Electrochemical and Fluorescent Properties of Crown Ether Functionalized Graphene Quantum Dots for Potassium and Sodium Ions Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of GQDs-15-Crown-5 and GQDs-18-Crown-6
2.3. Microstructural, Optical and Electrochemical Characterizations
3. Results and Discussion
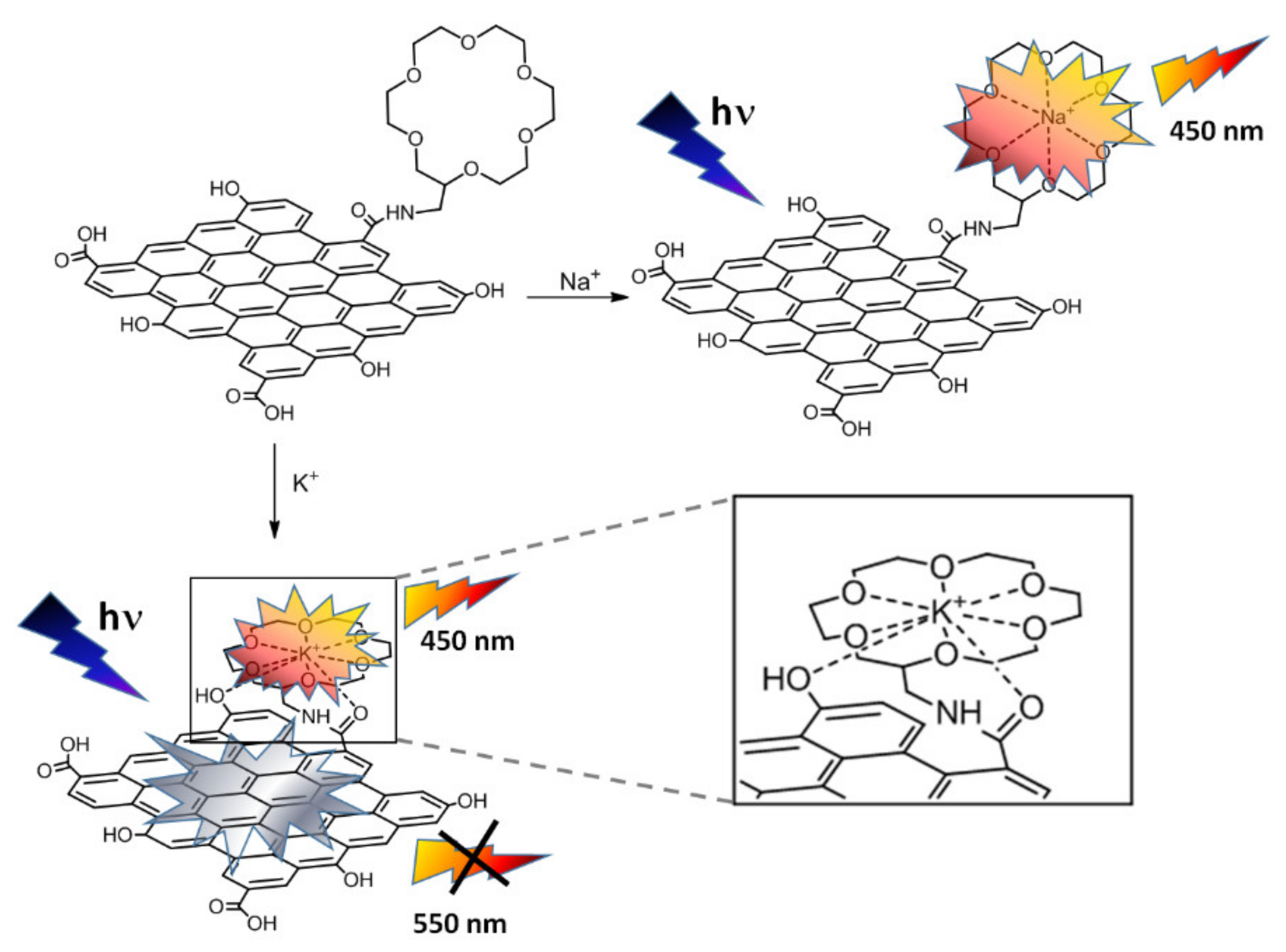
3.1. Synthesis and Characterization of GQDs-15-Crown-5 and GQDs-18-Crown-6
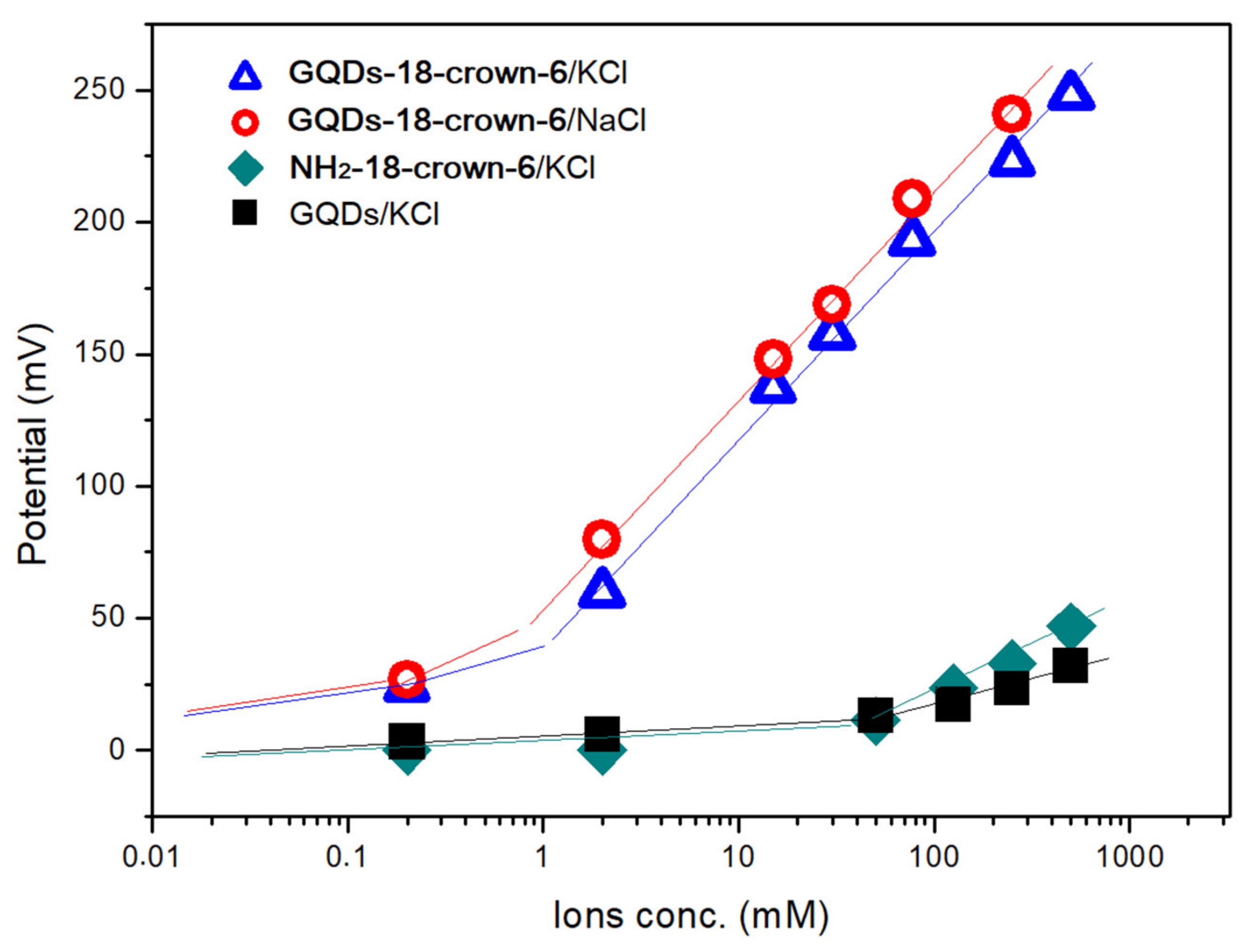
3.2. Electrochemical Studies
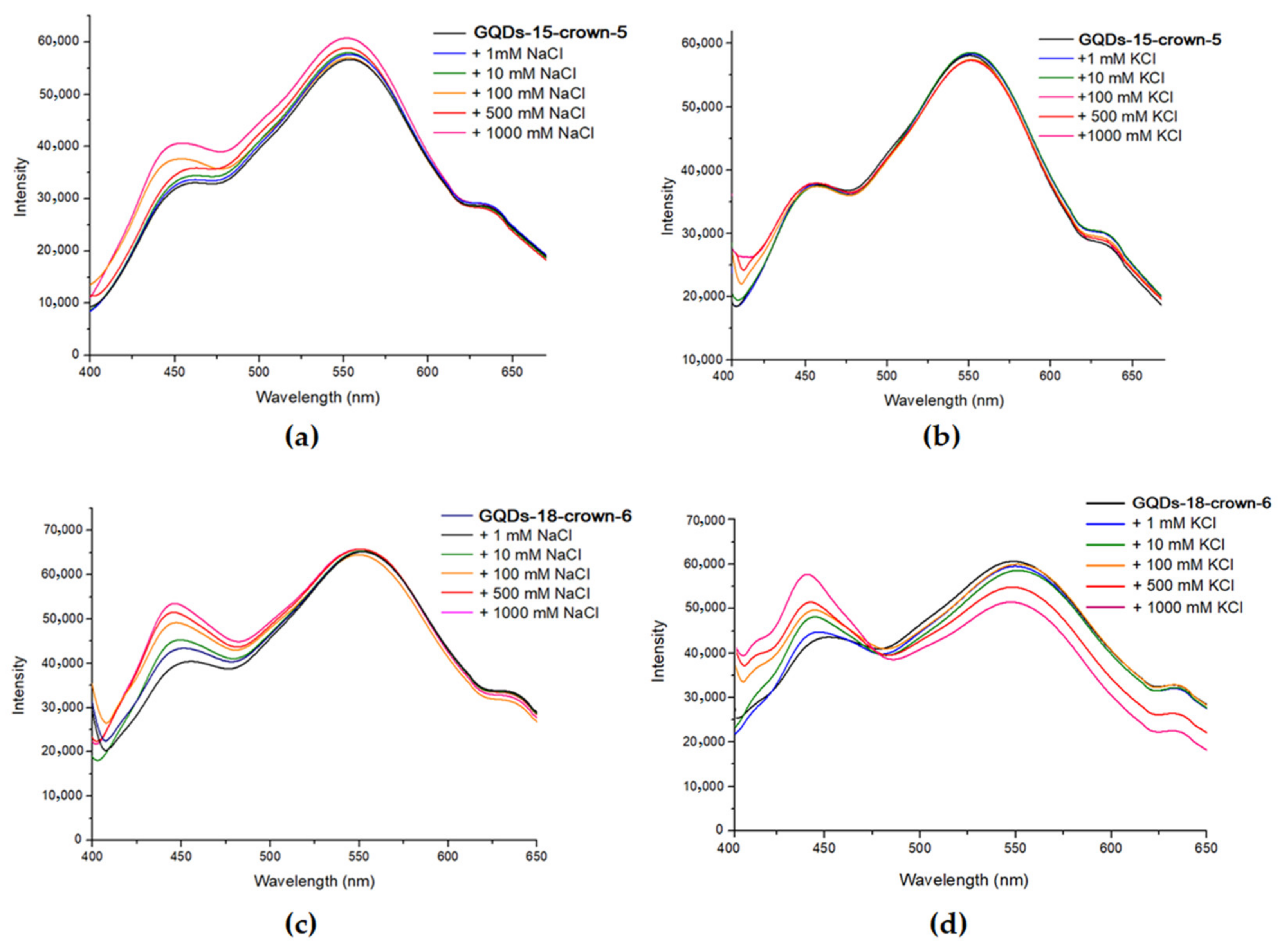
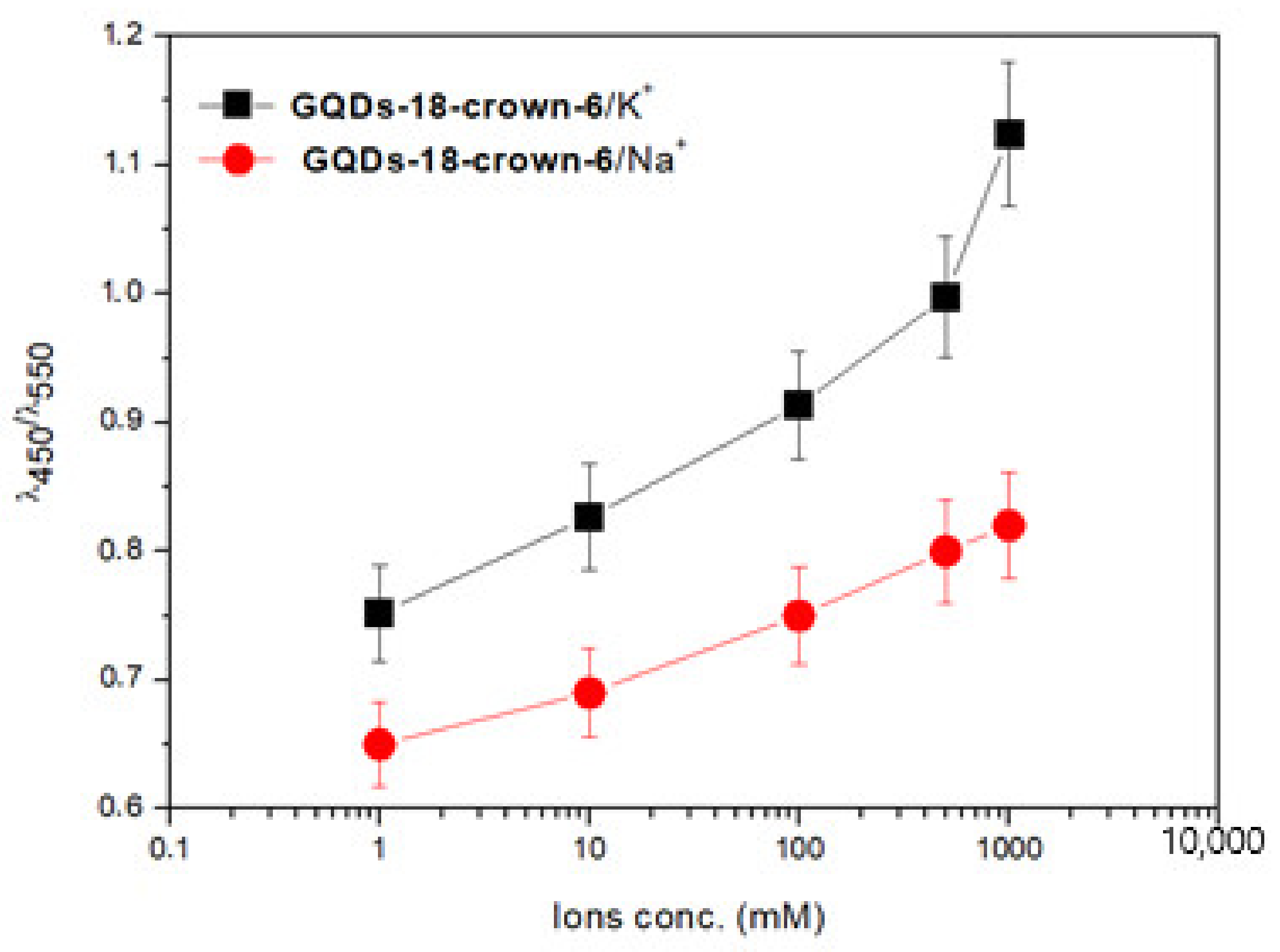
3.3. Photoluminescence Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pohl, H.R.; Wheeler, J.S.; Murray, H.E. Sodium and Potassium in Health and Disease. In Interrelations between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R., Eds.; Springer: Dordrecht, The Netherlands, 2013; Volume 13, pp. 29–47. [Google Scholar]
- Lim, H.R.; Kim, Y.-S.; Kwon, S.; Mahmood, M.; Kwon, Y.T.; Lee, Y.; Lee, S.M.; Yeo, W.-H. Wireless, Flexible, Ion-Selective Electrode System for Selective and Repeatable Detection of Sodium. Sensors 2020, 20, 3297. [Google Scholar] [CrossRef] [PubMed]
- Ghafar-Zadeh, E. Wireless Integrated Biosensors for Point-of- Care Diagnostic Applications. Sensors 2015, 15, 3236–3261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, J.P.; Butler, J.; Rossignol, P.; Pitt, B.; Anker, S.D.; Kosiborod, M.; Lund, L.H.; Bakris, G.L.; Weir, M.R.; Zannad, F. Abnormalities of Potassium in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2836–2850. [Google Scholar] [CrossRef] [PubMed]
