Zn Metal Anodes for Zn-Ion Batteries in Mild Aqueous Electrolytes: Challenges and Strategies
Abstract
:1. Introduction
2. Challenges in the Commercialization of Zn Metal Anodes
Zn Anode Reactions
3. Assembly and Test Technology of Zn-Ion Batteries with Zn Metal Anodes
3.1. Cell Assembly
3.2. Cell Test
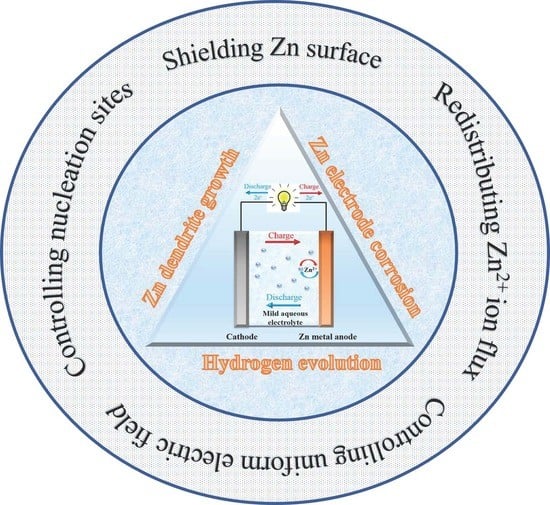
4. Drawbacks of Zn Metal Anodes in Mildly Acidic Electrolytes
4.1. Zn Dendrite Growth
4.2. Zn Electrode Corrosion
4.3. Hydrogen Evolution
5. Common Strategies for Modifying the Surface of Zn Metal Anodes
5.1. Shielding the Zn Surface
5.2. Regulating the Zn Deposition Behavior
5.2.1. Controlling the Nucleation Sites
5.2.2. Redistributing Zn2+ Ion Flux
5.3. Creating Uniform Electric Field
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiao, Y.; Kang, L.; Berry-Gair, J.; McColl, K.; Li, J.; Dong, H.; Jiang, H.; Wang, R.; Corà, F.; Brett, D.J.L.; et al. Enabling stable MnO2 matrix for aqueous zinc-ion battery cathodes. J. Mater. Chem. A 2020, 8, 22075–22082. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, J.; Zhao, S.; Zhao, F.; Xiong, S.; Brett, D.J.L.; He, G.; Parkin, I.P. An anti-aging polymer electrolyte for flexible rechargeable zinc-ion batteries. J. Mater. Chem. A 2020, 8, 22637–22644. [Google Scholar] [CrossRef]
- Dong, H.; Li, J.; Zhao, S.; Jiao, Y.; Chen, J.; Tan, Y.; Brett, D.J.L.; He, G.; Parkin, I.P. Investigation of a Biomass Hydrogel Electrolyte Naturally Stabilizing Cathodes for Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zhu, X.; Xue, L.; Ni, M.; Zhao, Y.; Liu, B.; Xia, H. The function of Mn2+ additive in aqueous electrolyte for Zn/δ-MnO2 battery. Electrochim. Acta 2020, 351, 136445. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Fuller, T.F. Batteries: Bigger and better. Nat. Energy 2016, 1, 16003. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Abdin, Z.; Khalilpour, K.R. Chapter 4—Single and Polystorage Technologies for Renewable-Based Hybrid Energy Systems. In Polygeneration with Polystorage for Chemical and Energy Hubs; Khalilpour, K.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 77–131. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Ramadass, P.; Fang, W. 18-Safety of Lithium-Ion Batteries. In Lithium-Ion Batteries; Pistoia, G., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2014; pp. 409–435. [Google Scholar] [CrossRef]
- Yoshino, A. 1-Development of the Lithium-Ion Battery and Recent Technological Trends. In Lithium-Ion Batteries; Pistoia, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–20. [Google Scholar] [CrossRef]
- Mun, Y.S.; Pham, T.N.; Hoang Bui, V.K.; Tanaji, S.T.; Lee, H.U.; Lee, G.-W.; Choi, J.S.; Kim, I.T.; Lee, Y.-C. Tin oxide evolution by heat-treatment with tin-aminoclay (SnAC) under argon condition for lithium-ion battery (LIB) anode applications. J. Power Sources 2019, 437, 226946. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Park, D.; Kim, I.T. FexSnyOz Composites as Anode Materials for Lithium-Ion Storage. J. Nanosci. Nanotechnol. 2019, 19, 6636–6640. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Kim, J.H.; Kim, I.T. Electrochemical Performance of Sn/SnO/Ni3Sn Composite Anodes for Lithium-Ion Batteries. J. Nanosci. Nanotechnol. 2019, 19, 1001–1005. [Google Scholar] [CrossRef]
- Pham, T.N.; Tanaji, S.T.; Choi, J.-S.; Lee, H.U.; Kim, I.T.; Lee, Y.-C. Preparation of Sn-aminoclay (SnAC)-templated Fe3O4 nanoparticles as an anode material for lithium-ion batteries. RSC Adv. 2019, 9, 10536–10545. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.P.; Kim, I.T. Ag Nanoparticle-Decorated MoS2 Nanosheets for Enhancing Electrochemical Performance in Lithium Storage. Nanomaterials 2021, 11, 626. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Kim, I.T. Self-Assembled Few-Layered MoS2 on SnO2 Anode for Enhancing Lithium-Ion Storage. Nanomaterials 2020, 10, 2558. [Google Scholar] [CrossRef]
