Nanocellulose-Based Materials for Water Treatment: Adsorption, Photocatalytic Degradation, Disinfection, Antifouling, and Nanofiltration
Abstract
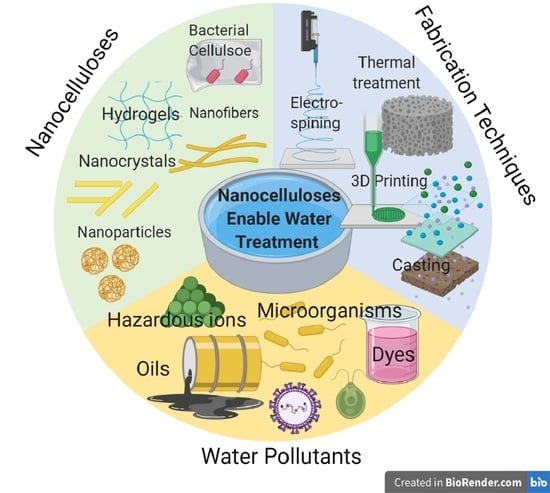
:1. Introduction
2. Production, Morphologies, and Unique Properties of Nanocelluloses
- (1)
- High dispersion stability of nanocelluloses in aqueous solutions can be achieved through sulfuric acid hydrolysis introducing negatively charged sulfate groups. However, this high dispersion stability of individual nanocellulose particles makes their separation from the water system difficult and necessitates the addition of salt or pH alteration to recover them after the water treatment process.
- (2)
- Dispersion of nanocellulose in hydrophobic polymer matrices (membranes) remains a critical issue. However, the dispersion of nanocelluloses in polymer blends for sustainable wastewater treatment applications can be achieved by surface grafting of nanocelluloses with low molecular weight polymers. Solution-casting is the most important method for preparing nanocellulose-polymer composite membranes, which, however, remains difficult for large-scale application.
- (3)
- Production of nanocellulose from plant sources is generally based on multi-step, top-down techniques that include physical (e.g., refining, mechanical grinding, ultrasonic grinding, thermal treatment), chemical (e.g., acid hydrolysis, alkali treatment, and chemical modification), biological (e.g., enzymatic hydrolysis and production of cellulose nanofibers from bacteria), and hybrid methods [43,44]. High water and energy consumption and yield are the main challenges in the preparation process, along with by-product toxicity [4]. For example, acid wastewater is typically generated from the washing process for neutralizing the pH value of the nanocellulose suspension [45].
| Nanocellulose Features | Effect on Adsorption | Reference |
|---|---|---|
| High surface area | Increases the specific surface area from micro- to nano-size, thus enhancing the nanocellulose adsorption capacity. The mixed aerogel (ratio of 1:3 CNC/CNF) can provide a higher specific surface area than pure CNC or CNF. | [43,45] |
| High aspect ratio | Aspect ratio of CNC (10–80) is generally smaller than that of CNF (up to 80–500), depending on nanocellulose sources and the treatment process. Favors the set-up of percolated CNCs and entangled CNF networks held by strong hydrogen bonding, thus enhancing the adsorbent mechanical strength | [45] |
| High mechanical stiffness | CNC and CNF high mechanical stiffness (modulus), ~130 and ~70 GPa, respectively, increases the adsorbent material stiffness and cohesion. High crystalline forms (CNCs and CNFs) are transparent, and gas-impermeable with a very high tensile strength up to 8 times that of steel. | [6,46] |
| High crystalline degree | Nanocellulose high crystallinity degree (60–80%) enhances the adsorbent chemical resistance and reduces cellulose solubility even in high polar solvents | [47] |
| Susceptible to surface functionalization | Hydroxyl group functionalization (oxidation, esterification, etherification, radical grafting, and silylation) increases the nanocellulose adsorption capacity | [48,49] |
| Stability in water | Reduces biofouling of nanocellulose-based adsorbents. The surface of cellulose-based water treatment materials is negatively charged due to the high concentration of hydroxyl and carboxylate groups, resulting in higher electrostatic repulsive forces between the surface layer and most model foulant. | [50] |