- Scurati-Manzoni, E.; Fossali, E.F.; Agostoni, C.; Riva, E.; Simonetti, G.D.; Zanolari-Calderari, M.; Bianchetti, M.G.; Lava, S.A.G. Electrolyte abnormalities in cystic fibrosis: Systematic review of the literature. Pediatr. Nephrol. 2014, 29, 1015–1023. [Google Scholar] [CrossRef] [Green Version]
- Piccoli, G.B.; Capobianco, M.; Odetto, L.; Deagostini, M.C.; Consiglio, V.; Radeschi, G. Acute renal failure, severe sodium and potassium imbalance and sudden tetraplegia. NDT Plus 2010, 3, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Usman, A. Initial Potassium Replacement in Diabetic Ketoacidosis: The Unnoticed Area of Gap. Front. Endocrinol. 2018, 9, 109. [Google Scholar] [CrossRef]
- Fraser, R. Disorders of the adrenal cortex: Their effects on electrolyte metabolism. Clin. Endocrinol. Metab. 1984, 13, 413–430. [Google Scholar] [CrossRef]
- Garcia, R.A.; Vanelli, C.P.; dos Santos Pereira, O.; do Amaral Corrêa, J.O. Comparative analysis for strength serum sodium and potassium in three different methods: Flame photometry, ion-selective electrode (ISE) and colorimetric enzymatic. J. Clin. Lab. Anal. 2018, 32, 22594. [Google Scholar] [CrossRef]
- Poulsen, H.; Nissen, P. The Inner Workings of a Dynamic Duo. Science 2012, 335, 416–417. [Google Scholar] [CrossRef]
- Jiang, N.; Yetisena, A.K.; Linhart, N.; Flisikowski, K.; Dong, J.; Dong, X.; Butt, H.; Jakobi, M.; Schnieke, A.; Koch, A.W. Fluorescent dermal tattoo biosensors for electrolyte analysis. Sens. Actuators B Chem. 2020, 320, 128378. [Google Scholar] [CrossRef]
