One-Pot Synthesis of W2C/WS2 Hybrid Nanostructures for Improved Hydrogen Evolution Reactions and Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of WS2 and W2C Nanostructures
2.2. Synthesis of W2C/WS2 Hybrids
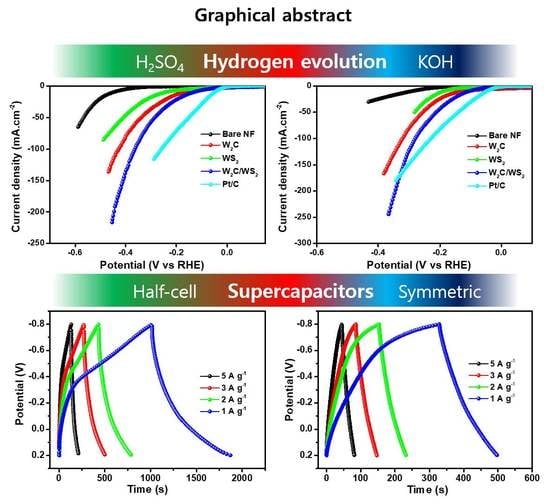
2.3. HER Performance
2.4. Supercapacitor Performance
2.5. Characterization Details
3. Results and Discussion
3.1. Materials Characteristics
3.2. Hydrogen Evolution Studies
3.3. Supercapacitor Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vikraman, D.; Akbar, K.; Hussain, S.; Yoo, G.; Jang, J.-Y.; Chun, S.-H.; Jung, J.; Park, H.J. Direct synthesis of thickness-tunable MoS2 quantum dot thin layers: Optical, structural and electrical properties and their application to hydrogen evolution. Nano Energy 2017, 35, 101–114. [Google Scholar] [CrossRef]
- Sarma, P.V.; Kayal, A.; Sharma, C.H.; Thalakulam, M.; Mitra, J.; Shaijumon, M. Electrocatalysis on edge-rich spiral WS2 for hydrogen evolution. ACS Nano 2019, 13, 10448–10455. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, D.; Karuppasamy, K.; Hussain, S.; Kathalingam, A.; Sanmugam, A.; Jung, J.; Kim, H.-S. One-pot facile methodology to synthesize MoS2-graphene hybrid nanocomposites for supercapacitors with improved electrochemical capacitance. Compos. Part. B Eng. 2019, 161, 555–563. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, X. Recent advances of supercapacitors based on two-dimensional materials. Appl. Mater. Today 2017, 8, 104–115. [Google Scholar] [CrossRef]
- Wazir, M.B.; Daud, M.; Ullah, N.; Hai, A.; Muhammad, A.; Younas, M.; Rezakazemi, M. Synergistic properties of molybdenum disulfide (MoS2) with electro-active materials for high-performance supercapacitors. Int. J. Hydrog Energy 2019, 44, 17470–17492. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Q.; Sun, Z.; Cheng, C. Three-dimensional macroporous W2C inverse opal arrays for the efficient hydrogen evolution reaction. Nanoscale 2019, 11, 11505–11512. [Google Scholar] [CrossRef]
- Attanayake, N.H.; Thenuwara, A.C.; Patra, A.; Aulin, Y.V.; Tran, T.M.; Chakraborty, H.; Borguet, E.; Klein, M.L.; Perdew, J.P.; Strongin, D.R. Effect of intercalated metals on the electrocatalytic activity of 1T-MoS2 for the hydrogen evolution reaction. ACS Energy Lett. 2017, 3, 7–13. [Google Scholar] [CrossRef]
- Hussain, S.; Vikraman, D.; Akbar, K.; Naqvi, B.A.; Abbas, S.M.; Kim, H.-S.; Chun, S.-H.; Jung, J. Fabrication of MoSe2 decorated three-dimensional graphene composites structure as a highly stable electrocatalyst for improved hydrogen evolution reaction. Renew. Energy 2019, 143, 1659–1669. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Karuppasamy, K.; Feroze, A.; Kathalingam, A.; Sanmugam, A.; Chun, S.-H.; Jung, J.; Kim, H.-S. Engineering the novel MoSe2-Mo2C hybrid nanoarray electrodes for energy storage and water splitting applications. Appl. Catal. B Environ. 2020, 264, 118531. [Google Scholar] [CrossRef]
- Huang, M.; Li, F.; Dong, F.; Zhang, Y.X.; Zhang, L.L. Mno2-based nanostructures for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 21380–21423. [Google Scholar] [CrossRef]
- Ramesh, S.; Vikraman, D.; Karuppasamy, K.; Yadav, H.M.; Sivasamy, A.; Kim, H.-S.; Kim, J.-H.; Kim, H.-S. Controlled synthesis of SnO2@NiCo2O4/nitrogen doped multiwalled carbon nanotube hybrids as an active electrode material for supercapacitors. J. Alloys Compd. 2019, 794, 186–194. [Google Scholar] [CrossRef]