- Preman, A.N.; Lee, H.; Yoo, J.; Kim, I.T.; Saito, T.; Ahn, S.-k. Progress of 3D network binders in silicon anodes for lithium ion batteries. J. Mater. Chem. A 2020, 8, 25548–25570. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. W2C/WS2 Alloy Nanoflowers as Anode Materials for Lithium-Ion Storage. Nanomaterials 2020, 10, 1336. [Google Scholar] [CrossRef]
- Kim, W.S.; Vo, T.N.; Kim, I.T. GeTe-TiC-C Composite Anodes for Li-Ion Storage. Materials 2020, 13, 4222. [Google Scholar] [CrossRef]
- Vo, T.N.; Kim, D.S.; Mun, Y.S.; Lee, H.J.; Ahn, S.-k.; Kim, I.T. Fast charging sodium-ion batteries based on Te-P-C composites and insights to low-frequency limits of four common equivalent impedance circuits. Chem. Eng. J. 2020, 398, 125703. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Y.; Dong, Y.; Zhang, Z.; Tang, Z. Recent progress in Ti-based nanocomposite anodes for lithium ion batteries. J. Adv. Ceram. 2019, 8, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Lu, J.; Ji, X.; Li, Y.; Shao, Y.; Chen, Z.; Zhong, C.; Amine, K. Design strategies for nonaqueous multivalent-ion and monovalent-ion battery anodes. Nat. Rev. Mater. 2020, 5, 276–294. [Google Scholar] [CrossRef]
- Li, X.; Chen, G.; Le, Z.; Li, X.; Nie, P.; Liu, X.; Xu, P.; Wu, H.B.; Liu, Z.; Lu, Y. Well-dispersed phosphorus nanocrystals within carbon via high-energy mechanical milling for high performance lithium storage. Nano Energy 2019, 59, 464–471. [Google Scholar] [CrossRef]
- Chao, D.; Ouyang, B.; Liang, P.; Huong, T.T.T.; Jia, G.; Huang, H.; Xia, X.; Rawat, R.S.; Fan, H.J. C-Plasma of Hierarchical Graphene Survives SnS Bundles for Ultrastable and High Volumetric Na-Ion Storage. Adv. Mater. 2018, 30, e1804833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Guo, Z. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci. Adv. 2019, 5, eaav7412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, K.M. How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Jothi, V.R.; Nichelson, A.; Vikraman, D.; Tanveer, W.H.; Kim, H.-S.; Yi, S.-C. Chapter 14—Nanostructured transition metal sulfide/selenide anodes for high-performance sodium-ion batteries. In Nanostructured, Functional, and Flexible Materials for Energy Conversion and Storage Systems; Pandikumar, A., Rameshkumar, P., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 437–464. [Google Scholar] [CrossRef]
- Jana, A.; Paul, R.; Roy, A.K. Chapter 2—Architectural design and promises of carbon materials for energy conversion and storage: In laboratory and industry. In Carbon Based Nanomaterials for Advanced Thermal and Electrochemical Energy Storage and Conversion; Paul, R., Etacheri, V., Wang, Y., Lin, C.-T., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 25–61. [Google Scholar] [CrossRef]
- Anoopkumar, V.; John, B.; Mercy, T.D. Potassium-Ion Batteries: Key to Future Large-Scale Energy Storage? ACS Appl. Energy Mater. 2020, 3, 9478–9492. [Google Scholar] [CrossRef]
- Min, X.; Xiao, J.; Fang, M.; Wang, W.; Zhao, Y.; Liu, Y.; Abdelkader, A.M.; Xi, K.; Kumar, R.V.; Huang, Z. Potassium-ion batteries: Outlook on present and future technologies. Energy Environ. Sci. 2021, 14, 2186–2243. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Hu, J.; Jung, E.; Zhang, J.; Jun, S.C.; Yamauchi, Y. Unlocking the Potential of Oxygen-Deficient Copper-Doped Co3O4 Nanocrystals Confined in Carbon as an Advanced Electrode for Flexible Solid-State Supercapacitors. ACS Energy Lett. 2021, 6, 3011–3019. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Kim, J.M.; Chun, Y.T.; Zhang, J.; Jun, S.C. Recent Advances in Vanadium-Based Aqueous Rechargeable Zinc-Ion Batteries. Adv. Energy Mater. 2020, 10, 2000477. [Google Scholar] [CrossRef]