- Pirovano, P.; Dorrian, M.; Shinde, A.; Donohoe, A.; Brady, A.J.; Moyna, N.M.; Wallace, G.; Diamond, D.; McCaula, M. A wearable sensor for the detection of sodium and potassium in human sweat during exercise. Talanta 2020, 219, 121145. [Google Scholar] [CrossRef]
- Banerjee, P.; Prasad, B. Determination of concentration of total sodium and potassium in surface and ground water using a flame photometer. Appl. Water Sci. 2020, 10, 113. [Google Scholar] [CrossRef]
- Stekolshchikova, A.A.; Radaev, A.V.; Orlova, O.Y.; Nikolaev, K.G.; Skorb, E.V. Thin and Flexible Ion Sensors Based on Polyelectrolyte Multilayers Assembled onto the Carbon Adhesive Tape. ACS Omega 2019, 4, 15421–15427. [Google Scholar] [CrossRef] [Green Version]
- Kabaa, E.A.; Abdulateef, S.A.; Ahmed, N.M.; Hassan, Z.; Sabah, F.A.A. novel porous silicon multi-ions selective electrode based extended gate field effect transistor for sodium, potassium, calcium, and magnesium sensor. Appl. Phys. A 2019, 125, 753. [Google Scholar] [CrossRef]
- Jarolımova, Z.; Vishe, M.; Lacour, J.; Bakker, E. Potassium ion-selective fluorescent and pH independent nanosensors based on functionalized polyether macrocycles. Chem. Sci. 2016, 7, 525. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Xu, H.; Ji, H.-F. From New fluorescent probes for the detection of mixed sodium and potassium metal ions. Chem. Comm. 2001, 20, 2092–2093. [Google Scholar] [CrossRef]
- Chard, A.N.; Trinies, V.; Edmonds, C.J.; Sogore, A.; Freeman, M.C. The impact of water consumption on hydration and cognition among schoolchildren: Methods and results from a crossover trial in rural Mali. PLoS ONE 2019, 14, 0210568. [Google Scholar] [CrossRef] [Green Version]