- Hussain, S.; Akbar, K.; Vikraman, D.; Afzal, R.A.; Song, W.; An, K.S.; Farooq, A.; Park, J.Y.; Chun, S.H.; Jung, J. Ws(1−x)sex nanoparticles decorated three-dimensional graphene on nickel foam: A robust and highly efficient electrocatalyst for the hydrogen evolution reaction. Nanomaterials 2018, 8, 929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.; Chae, J.; Akbar, K.; Vikraman, D.; Truong, L.; Naqvi, A.B.; Abbas, Y.; Kim, H.-S.; Chun, S.-H.; Kim, G.; et al. Fabrication of robust hydrogen evolution reaction electrocatalyst using Ag2Se by vacuum evaporation. Nanomaterials 2019, 9, 1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, C.-C.; Lin, L.-Y.; Xiao, B.-C.; Chen, Y.-S. Highly efficient supercapacitor electrode with two-dimensional tungsten disulfide and reduced graphene oxide hybrid nanosheets. J. Power Sources 2016, 320, 78–85. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Akbar, K.; Karuppasamy, K.; Chun, S.H.; Jung, J.; Kim, H.S. Design of basal plane edges in metal-doped nanostripes-structured MoSe2 atomic layers to enhance hydrogen evolution reaction activity. ACS Sustain. Chem. Eng. 2019, 7, 458–469. [Google Scholar] [CrossRef]
- Esposito, D.V.; Hunt, S.T.; Kimmel, Y.C.; Chen, J.G. A new class of electrocatalysts for hydrogen production from water electrolysis: Metal monolayers supported on low-cost transition metal carbides. J. Am. Chem. Soc. 2012, 134, 3025–3033. [Google Scholar] [CrossRef]
- Hunt, S.T.; Milina, M.; Alba-Rubio, A.C.; Hendon, C.H.; Dumesic, J.A.; Román-Leshkov, Y. Self-assembly of noble metal monolayers on transition metal carbide nanoparticle catalysts. Science 2016, 352, 974–978. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Vikraman, D.; Feroze, A.; Song, W.; An, K.-S.; Kim, H.-S.; Chun, S.-H.; Jung, J. Synthesis of Mo2C and W2C nanoparticle electrocatalysts for the efficient hydrogen evolution reaction in alkali and acid electrolytes. Front. Chem. 2019, 7, 716. [Google Scholar] [CrossRef] [Green Version]
- Ratha, S.; Rout, C.S. Supercapacitor electrodes based on layered tungsten disulfide-reduced graphene oxide hybrids synthesized by a facile hydrothermal method. ACS Appl. Mater. Interfaces 2013, 5, 11427–11433. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xu, D.; Zhu, Y.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Hierarchical “nanoroll” like MoS2/Ti3C2Tx hybrid with high electrocatalytic hydrogen evolution activity. Appl. Catal. B Environ. 2019, 241, 89–94. [Google Scholar] [CrossRef]
- Lin, T.W.; Sadhasivam, T.; Wang, A.Y.; Chen, T.Y.; Lin, J.Y.; Shao, L.D. Ternary composite nanosheets with MoS2/WS2/graphene heterostructures as high-performance cathode materials for supercapacitors. Chem. Electro. Chem. 2018, 5, 1024–1031. [Google Scholar] [CrossRef]
- Zhao, Z.; Qin, F.; Kasiraju, S.; Xie, L.; Alam, M.K.; Chen, S.; Wang, D.; Ren, Z.; Wang, Z.; Grabow, L.C. Vertically aligned MoS2/Mo2C hybrid nanosheets grown on carbon paper for efficient electrocatalytic hydrogen evolution. ACS Catal. 2017, 7, 7312–7318. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Huang, Z.; Guo, P.; Wang, Z. In-situ solution synthesis of graphene supported lamellar 1T’-MoTe2 for enhanced pseudocapacitors. Mater. Lett. 2017, 206, 229–232. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, Y.; Ge, Z.; Zhao, C.; Qian, X. Facile construction of MoS2/RCF electrode for high-performance supercapacitor. Carbon 2018, 127, 699–706. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Choi, K.S.; Kim, S.Y.; Lee, T.H.; Jang, H.W.; Van Le, Q.; Kim, I.T. Strategy for controlling the morphology and work function of W2C/WS2 nanoflowers. J. Alloys Compd. 2020, 829, 154582. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, S.Y.; Lee, T.H.; Jang, H.W.; Le, Q.V.; Kim, I.T. Facile synthesis of W2C@WS2 alloy nanoflowers and their hydrogen generation performance. Appl. Surf. Sci. 2020, 504, 144389. [Google Scholar] [CrossRef]
- Wang, F.; He, P.; Li, Y.; Shifa, T.A.; Deng, Y.; Liu, K.; Wang, Q.; Wang, F.; Wen, Y.; Wang, Z. Interface engineered WxC@WS2 nanostructure for enhanced hydrogen evolution catalysis. Adv. Funct. Mater. 2017, 27, 1605802. [Google Scholar] [CrossRef]
- Chen, Z.; Gong, W.; Cong, S.; Wang, Z.; Song, G.; Pan, T.; Tang, X.; Chen, J.; Lu, W.; Zhao, Z. Eutectoid-structured WC/W2C heterostructures: A new platform for long-term alkaline hydrogen evolution reaction at low overpotentials. Nano Energy 2020, 68, 104335. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Zhang, H.; Zhang, J. Interface designing over WS2/W2C for enhanced hydrogen evolution catalysis. ACS Appl. Energy Mater. 2018, 1, 3377–3384. [Google Scholar] [CrossRef]
- Javed, M.S.; Dai, S.; Wang, M.; Guo, D.; Chen, L.; Wang, X.; Hu, C.; Xi, Y. High performance solid state flexible supercapacitor based on molybdenum sulfide hierarchical nanospheres. J. Power Sources 2015, 285, 63–69. [Google Scholar] [CrossRef]
- Dash, T.; Nayak, B. Preparation of WC–W2C composites by arc plasma melting and their characterisations. Ceram. Int. 2013, 39, 3279–3292. [Google Scholar] [CrossRef]
- Yan, G.; Wu, C.; Tan, H.; Feng, X.; Yan, L.; Zang, H.; Li, Y. N-carbon coated pw 2 c composite as efficient electrocatalyst for hydrogen evolution reactions over the whole ph range. J. Mater. Chem. A 2017, 5, 765–772. [Google Scholar] [CrossRef]
- Voiry, D.; Yamaguchi, H.; Li, J.; Silva, R.; Alves, D.C.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 2013, 12, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, N.; Su, C.; Zhao, H.; Xu, L.; Yin, Z.; Li, J.; Du, Y. Colloidal synthesis of 1T’phase dominated WS2 towards endurable electrocatalysis. Nano Energy 2018, 50, 176–181. [Google Scholar] [CrossRef]
- Gao, M.-R.; Chan, M.K.; Sun, Y. Edge-terminated molybdenum disulfide with a 9.4-Å interlayer spacing for electrochemical hydrogen production. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Lu, Z.; Kong, D.; Sun, J.; Hymel, T.M.; Cui, Y. Electrochemical tuning of MoS2 nanoparticles on three-dimensional substrate for efficient hydrogen evolution. ACS Nano 2014, 8, 4940–4947. [Google Scholar] [CrossRef]
- Vikraman, D.; Park, H.J.; Kim, S.I.; Thaiyan, M. Magnetic, structural and optical behavior of cupric oxide layers for solar cells. J. Alloys Compd. 2016, 686, 616–627. [Google Scholar] [CrossRef]
- Sebastian, S.; Kulandaisamy, I.; Valanarasu, S.; Yahia, I.S.; Kim, H.-S.; Vikraman, D. Microstructural and electrical properties evaluation of lead doped tin sulfide thin films. J. Sol. Gel Sci. Technol. 2020, 93, 52–61. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, Y.; Hu, Q.; Zhou, J.; Feng, R.; Duchesne, P.N.; Zhang, P.; Chen, F.; Han, N.; Li, Y. Ultrasmall and phase-pure W2C nanoparticles for efficient electrocatalytic and photoelectrochemical hydrogen evolution. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-N.; Ma, Y.-Y.; Lang, Z.-L.; Wang, Y.-H.; Khan, S.U.; Yan, G.; Tan, H.-Q.; Zang, H.-Y.; Li, Y.-G. Ultrafine cable-like WC/W2C heterojunction nanowires covered by graphitic carbon towards highly efficient electrocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 15395–15403. [Google Scholar] [CrossRef]