- Kang, L.; Zhang, M.; Zhang, J.; Liu, S.; Zhang, N.; Yao, W.; Ye, Y.; Luo, C.; Gong, Z.; Wang, C.; et al. Dual-defect surface engineering of bimetallic sulfide nanotubes towards flexible asymmetric solid-state supercapacitors. J. Mater. Chem. A 2020, 8, 24053–24064. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Jun, S.C. Challenges and Strategies toward Cathode Materials for Rechargeable Potassium-Ion Batteries. Adv. Mater. 2021, 2004689. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yamada, Y.; Sodeyama, K.; Watanabe, E.; Takada, K.; Tateyama, Y.; Yamada, A. Fire-extinguishing organic electrolytes for safe batteries. Nat. Energy 2018, 3, 22–29. [Google Scholar] [CrossRef]
- Chen, L.; Cao, L.; Ji, X.; Hou, S.; Li, Q.; Chen, J.; Yang, C.; Eidson, N.; Wang, C. Enabling safe aqueous lithium ion open batteries by suppressing oxygen reduction reaction. Nat. Commun. 2020, 11, 2638. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Lv, C.; Wang, L.; Cui, W.; Zhang, L.; Dinh, K.N.; Tan, H.; Wu, C.; Wu, T.; Ren, Y.; et al. Architecting a Stable High-Energy Aqueous Al-Ion Battery. J. Am. Chem. Soc. 2020, 142, 15295–15304. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, V.; Arora, H.; Manalastas, W.; Srinivasan, M. Rechargeable Al-Metal Aqueous Battery Using NaMnHCF as a Cathode: Investigating the Role of Coated-Al Anode Treatments for Superior Battery Cycling Performance. ACS Appl. Energy Mater. 2020, 3, 8627–8635. [Google Scholar] [CrossRef]
- Tian, H.; Li, Z.; Feng, G.; Yang, Z.; Fox, D.; Wang, M.; Zhou, H.; Zhai, L.; Kushima, A.; Du, Y.; et al. Stable, high-performance, dendrite-free, seawater-based aqueous batteries. Nat. Commun. 2021, 12, 237. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Park, K.-Y.; Kim, H.; Kim, S.-W.; Kang, K. Aqueous Rechargeable Li and Na Ion Batteries. Chem. Rev. 2014, 114, 11788–11827. [Google Scholar] [CrossRef]
- Chao, D.; Zhou, W.; Xie, F.; Ye, C.; Li, H.; Jaroniec, M.; Qiao, S.-Z. Roadmap for advanced aqueous batteries: From design of materials to applications. Sci. Adv. 2020, 6, eaba4098. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.; Park, Y.; Choi, J.W. Aqueous zinc ion batteries: Focus on zinc metal anodes. Chem. Sci. 2020, 11, 2028–2044. [Google Scholar] [CrossRef] [Green Version]
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.-K.; Myung, S.-T. Present and Future Perspective on Electrode Materials for Rechargeable Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent Advances in Zn-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, T.; van den Bergh, W.; Stefik, M.; Huang, K. A High Performing Zn-Ion Battery Cathode Enabled by In Situ Transformation of V(2) O(5) Atomic Layers. Angew. Chem. Int. Ed. 2020, 59, 17004–17011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Fu, H. Recent advances in rechargeable Zn-based batteries. J. Power Sources 2021, 493, 229677. [Google Scholar] [CrossRef]
- Li, C.; Xie, X.; Liang, S.; Zhou, J. Issues and Future Perspective on Zinc Metal Anode for Rechargeable Aqueous Zinc-ion Batteries. Energy Environ. Mater. 2020, 3, 146–159. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Z.; Tawiah, B.; Wang, Y.; Chan, C.-Y.; Fei, B.; Pan, F. Recent advances in zinc anodes for high-performance aqueous Zn-ion batteries. Nano Energy 2020, 70, 104523. [Google Scholar] [CrossRef]
- Han, C.; Li, W.; Liu, H.K.; Dou, S.; Wang, J. Principals and strategies for constructing a highly reversible zinc metal anode in aqueous batteries. Nano Energy 2020, 74, 104880. [Google Scholar] [CrossRef]
- Xie, C.; Li, Y.; Wang, Q.; Sun, D.; Tang, Y.; Wang, H. Issues and solutions toward zinc anode in aqueous zinc-ion batteries: A mini review. Carbon Energy 2020, 2, 540–560. [Google Scholar] [CrossRef]
- Guo, L.; Guo, H.; Huang, H.; Tao, S.; Cheng, Y. Inhibition of Zinc Dendrites in Zinc-Based Flow Batteries. Front. Chem. 2020, 8, 557. [Google Scholar] [CrossRef]