- Takami, T.; Son, J.W.; Lee, J.K.; Park, B.H.; Kawai, T. Separate detection of sodium and potassium ions with sub-micropipette probe. Jpn. J. Appl. Phys. 2011, 50, 08LB13. [Google Scholar] [CrossRef]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 2495–2496. [Google Scholar] [CrossRef]
- Zhou, D.; Hao, H.; Ma, Y.; Zhong, H.; Dai, Y.; Cai, K.; Mukherjee, S.; Liu, J.; Bian, H. Specific Host–Guest Interactions in the Crown Ether Complexes with K+ and NH4+ Revealed from the Vibrational Relaxation Dynamics of the Counteranion. J. Phys. Chem. B 2020, 124, 9154–9162. [Google Scholar] [CrossRef]
- Zhou, X.; Su, F.; Tian, Y.; Youngbull, C.; Johnson, R.H.; Meldrum, D.R. A New Highly Selective Fluorescent K+ Sensor. J. Am. Chem. Soc. 2011, 133, 18530–18533. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Xu, C.; Ren, J.; Xu, B.; Qu, X. Sensing metal ions with ion selectivity of a crown ether and fluorescence resonance energy transfer between carbon dots and graphene. Chem. Commun. 2012, 48, 1284–1286. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, S.L.; Xu, H.L.; Zhao, L.; Su, Z.M. N-Methylbenzoaza-18-crown-6-ether derivatives as efficient alkali metal cations sensors: The dipole moment and first hyperpolarizability. RSC Adv. 2014, 4, 24433–24438. [Google Scholar] [CrossRef]
- Olsen, G.; Ulstrup, J.; Chi, Q. Crown-Ether Derived Graphene Hybrid Composite for Membrane- Free Potentiometric Sensing of Alkali Metal Ions. ACS Appl. Mater. Interfaces 2016, 8, 37–41. [Google Scholar] [CrossRef]
- Zhang, H.X.; Huang, Z.X.; Zhao, P.Y.; Hou, Y.; Guo, J.J.; Wu, Y.C. Crown ether functionalized graphene oxide as ultrasensitive electrochemical sensor for detection of potassium ions. Mater. Res. Express 2019, 6, 125095. [Google Scholar] [CrossRef]