| High surface tension | High surface tension (nanocellulose surface energy is ~60 mJ m−2) of nanocellulose-based adsorbents by water improve the wetting characteristics and reduce the bio-fouling | [51] |
3. Adsorbents for Hazardous Metal Removal
4. Adsorbents for Hazardous Organic Pollutants Removal
5. Wettable Materials for Oil/Water Separation
6. Flocculants and Coagulants for Suspended Materials
| No. | Nanocellulsoe Flocculants | Contaminants | Optimum Flocculation Conditions | Highest Removal Efficiency | Ref. No. |
|---|---|---|---|---|---|
| 1 | Pristine CNCs | Pseudomonas aeruginosa (Gram-negative Bacteria) | Flocculant concentration (CNC:Bacteria) = 100,000:1, reaction time = 24 h | 100% | [179] |
| 2 | Carboxylated CNCs | Kaolin clay (suspended filler particles) | Flocculant concentration = 40 mg L−1, pH = 4–10, reaction time = 30 min | 95.4% | [128] |
| 3 | Pyridinium grafted CNCs | Chlorella vulgaris (Microalgae) | Flocculant concentration = 0.1 g flocculant per g microalgae, pH = 4–11, reaction time = 30 min | 95% | [180] |
| 4 | Amine-functionalized CNCs | Sodium dodecyl sulfate (anionic surfactant) | Optimum pH = 4, | - | [181] |
| 5 | Carboxylated CNFs | Suspended particles | Flocculant concentration = 2.5–5.0 mg.dm−3, pH = 6–8, reaction time = 30 min | 40–80% | [174] |
| 6 | Sulfonated CNFs | Suspended particles | Flocculant concentration = 2.5 mg dm−3, reaction time = 30 min | - | [182] |
| 7 | Quaternized CNFs | Reactive orange 16 (reactive dye) | Flocculant concentration = 150 mg.L−1, reaction time = 12 h | qmax = 0.477 mmol.g−1 for the reactive dye | [183] |
7. Photocatalytic Materials for Hazardous Pollutants Degradation
8. Membrane Materials for Wastewater Treatment
- (1)
- Microfiltration membranes with microspores of 10 µm to 100 nm; to be used for removal of suspended particles and Bacteria (200 nm–30 micron)
- (2)
- Ultrafiltration membranes with nanopore range from 100 nm to 2 nm; it is suitable for removal of nanoparticles and viruses with sizes 50–200 nm
- (3)
- Nanofiltration membranes with nanopore size range from 2 nm to 1 nm; to be used for removal of organic pollutants (0.7–1.5 nm)
- (4)
- Reverse osmosis with sub-nanopore size from 0.1 to 1 nm; to be used for hazardous metal ion (0.1–0.7) removal.
9. Water Disinfection Materials from Pathogenic Microorganisms
10. Current Challenges and Limitations
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ranade, V.V.; Bhandari, V.M. Industrial Wastewater Treatment, Recycling, and Reuse: An Overview. In Industrial Wastewater Treatment, Recycling and Reuse; Butterworth-Heinemann: Oxford, UK, 2014; pp. 1–80. ISBN 9780444634030. [Google Scholar]
- Wiesmann, U.; Choi, I.S.; Dombrowski, E.M. Fundamentals of Biological Wastewater Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 9783527312191. [Google Scholar]
- Ahankari, S.; George, T.; Subhedar, A.; Kar, K.K. Nanocellulose as a sustainable material for water purification. SPE Polym. 2020, 1, 69–80. [Google Scholar] [CrossRef]
- Barhoum, A.; Jeevanandam, J.; Rastogi, A.; Samyn, P.; Boluk, Y.; Dufresne, A.; Danquah, M.K.; Bechelany, M. Plant celluloses, hemicelluloses, lignins, and volatile oils for the synthesis of nanoparticles and nanostructured materials. Nanoscale 2020, 12, 22845–22890. [Google Scholar] [CrossRef]