- Du, W.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Challenges in the material and structural design of zinc anode towards high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2020, 13, 3330–3360. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Mo, F.; Wang, D.; Yang, Q.; Huang, Z.; Liang, G.; Chen, A.; Zhi, C. Dendrites issues and advances in Zn anode for aqueous rechargeable Zn-based batteries. EcoMat 2020, 2, e12035. [Google Scholar] [CrossRef]
- Hao, J.; Li, X.; Zeng, X.; Li, D.; Mao, J.; Guo, Z. Deeply understanding the Zn anode behaviour and corresponding improvement strategies in different aqueous Zn-based batteries. Energy Environ. Sci. 2020, 13, 3917–3949. [Google Scholar] [CrossRef]
- Yi, Z.; Chen, G.; Hou, F.; Wang, L.; Liang, J. Zinc-Ion Batteries: Strategies for the Stabilization of Zn Metal Anodes for Zn-Ion Batteries (Adv. Energy Mater. 1/2021). Adv. Energy Mater. 2021, 11, 2170001. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Shao, C.; Li, G.; Wang, J.; Liu, D.; Dong, X. The strategies of boosting the performance of highly reversible zinc anodes in zinc-ion batteries: Recent progress and future perspectives. Sustain. Energy Fuels 2021, 5, 332–350. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Zhang, Y.; Li, Y.; Sun, R.; Wang, Z.; Wang, H. Strategies towards the challenges of zinc metal anode in rechargeable aqueous zinc ion batteries. Energy Storage Mater. 2021, 35, 19–46. [Google Scholar] [CrossRef]
- Zheng, J.; Archer, L.J.S.A. Controlling electrochemical growth of metallic zinc electrodes: Toward affordable rechargeable energy storage systems. Sci. Adv. 2021, 7, eabe0219. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xiao, P.; Xue, L.; Li, H.; Zhai, T. The rising zinc anodes for high-energy aqueous batteries. EnergyChem 2021, 3, 100052. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, X.; Ding, J.; Hu, W.; Zhong, C.; Lu, J. Challenges in Zinc Electrodes for Alkaline Zinc–Air Batteries: Obstacles to Commercialization. ACS Energy Lett. 2019, 4, 2259–2270. [Google Scholar] [CrossRef]
- Wu, T.H.; Zhang, Y.; Althouse, Z.D.; Liu, N. Nanoscale design of zinc anodes for high-energy aqueous rechargeable batteries. Mater. Today Nano 2019, 6, 100032. [Google Scholar] [CrossRef]
- Tan, P.; Chen, B.; Xu, H.; Cai, W.; He, W.; Zhang, H.; Liu, M.; Shao, Z.; Ni, M. Integration of Zn–Ag and Zn–Air Batteries: A Hybrid Battery with the Advantages of Both. ACS Appl. Mater. Interfaces 2018, 10, 36873–36881. [Google Scholar] [CrossRef]
- Li, P.-C.; Hu, C.-C.; You, T.-H.; Chen, P.-Y. Development and characterization of bi-functional air electrodes for rechargeable zinc-air batteries: Effects of carbons. Carbon 2017, 111, 813–821. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, D.; He, J.; Li, J.; Chen, H.; Chen, Y.; Chao, D. A scalable top-down strategy toward practical metrics of Ni–Zn aqueous batteries with total energy densities of 165 W h kg−1 and 506 W h L−1. Energy Environ. Sci. 2020, 13, 4157–4167. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry With H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Dong, Y.; Jia, M.; Bian, X.; Wang, Y.; Qiu, M.; Xu, J.; Liu, Y.; Jiao, L.; Cheng, F. Rechargeable Aqueous Zn–V2O5 Battery with High Energy Density and Long Cycle Life. ACS Energy Lett. 2018, 3, 1366–1372. [Google Scholar] [CrossRef]
- Xiaowei, C.; Yanliang, L.; Yan, Y. Electrolyte dictated materials design for beyond lithium ion batteries. In Energy Harvesting and Storage: Materials, Devices, and Applications VIII; International Society for Optics and Photonics: Bellingham, WA, USA, 2018; Volume 10663. [Google Scholar]
- Liang, Y.; Jing, Y.; Gheytani, S.; Lee, K.-Y.; Liu, P.; Facchetti, A.; Yao, Y. Universal quinone electrodes for long cycle life aqueous rechargeable batteries. Nat. Mater. 2017, 16, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, C.F.; Fitz, O.S.; Burns, J.; Bauer, M.; Gentischer, H.; Birke, K.P.; Henning, H.-M.; Biro, D. Revealing the Local pH Value Changes of Acidic Aqueous Zinc Ion Batteries with a Manganese Dioxide Electrode during Cycling. J. Electrochem. Soc. 2020, 167, 020545. [Google Scholar] [CrossRef]