- Du, D.; Yuqi, Y.; Lin, Y. Graphene-based Materials for Biosensing and Bioimaging. MRS Bull. 2012, 37, 1290–1296. [Google Scholar] [CrossRef]
- Wu, L.; Wang, J.; Yin, M.; Ren, J.; Miyoshi, D.; Sugimoto, N.; Qu, X. Reduced Graphene Oxide Upconversion Nanoparticle Hybrid for Electrochemiluminescent Sensing of a Prognostic Indicator in Early- Stage Cancer. Small 2014, 10, 330–336. [Google Scholar] [CrossRef]
- Vasu, K.S.; Sridevi, S.; Sampath, S.; Sood, A.K. Non-Enzymatic Electronic Detection of Glucose Using Aminophenylboronic Acid Functionalized Reduced Graphene Oxide. Sens. Actuators B 2015, 221, 1209–1214. [Google Scholar] [CrossRef]
- Guo, J.; Lee, J.; Contescu, C.I.; Gallego, N.C.; Pantelides, S.T.; Pennycook, S.K.; Moyer, B.A.; Chisholm, M.F. Crown ethers in graphene. Nat. Commun. 2014, 5, 5389. [Google Scholar] [CrossRef] [Green Version]
- Iannazzo, D.; Ziccarelli, I.; Pistone, A. Graphene quantum dots: Multifunctional nanoplatforms for anticancer therapy. J. Mater. Chem. B 2017, 5, 6471–6489. [Google Scholar] [CrossRef]
- Iannazzo, D.; Celesti, C.; Espro, C. Recent Advances on Graphene Quantum Dots as Multifunctional Nanoplatforms for Cancer Treatment. Biotechnol. J. 2021, 16, 1900422. [Google Scholar] [CrossRef] [PubMed]
- Mansuriya, B.D.; Altintas, Z. Applications of Graphene Quantum Dots in Biomedical Sensors. Sensors 2020, 20, 1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, P.; Ganguly, S.; Banerjee, S.; Das, N.C. Graphene based emergent nanolights: A short review on the synthesis, properties and application. Res. Chem. Intermed. 