- Lewin, E.; Persson, P.Å.; Lattemann, M.; Stüber, M.; Gorgoi, M.; Sandell, A.; Ziebert, C.; Schäfers, F.; Braun, W.; Halbritter, J. On the origin of a third spectral component of C1s XPS-spectra for nc-TiC/a-C nanocomposite thin films. Surf. Coat. Technol. 2008, 202, 3563–3570. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Mu, J.; Yang, E.-C.; Zhao, X.-J. In situ synthesis of molybdenum carbide/n-doped carbon hybrids as an efficient hydrogen-evolution electrocatalyst. RSC Adv. 2018, 8, 17202–17208. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Zhao, T.; Wu, W.; Wu, F.; Li, L.; Qian, J.; Xu, R.; Wu, H.; Albishri, H.M.; Al-Bogami, A. Free-standing hierarchically sandwich-type tungsten disulfide nanotubes/graphene anode for lithium-ion batteries. Nano Lett. 2014, 14, 5899–5904. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, D.; Hussain, S.; Truong, L.; Karuppasamy, K.; Kim, H.-J.; Maiyalagan, T.; Chun, S.-H.; Jung, J.; Kim, H.-S. Fabrication of MoS2/WSe2 heterostructures as electrocatalyst for enhanced hydrogen evolution reaction. Appl. Surf. Sci. 2019, 480, 611–620. [Google Scholar] [CrossRef]
- Fan, X.; Zhou, H.; Guo, X. Wc nanocrystals grown on vertically aligned carbon nanotubes: An efficient and stable electrocatalyst for hydrogen evolution reaction. ACS Nano 2015, 9, 5125–5134. [Google Scholar] [CrossRef]
- Xu, K.; Wang, F.; Wang, Z.; Zhan, X.; Wang, Q.; Cheng, Z.; Safdar, M.; He, J. Component-controllable WS2(1–x)Se2x nanotubes for efficient hydrogen evolution reaction. ACS Nano 2014, 8, 8468–8476. [Google Scholar] [CrossRef]
- Ma, L.; Ting, L.R.L.; Molinari, V.; Giordano, C.; Yeo, B.S. Efficient hydrogen evolution reaction catalyzed by molybdenum carbide and molybdenum nitride nanocatalysts synthesized via the urea glass route. J. Mater. Chem. A 2015, 3, 8361–8368. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, Y.; Fu, D.; Chen, Y. Molybdenum carbide nanocrystal embedded n-doped carbon nanotubes as electrocatalysts for hydrogen generation. J. Mater. Chem. A 2015, 3, 5783–5788. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Akbar, K.; Truong, L.; Kathalingam, A.; Chun, S.-H.; Jung, J.; Park, H.J.; Kim, H.-S. Improved hydrogen evolution reaction performance using MoS2–WS2 heterostructures by physicochemical process. ACS Sustain. Chem. Eng. 2018, 6, 8400–8409. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Li, C.; Zhao, Y.; Yu, X.; Chen, Y. Porous one-dimensional Mo2C–amorphous carbon composites: High-efficient and durable electrocatalysts for hydrogen generation. Phys. Chem. Chem. Phys. 2015, 17, 16609–16614. [Google Scholar] [CrossRef] [PubMed]
- Šljukić, B.; Vujković, M.; Amaral, L.; Santos, D.; Rocha, R.; Sequeira, C.; Figueiredo, J.L. Carbon-supported Mo2C electrocatalysts for hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 15505–15512. [Google Scholar] [CrossRef]
- Soon, J.M.; Loh, K.P. Electrochemical double-layer capacitance of MoS2 nanowall films. Electrochem. Solid State Lett. 2007, 10, A250–A254. [Google Scholar] [CrossRef]