- Higashi, S.; Lee, S.W.; Lee, J.S.; Takechi, K.; Cui, Y. Avoiding short circuits from zinc metal dendrites in anode by backside-plating configuration. Nat. Commun. 2016, 7, 11801. [Google Scholar] [CrossRef]
- Blanc, L.E.; Kundu, D.; Nazar, L.F. Scientific Challenges for the Implementation of Zn-Ion Batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent progress and perspectives on aqueous Zn-based rechargeable batteries with mild aqueous electrolytes. Energy Storage Mater. 2019, 20, 410–437. [Google Scholar] [CrossRef]
- Wippermann, K.; Schultze, J.W.; Kessel, R.; Penninger, J. The inhibition of zinc corrosion by bisaminotriazole and other triazole derivatives. Corros. Sci. 1991, 32, 205–230. [Google Scholar] [CrossRef]
- Hwang, B.; Oh, E.-S.; Kim, K. Observation of electrochemical reactions at Zn electrodes in Zn-air secondary batteries. Electrochim. Acta 2016, 216, 484–489. [Google Scholar] [CrossRef]
- Toussaint, G.; Stevens, P.; Akrour, L.; Rouget, R.; Fourgeot, F. Development of a Rechargeable Zinc-Air Battery. ECS Trans. 2019, 28, 25–34. [Google Scholar] [CrossRef]
- Zhang, W.; Zhai, X.; Zhang, Y.; Wei, H.; Ma, J.; Wang, J.; Liang, L.; Liu, Y.; Wang, G.; Ren, F.; et al. Application of Manganese-Based Materials in Aqueous Rechargeable Zinc-Ion Batteries. Front. Energy Res. 2020, 8, 195. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Zhang, X. Challenges and perspectives for manganese-based oxides for advanced aqueous zinc-ion batteries. InfoMat 2020, 2, 237–260. [Google Scholar] [CrossRef]
- Yang, H.; Chang, Z.; Qiao, Y.; Deng, H.; Mu, X.; He, P.; Zhou, H. Constructing a Super-Saturated Electrolyte Front Surface for Stable Rechargeable Aqueous Zinc Batteries. Angew. Chem. Int. Ed. 2020, 59, 9377–9381. [Google Scholar] [CrossRef]
- Yuksel, R.; Buyukcakir, O.; Seong, W.K.; Ruoff, R.S. Metal-Organic Framework Integrated Anodes for Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2020, 10, 1904215. [Google Scholar] [CrossRef]
- Hou, Z.; Gao, Y.; Tan, H.; Zhang, B. Realizing high-power and high-capacity zinc/sodium metal anodes through interfacial chemistry regulation. Nat. Commun. 2021, 12, 3083. [Google Scholar] [CrossRef]
- Yu, Y.; Xie, J.; Zhang, H.; Qin, R.; Liu, X.; Lu, X. High-Voltage Rechargeable Aqueous Zinc-Based Batteries: Latest Progress and Future Perspectives. Small Sci. 2021, 1, 2000066. [Google Scholar] [CrossRef]
- Li, G.; Liu, Z.; Huang, Q.; Gao, Y.; Regula, M.; Wang, D.; Chen, L.-Q.; Wang, D. Stable metal battery anodes enabled by polyethylenimine sponge hosts by way of electrokinetic effects. Nat. Energy 2018, 3, 1076–1083. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, J.; Guo, Z.; Dong, X.; Liu, Y.; Wang, Y.; Xia, Y. A Metal-Organic Framework Host for Highly Reversible Dendrite-free Zinc Metal Anodes. Joule 2019, 3, 1289–1300. [Google Scholar] [CrossRef]
- Pei, A.; Zheng, G.; Shi, F.; Li, Y.; Cui, Y. Nanoscale Nucleation and Growth of Electrodeposited Lithium Metal. Nano Lett. 2017, 17, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Luan, J.; Tang, Y.; Ji, X.; Wang, S.; Wang, H. A facile annealing strategy for achieving in situ controllable Cu2O nanoparticle decorated copper foil as a current collector for stable lithium metal anodes. J. Mater. Chem. A 2018, 6, 18444–18448. [Google Scholar] [CrossRef]
- Sagane, F.; Ikeda, K.-i.; Okita, K.; Sano, H.; Sakaebe, H.; Iriyama, Y. Effects of current densities on the lithium plating morphology at a lithium phosphorus oxynitride glass electrolyte/copper thin film interface. J. Power Sources 2013, 233, 34–42. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, G.; Guo, Y.; Liu, Z.; Yan, B.; Wang, D.; Huang, Z.; Li, X.; Fan, J.; Zhi, C. Do Zinc Dendrites Exist in Neutral Zinc Batteries: A Developed Electrohealing Strategy to In Situ Rescue In-Service Batteries. Adv. Mater. 2019, 31, 1903778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Luan, J.; Tang, Y.; Ji, X.; Wang, H. Interfacial Design of Dendrite-Free Zinc Anodes for Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 13180–13191. [Google Scholar] [CrossRef]