2019, 45, 3823–3853. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Mondal, S.; Ghorai, U.K.; Maity, P.P.; Choudhary, S.; Gangopadhyay, S.; Dhara, S.; Banerjee, S.; Das, N.C. Dual doped biocompatible multicolor luminescent carbon dots for bio labeling, UV-active marker and fluorescent polymer composite. Luminescence 2018, 33, 1136–1145. [Google Scholar] [CrossRef]
- Huang, H.; Liao, L.; Xu, X.; Zou, M.; Liu, F.; Li, N. The electron-transfer based interaction between transition metal ions and photoluminescent graphene quantum dots (GQDs): A platform for metal ion sensing. Talanta 2013, 117, 152–157. [Google Scholar] [CrossRef]
- Giofrè, S.V.; Celesti, C.; Patanè, S.; Triolo, C.; Gulino, A.; Spitaleri, L.; Scalese, S.; Scuderi, M.; Iannazzo, D. Eco-Friendly 1,3-Dipolar Cycloaddition Reactions on Graphene Quantum Dots in Natural Deep Eutectic Solvent. Nanomaterials 2020, 10, 2549. [Google Scholar] [CrossRef]
- Rajender, G.; Giri, P.K. Formation mechanism of graphene quantum dots and their edge state conversion probed by photoluminescence and Raman spectroscopy. J. Mater. Chem. C 2016, 4, 10852–10865. [Google Scholar] [CrossRef]
- Suzuki, K.; Sato, K.; Hisamoto, H.; Siswanta, D.; Hayaski, K.; Kasahara, N.; Watanabe, K.; Yamamoto, N.; Sasakura, H. Design and synthesis of sodium ion-selective ionophores based on 16-crown-5 derivatives for an ion-selective electrode. Anal. Chem. 1996, 68, 208–215. [Google Scholar] [CrossRef]
