Fe75Cu25/CNT | 2.9 | - | - |
| Fe50Cu50/CNT | 3.5 | 18.7 | - |
| Fe25Cu75/CNT | - | 24.9 | 15.0 |
| Cu100/CNT | - | 29.0 | 20.0 |
| Catalyst | Fe (wt % ± sd) | Cu (wt % ± sd) | (Fe + Cu) (wt %± sd) |
|---|---|---|---|
| Fe100/CNT | 5.87 ± 0.06 (7) | - | 5.87 ± 0.06 |
| Fe75Cu25/CNT | 4.55 ± 0.04 (5.25) | 1.93 ± 0.01 (1.75) | 6.48 ± 0.04 |
| Fe50Cu50/CNT | 2.91 ± 0.04 (3.5) | 3.82 ± 0.01 (3.5) | 6.73 ± 0.04 |
| Fe25Cu75/CNT | 1.66 ± 0.01 (1.75) | 6.51 ± 0.09 (5.25) | 8.17 ± 0.09 |
| Cu100/CNT | - | 8.07 ± 0.11 (7) | 8.07 ± 0.11 |
| Catalyst | SBET (m²/g) | Smic (m²/g) | VP (cm³/g) | Vmes (cm³/g) | dmes (nm) |
|---|---|---|---|---|---|
| CNT | 248.0 | 24.7 | 0.799 | 0.518 | 12.9 |
| CNTO | 329.7 | 24.2 | 1.136 | 0.956 | 13.8 |
| Fe100/CNT | 254.6 | 15.4 | 0.817 | 0.707 | 12.8 |
| Fe75Cu25/CNT | 237.8 | 4.8 | 0.775 | 0.674 | 13.0 |
| Fe50Cu50/CNT | 273.6 | - | 0.946 | 0.839 | 13.8 |
| Fe25Cu75/CNT | 323.5 | 0.4 | 1.073 | 0.923 | 13.3 |
| Cu100/CNT | 306.3 | 3.3 | 0.969 | 0.865 | 12.7 |
| pH = 6.3–6.6 C0 H2O2 = 13.8 × 10−3 mol/L | pH = 3 C0 H2O2 = 13.8 × 10−3 mol/L | pH = 6.3–6.6 C0 H2O2 = 6.9 × 10−3 mol/L | ||
|---|---|---|---|---|
| Catalyst | k (h−1) | k1 (h−1) * | k2 (h−1) * | k (h−1) |
| Fe100/CNT | 0.53 | 7.36 | 0.34 | 0.15 (k1*), 1.05 (k2*) |
| Fe75Cu25/CNT | 0.17 | 4.20 | 0.03 | 0.05 |
| Fe50Cu50/CNT | 0.45 | 2.15 | 0.22 | 0.10 |
| Fe25Cu75/CNT | 0.78 | 2.29 | 0.06 | 0.29 |
| Cu100/CNT | 0.54 | 0.61 | 0.005 | 0.12 |
| Catalyst | Leaching of Iron (mg/L) | Leaching of Iron * (%) | Leaching of Copper (mg/L) | Leaching of Copper * (%) |
|---|---|---|---|---|
| Fe100/CNT | 3.99 | 17 | - | - |
| Fe75Cu25/CNT | 0.10 | 0.5 | 2.26 | 29.2 |
| Fe50Cu50/CNT | 0.58 | 4.95 | 10.56 | 69.1 |
| Fe25Cu75/CNT | 0.10 | 1.4 | 10.57 | 40.6 |
| Cu100/CNT | - | - | 2.17 | 6.7 |
| Fe100/CNT, pH 3 | 9.25 | 39.4 | - | - |
| Cu100/CNT, pH 3 | - | - | 18.61 | 57.6 |
| TOC (%) | pH | |||||
|---|---|---|---|---|---|---|
| Catalyst | 15 min | 60 min | 180 min | 300 min | Initial | Final |
| Fe100/CNT | - | 88.4 | 70.1 | 7.1 | 6.31 | 5.12 |
| Fe75Cu25/CNT | - | 77.9 | 54.5 | 52.5 | 6.31 | 4.53 |
| Fe50Cu50/CNT | 79.9 | 73.7 | 60.3 | 59.4 | 6.31 | 4.92 |
| Fe25Cu75/CNT | 85.6 | 43.7 | 31.3 | 14.4 | 6.60 | 4.35 |
| Cu100/CNT | 80.7 | 68.1 | 62.2 | 54.0 | 6.60 | 4.51 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrios-Bermúdez, N.; González-Avendaño, M.; Lado-Touriño, I.; Cerpa-Naranjo, A.; Rojas-Cervantes, M.L. Fe-Cu Doped Multiwalled Carbon Nanotubes for Fenton-like Degradation of Paracetamol Under Mild Conditions. Nanomaterials 2020, 10, 749. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10040749
Barrios-Bermúdez N, González-Avendaño M, Lado-Touriño I, Cerpa-Naranjo A, Rojas-Cervantes ML. Fe-Cu Doped Multiwalled Carbon Nanotubes for Fenton-like Degradation of Paracetamol Under Mild Conditions. Nanomaterials. 2020; 10(4):749. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10040749
Chicago/Turabian StyleBarrios-Bermúdez, Niurka, Marta González-Avendaño, Isabel Lado-Touriño, Arisbel Cerpa-Naranjo, and María Luisa Rojas-Cervantes. 2020. "Fe-Cu Doped Multiwalled Carbon Nanotubes for Fenton-like Degradation of Paracetamol Under Mild Conditions" Nanomaterials 10, no. 4: 749. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10040749