- Kang, L.; Cui, M.; Jiang, F.; Gao, Y.; Luo, H.; Liu, J.; Liang, W.; Zhi, C. Nanoporous CaCO3 Coatings Enabled Uniform Zn Stripping/Plating for Long-Life Zinc Rechargeable Aqueous Batteries. Adv. Energy Mater. 2018, 8, 1801090. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, J.; Tian, J.; Niu, Z. Recent Progress in the Electrolytes of Aqueous Zinc-Ion Batteries. Chem. A Eur. J. 2019, 25, 14480–14494. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhao, J.; Hu, Z.; Li, J.; Li, J.; Zhang, Y.; Wang, C.; Cui, G. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ. Sci. 2019, 12, 1938–1949. [Google Scholar] [CrossRef]
- Kim, D.; Lee, C.; Jeong, S. A concentrated electrolyte for zinc hexacyanoferrate electrodes in aqueous rechargeable zinc-ion batteries. IOP Conf. Ser. Mater. Sci. Eng. 2018, 284, 012001. [Google Scholar] [CrossRef]
- Kasiri, G.; Trócoli, R.; Bani Hashemi, A.; La Mantia, F. An electrochemical investigation of the aging of copper hexacyanoferrate during the operation in zinc-ion batteries. Electrochim. Acta 2016, 222, 74–83. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Meng, Y.; Yu, M.; Yi, J.; Wu, Y.; Lu, X.; Tong, Y. Achieving Ultrahigh Energy Density and Long Durability in a Flexible Rechargeable Quasi-Solid-State Zn–MnO2 Battery. Adv. Mater. 2017, 29, 1700274. [Google Scholar] [CrossRef]
- Lee, B.; Seo, H.R.; Lee, H.R.; Yoon, C.S.; Kim, J.H.; Chung, K.Y.; Cho, B.W.; Oh, S.H. Critical Role of pH Evolution of Electrolyte in the Reaction Mechanism for Rechargeable Zinc Batteries. ChemSusChem 2016, 9, 2948–2956. [Google Scholar] [CrossRef]
- Jiang, B.; Xu, C.; Wu, C.; Dong, L.; Li, J.; Kang, F. Manganese Sesquioxide as Cathode Material for Multivalent Zinc Ion Battery with High Capacity and Long Cycle Life. Electrochim. Acta 2017, 229, 422–428. [Google Scholar] [CrossRef]
- Zhao, S.; Han, B.; Zhang, D.; Huang, Q.; Xiao, L.; Chen, L.; Ivey, D.G.; Deng, Y.; Wei, W. Unravelling the reaction chemistry and degradation mechanism in aqueous Zn/MnO2 rechargeable batteries. J. Mater. Chem. A 2018, 6, 5733–5739. [Google Scholar] [CrossRef]
- Cai, Z.; Ou, Y.; Wang, J.; Xiao, R.; Fu, L.; Yuan, Z.; Zhan, R.; Sun, Y. Chemically resistant Cu–Zn/Zn composite anode for long cycling aqueous batteries. Energy Storage Mater. 2020, 27, 205–211. [Google Scholar] [CrossRef]
- Chao, D.; Zhou, W.; Ye, C.; Zhang, Q.; Chen, Y.; Gu, L.; Davey, K.; Qiao, S.-Z. An Electrolytic Zn–MnO2 Battery for High-Voltage and Scalable Energy Storage. Angew. Chem. Int. Ed. 2019, 58, 7823–7828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tang, Y.; Guo, S.; Cao, X.; Pan, A.; Fang, G.; Zhou, J.; Liang, S. Fundamentals and perspectives in developing zinc-ion battery electrolytes: A comprehensive review. Energy Environ. Sci. 2020, 13, 4625–4665. [Google Scholar] [CrossRef]
- Lu, J.; Xiong, T.; Zhou, W.; Yang, L.; Tang, Z.; Chen, S. Metal Nickel Foam as an Efficient and Stable Electrode for Hydrogen Evolution Reaction in Acidic Electrolyte under Reasonable Overpotentials. ACS Appl. Mater. Interfaces 2016, 8, 5065–5069. [Google Scholar] [CrossRef] [PubMed]
- Barton, G.W.; Scott, A.C. Industrial applications of a mathematical model for the zinc electrowinning process. J. Appl. Electrochem. 1994, 24, 377–383. [Google Scholar] [CrossRef]
- Selvakumaran, D.; Pan, A.; Liang, S.; Cao, G. A review on recent developments and challenges of cathode materials for rechargeable aqueous Zn-ion batteries. J. Mater. Chem. A 2019, 7, 18209–18236. [Google Scholar] [CrossRef]