- Buck, R.P.; Lindner, E. Recommendations for nomenclature of ion selective electrodes (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Miyake, M.; Chen, L.D.; Pozzi, G.; Bühlmann, P. Ion-Selective Electrodes with Unusual Response Functions: Simultaneous Formation of Ionophore–Primary Ion Complexes with Different Stoichiometries. Anal. Chem. 2012, 84, 1104–1111. [Google Scholar] [CrossRef] [Green Version]
- Sarfo, D.K.; Sivanesan, A.; Izake, E.L.; Ayoko, G.A. Rapid detection of mercury contamination in water by surface enhanced Raman spectroscopy. RSC Adv. 2017, 7, 21567–21575. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.L.; Huang, C.C.; Mai, F.D.; Yen, C.L.; Tzing, S.H.; Hsieh, H.T.; Ling, Y.C.; Chang, J.Y. Application of paramagnetic graphene quantum dots as a platform for simultaneous dual-modality bioimaging and tumor-targeted drug delivery. J. Mater. Chem. B 2015, 3, 651–664. [Google Scholar] [CrossRef]
- Hausmann, D.; Kuzmanoski, A.; Feldmann, C. MnBr2/18-crown-6 coordination complexes showing high room temperature luminescence and quantum yield. Dalton Trans. 2016, 45, 6541–6547. [Google Scholar] [CrossRef]
- Merzlyakova, E.; Wolf, S.; Lebedkin, S.; Bayarjargal, L.; Neumeier, L.; Bartenbach, D.; Holzer, C.; Klopper, W.; Winkler, B.; Kappes, M.; et al. 18-Crown-6 Coordinated Metal Halides with Bright Luminescence and Nonlinear Optical Effects. Am. Chem. Soc. 2021, 143, 798–804. [Google Scholar] [CrossRef]
- Li, P.; Li, S.F.Y. Recent advances in fluorescence probes based on carbon dots for sensing and speciation of heavy metals. Nanophotonics 2020, 10, 877–908. [Google Scholar] [CrossRef]