- Chen, W.; Yu, X.; Zhao, Z.; Ji, S.; Feng, L. Hierarchical architecture of coupling graphene and 2D WS2 for high-performance supercapacitor. Electrochim. Acta 2019, 298, 313–320. [Google Scholar] [CrossRef]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon materials for chemical capacitive energy storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ju, P.; Zhao, C.; Qian, X. In-situ grown of MoS2/rGO/ MoS2@Mo nanocomposite and its supercapacitor performance. Electrochim. Acta 2016, 219, 693–700. [Google Scholar] [CrossRef]
- Huang, K.-J.; Wang, L.; Zhang, J.-Z.; Wang, L.-L.; Mo, Y.-P. One-step preparation of layered molybdenum disulfide/multi-walled carbon nanotube composites for enhanced performance supercapacitor. Energy 2014, 67, 234–240. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, C.; Huang, Y.; Gao, Y.; Wang, Y.; Li, Z.; Yan, Y.; Zhang, M. Oxygen-vacancy-rich NiCo2O4 nanoneedles electrode with poor crystallinity for high energy density all-solid-state symmetric supercapacitors. J. Power Sources 2020, 449, 227571. [Google Scholar] [CrossRef]
- Balasingam, S.K.; Lee, M.; Kim, B.H.; Lee, J.S.; Jun, Y. Freeze-dried MoS2 sponge electrodes for enhanced electrochemical energy storage. Dalton Trans. 2017, 46, 2122–2128. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Manoharan, S.; Kim, S.-J. High energy symmetric supercapacitor based on mechanically delaminated few-layered MoS2 sheets in organic electrolyte. J. Alloys Compd. 2019, 771, 803–809. [Google Scholar] [CrossRef]
- Navarro-Suárez, A.M.; Van Aken, K.L.; Mathis, T.; Makaryan, T.; Yan, J.; Carretero-González, J.; Rojo, T.; Gogotsi, Y. Development of asymmetric supercapacitors with titanium carbide-reduced graphene oxide couples as electrodes. Electrochim. Acta 2018, 259, 752–761. [Google Scholar] [CrossRef]
- Khawula, T.N.; Raju, K.; Franklyn, P.J.; Sigalas, I.; Ozoemena, K.I. Symmetric pseudocapacitors based on molybdenum disulfide (MoS2)-modified carbon nanospheres: Correlating physicochemistry and synergistic interaction on energy storage. J. Mater. Chem. A 2016, 4, 6411–6425. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.; Kumar, S.; Agarwal, K.; Soni, K.; Gedela, V.R.; Ghosh, K. Three-dimensional graphene with MoS2 nanohybrid as potential energy storage/transfer device. Sci. Rep. 2017, 7, 1–12. [Google Scholar]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Rabani, I.; Vikraman, D.; Feroze, A.; Ali, M.; Seo, Y.-S.; Kim, H.-S.; Chun, S.-H.; Jung, J. One-Pot Synthesis of W2C/WS2 Hybrid Nanostructures for Improved Hydrogen Evolution Reactions and Supercapacitors. Nanomaterials 2020, 10, 1597. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10081597
Hussain S, Rabani I, Vikraman D, Feroze A, Ali M, Seo Y-S, Kim H-S, Chun S-H, Jung J. One-Pot Synthesis of W2C/WS2 Hybrid Nanostructures for Improved Hydrogen Evolution Reactions and Supercapacitors. Nanomaterials. 2020; 10(8):1597. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10081597
Chicago/Turabian StyleHussain, Sajjad, Iqra Rabani, Dhanasekaran Vikraman, Asad Feroze, Muhammad Ali, Young-Soo Seo, Hyun-Seok Kim, Seung-Hyun Chun, and Jongwan Jung. 2020. "One-Pot Synthesis of W2C/WS2 Hybrid Nanostructures for Improved Hydrogen Evolution Reactions and Supercapacitors" Nanomaterials 10, no. 8: 1597. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10081597