- Ibrahim, M.A.M. Improving the throwing power of acidic zinc sulfate electroplating baths. J. Chem. Technol. Biotechnol. 2000, 75, 745–755. [Google Scholar] [CrossRef]
- Kühne, H.M.; Schefold, J. Tafel Plots from Illuminated Photoelectrodes: A New Insight Into Charge Transfer Mechanism. J. Electrochem. Soc. 1990, 137, 568–575. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised pourbaix diagrams for zinc at 25–300 °C. Corros. Sci. 1997, 39, 107–114. [Google Scholar] [CrossRef]
- Dong, H.; Lei, T.; He, Y.; Xu, N.; Huang, B.; Liu, C.T. Electrochemical performance of porous Ni3Al electrodes for hydrogen evolution reaction. Int. J. Hydrog. Energy 2011, 36, 12112–12120. [Google Scholar] [CrossRef]
- Miles, M.H.; Kissel, G.; Lu, P.W.T.; Srinivasan, S. Effect of Temperature on Electrode Kinetic Parameters for Hydrogen and Oxygen Evolution Reactions on Nickel Electrodes in Alkaline Solutions. J. Electrochem. Soc. 1976, 123, 332–336. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Hua, Y.X.; Dong, T.G.; Zhou, D.G. Effects of temperature and current density on zinc electrodeposition from acidic sulfate electrolyte with [BMIM]HSO4 as additive. J. Appl. Electrochem. 2009, 39, 1207. [Google Scholar] [CrossRef]
- Pan, D.; Ma, L.; Xie, Y.; Jen, T.C.; Yuan, C. On the physical and chemical details of alumina atomic layer deposition: A combined experimental and numerical approach. J. Vac. Sci. Technol. A 2015, 33, 021511. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.-B.-R.; Maeng, W.J. Applications of atomic layer deposition to nanofabrication and emerging nanodevices. Thin Solid Film. 2009, 517, 2563–2580. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, C.; Yu, Y.; Yan, M.; Wei, Q.; He, P.; Dong, Y.; Zhang, Z.; Wang, X.; Mai, L. Ultrathin Surface Coating Enables Stabilized Zinc Metal Anode. Adv. Mater. Interfaces 2018, 5, 1800848. [Google Scholar] [CrossRef]
- He, H.; Tong, H.; Song, X.; Song, X.; Liu, J. Highly stable Zn metal anodes enabled by atomic layer deposited Al2O3 coating for aqueous zinc-ion batteries. J. Mater. Chem. A 2020, 8, 7836–7846. [Google Scholar] [CrossRef]
- Xie, X.; Liang, S.; Gao, J.; Guo, S.; Guo, J.; Wang, C.; Xu, G.; Wu, X.; Chen, G.; Zhou, J. Manipulating the ion-transfer kinetics and interface stability for high-performance zinc metal anodes. Energy Environ. Sci. 2020, 13, 503–510. [Google Scholar] [CrossRef]
- Xia, A.; Pu, X.; Tao, Y.; Liu, H.; Wang, Y. Graphene oxide spontaneous reduction and self-assembly on the zinc metal surface enabling a dendrite-free anode for long-life zinc rechargeable aqueous batteries. Appl. Surf. Sci. 2019, 481, 852–859. [Google Scholar] [CrossRef]
- Cui, M.; Xiao, Y.; Kang, L.; Du, W.; Gao, Y.; Sun, X.; Zhou, Y.; Li, X.; Li, H.; Jiang, F.; et al. Quasi-Isolated Au Particles as Heterogeneous Seeds To Guide Uniform Zn Deposition for Aqueous Zinc-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 6490–6496. [Google Scholar] [CrossRef]
- Liang, P.; Yi, J.; Liu, X.; Wu, K.; Wang, Z.; Cui, J.; Liu, Y.; Wang, Y.; Xia, Y.; Zhang, J. Highly Reversible Zn Anode Enabled by Controllable Formation of Nucleation Sites for Zn-Based Batteries. Adv. Funct. Mater. 2020, 30, 1908528. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Qin, R.; Liu, X.; Fang, P.; Zheng, D.; Tong, Y.; Lu, X. Dendrite-Free Zinc Deposition Induced by Multifunctional CNT Frameworks for Stable Flexible Zn-Ion Batteries. Adv. Mater. 2019, 31, 1903675. [Google Scholar] [CrossRef]
- Hieu, L.T.; So, S.; Kim, I.T.; Hur, J. Zn anode with flexible β-PVDF coating for aqueous Zn-ion batteries with long cycle life. Chem. Eng. J. 2021, 411, 128584. [Google Scholar] [CrossRef]
- Liu, M.; Yang, L.; Liu, H.; Amine, A.; Zhao, Q.; Song, Y.; Yang, J.; Wang, K.; Pan, F. Artificial Solid-Electrolyte Interface Facilitating Dendrite-Free Zinc Metal Anodes via Nanowetting Effect. ACS Appl. Mater. Interfaces 2019, 11, 32046–32051. [Google Scholar] [CrossRef]