- Lu, S.; Wu, D.; Li, G.; Lv, Z.; Chen, Z.; Chen, L.; Chen, G.; Xia, L.; You, J.; Wu, Y. Carbon dots-based ratiometric nanosensor for highly sensitive and selective detection of mercury(ii) ions and glutathione. RSC Adv. 2016, 6, 103169–103177. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Celesti, C.; Triolo, C.; Patanè, S.; Giofrè, S.; Romeo, R.; Ziccarelli, I.; Mancuso, R.; Gabriele, B.; et al. A Smart Nanovector for Cancer Targeted Drug Delivery Based on Graphene Quantum Dots. Nanomaterials 2019, 9, 282. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Li, Y.; Wang, L.; Su, X. A label-free fluorescence nanosensor for the determination of adrenaline based on graphene quantum dots. Anal. Methods 2017, 9, 4434–4438. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iannazzo, D.; Espro, C.; Ferlazzo, A.; Celesti, C.; Branca, C.; Neri, G. Electrochemical and Fluorescent Properties of Crown Ether Functionalized Graphene Quantum Dots for Potassium and Sodium Ions Detection. Nanomaterials 2021, 11, 2897. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11112897
Iannazzo D, Espro C, Ferlazzo A, Celesti C, Branca C, Neri G. Electrochemical and Fluorescent Properties of Crown Ether Functionalized Graphene Quantum Dots for Potassium and Sodium Ions Detection. Nanomaterials. 2021; 11(11):2897. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11112897
Chicago/Turabian StyleIannazzo, Daniela, Claudia Espro, Angelo Ferlazzo, Consuelo Celesti, Caterina Branca, and Giovanni Neri. 2021. "Electrochemical and Fluorescent Properties of Crown Ether Functionalized Graphene Quantum Dots for Potassium and Sodium Ions Detection" Nanomaterials 11, no. 11: 2897. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11112897