- Wu, K.; Yi, J.; Liu, X.; Sun, Y.; Cui, J.; Xie, Y.; Liu, Y.; Xia, Y.; Zhang, J. Regulating Zn Deposition via an Artificial Solid–Electrolyte Interface with Aligned Dipoles for Long Life Zn Anode. Nano-Micro Lett. 2021, 13, 79. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.; Huang, X.; Zhu, L.; Tang, Y.; Ji, X.; Wang, H. Simultaneously Regulating the Ion Distribution and Electric Field to Achieve Dendrite-Free Zn Anode. Small 2020, 16, 2000929. [Google Scholar] [CrossRef]
- He, M.; Shu, C.; Zheng, R.; Xiang, W.; Hu, A.; Yan, Y.; Ran, Z.; Li, M.; Wen, X.; Zeng, T.; et al. Manipulating the ion-transference and deposition kinetics by regulating the surface chemistry of zinc metal anodes for rechargeable zinc-air batteries. Green Energy Environ. 2021. [Google Scholar] [CrossRef]


















| Components | Representative Material |
|---|---|
| Anode material | Zn foil (80.0 mm in diameter, 0.25 mm in thickness) |
| Cathode material | α-MnO2, β-MnO2, V-based materials, Prussian blue analogues |
| Cathode current collector | Stainless steel spring (15.4 mm in diameter and 1.1 mm in thickness) |
| Separator | Whatman glass fiber filter |
| Electrolyte | 2 M ZnSO4 with 0.1 M MnSO4 |
| Anode | Mechanism | Corrosion Potential (V) (vs. Ag/AgCl) | Symmetrical Cell Performance:Lifespan (h), Capacity, and Current Density | Full-cell Performance:Capacity (mAh g−1)/Cycle/Capacity Retention (%)/Current Density (mA g−1) | Ref |
|---|---|---|---|---|---|
| TiO2@Zn | Shielding Zn surface | −0.89 | 150 h 1 mA cm−2, 1 mAhcm−2 | 134 mAh g−1/1000 cycles/85%/1 A g−1 | [116] |
| Al2O3@Zn | Shielding Zn surface | −0.88 | 500 h 1 mA cm−2, 1 mAh cm−2 | 158 mAh g−1/1000 cycles/89%/1 A g−1 | [117] |
| Cu-Zn/Zn | Shielding Zn surface | −0.96 | 1500 h 1 mA cm−2, 0.5 mAh cm−2 | - | [101] |
| rGO@Zn | Shielding Zn surface | - | 300 h 1 mA cm−2, 0.5 mAh cm−2 | 61 mAh g−1/5000 cycles/86%/1 A g−1 | [119] |
| NA-Zn | Controlling nucleation sites | - | 2000 h 0.25 mA cm−2, 0.05 mAh cm−2 | 67 mAh g−1/2000 cycles/-/0.5 A g−1 | [120] |
| ZrO2@ Zn | Controlling nucleation sites | - | 2100 h 5 mA cm−2, 1 mAh cm−2 | 52 mAh g−1/3000 cycles/42%/1 A g−1 | [121] |
| CaCO3@Zn | Controlling nucleation sites | - | 900 h 0.25 mA cm−2, 0.05 mAh cm−2 | 177 mAh g−1/1000 cycles/86%/1 A g−1 | [92] |
| CNT@Zn | Controlling nucleation sites | - | 200 h 2 mA cm−2, 2 mAh cm−2 | 167 mAh g−1/1000 cycles/89%/- | [122] |
| PA@Zn | Redistributing Zn2+ ion flux | −0.96 | 8000 h 0.5 mA cm−2, 0.25 mAh cm−2 | 154 mAh g−1/1000 cycles/88%/0.6 A g−1 | [94] |
| PVDF@Zn | Redistributing Zn2+ ion flux | - | 2000 h 0.25 mA cm−2, 0.05 mAh cm−2 | 57 mAh g−1/2000 cycles/-/1 A g−1 | [123] |
| MOF-PVDF@Zn | Redistributing Zn2+ ion flux | - | 500 h 1 mA cm−2, 0.5 mAh cm−2 | - | [124] |
| BTO@Zn | Creating uniform electric field | - | 1500 h 5 mA cm−2, 0.5 mAh cm−2 | 74 mAh g−1/300 cycles/67%/2 A g−1 | [125] |
| CM@CuO@Zn | Creating uniform electric field | - | 350 h 1 mA cm−2, 1 mAh cm−2 | - | [126] |
| NC@Zn | Creating uniform electric field | - | 300 h 1 mA cm−2, 0.5 mAh cm−2 | - | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang Huy, V.P.; Hieu, L.T.; Hur, J. Zn Metal Anodes for Zn-Ion Batteries in Mild Aqueous Electrolytes: Challenges and Strategies. Nanomaterials 2021, 11, 2746. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11102746
Hoang Huy VP, Hieu LT, Hur J. Zn Metal Anodes for Zn-Ion Batteries in Mild Aqueous Electrolytes: Challenges and Strategies. Nanomaterials. 2021; 11(10):2746. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11102746
Chicago/Turabian StyleHoang Huy, Vo Pham, Luong Trung Hieu, and Jaehyun Hur. 2021. "Zn Metal Anodes for Zn-Ion Batteries in Mild Aqueous Electrolytes: Challenges and Strategies" Nanomaterials 11, no. 10: 2746. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11102746