- Barhoum, A.; Li, H.; Chen, M.; Cheng, L.; Yang, W.; Dufresne, A. Emerging Applications of Cellulose Nanofibers. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2019; pp. 1131–1156. [Google Scholar]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.; Hassan, M.; Abou-Zeid, R.; Berglund, L.; Oksman, K. Use of Bacterial Cellulose and Crosslinked Cellulose Nanofibers Membranes for Removal of Oil from Oil-in-Water Emulsions. Polymers 2017, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Nnaji, C.O.; Jeevanandam, J.; Chan, Y.S.; Danquah, M.K.; Pan, S.; Barhoum, A. Engineered nanomaterials for wastewater treatment: Current and future trends. In Fundamentals of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 129–168. [Google Scholar]
- Hassan, M.L.; Fadel, S.M.; Abouzeid, R.E.; Elseoud, W.S.A.; Hassan, E.A.; Berglund, L.; Oksman, K. Water purification ultrafiltration membranes using nanofibers from unbleached and bleached rice straw. Sci. Rep. 2020, 10, 11278. [Google Scholar] [CrossRef] [PubMed]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Mautner, A. Nanocellulose water treatment membranes and filters: A review. Polym. Int. 2020, 69, 741–751. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.; Abou-Zeid, R.; Hassan, E.; Berglund, L.; Aitomäki, Y.; Oksman, K. Membranes Based on Cellulose Nanofibers and Activated Carbon for Removal of Escherichia coli Bacteria from Water. Polymers 2017, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.; Hassan, E.; Fadel, S.M.; Abou-Zeid, R.E.; Berglund, L.; Oksman, K. Metallo-Terpyridine-Modified Cellulose Nanofiber Membranes for Papermaking Wastewater Purification. J. Inorg. Organomet. Polym. Mater. 2018, 28, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, N.; Grishkewich, N.; Tam, K.C. Cellulose nanomaterials: Promising sustainable nanomaterials for application in water/wastewater treatment processes. Environ. Sci. Nano 2018, 5, 623–658. [Google Scholar] [CrossRef]
- Shak, K.P.Y.; Pang, Y.L.; Mah, S.K. Nanocellulose: Recent advances and its prospects in environmental remediation. Beilstein J. Nanotechnol. 2018, 9, 2479–2498. [Google Scholar] [CrossRef]
- Zhang, K.; Barhoum, A.; Xiaoqing, C.; Li, H.; Samyn, P. Cellulose Nanofibers: Fabrication and Surface Functionalization Techniques. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2019; pp. 409–449. [Google Scholar]
- Li, R.; Zhang, L.; Wang, P. Rational design of nanomaterials for water treatment. Nanoscale 2015, 7, 17167–17194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Gendy, A.; Abou-Zeid, R.E.; Salama, A.; Diab, M.; El-Sakhawy, M. TEMPO-oxidized cellulose nanofibers/polylactic acid/TiO2 as antibacterial bionanocomposite for active packaging. Egypt. J. Chem. 2017, 60, 1007–1014. [Google Scholar] [CrossRef] [Green Version]
- Abouzeid, R.E.; Khiari, R.; Salama, A.; Diab, M.; Beneventi, D.; Dufresne, A. In situ mineralization of nano-hydroxyapatite on bifunctional cellulose nanofiber/polyvinyl alcohol/sodium alginate hydrogel using 3D printing. Int. J. Biol. Macromol. 2020, 160, 538–547. [Google Scholar] [CrossRef]
- Hassan, M.; Berglund, L.; Hassan, E.; Abou-Zeid, R.; Oksman, K. Effect of xylanase pretreatment of rice straw unbleached soda and neutral sulfite pulps on isolation of nanofibers and their properties. Cellulose 2018, 25, 2939–2953. [Google Scholar] [CrossRef]
- Ho, T.T.T.; Zimmermann, T.; Hauert, R.; Caseri, W. Preparation and characterization of cationic nanofibrillated cellulose from etherification and high-shear disintegration processes. Cellulose 2011, 18, 1391–1406. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; He, Y.; Fan, D.; Han, Y.; Li, G.; Wang, S. An Efficient Method for Cellulose Nanofibrils Length Shearing via Environmentally Friendly Mixed Cellulase Pretreatment. J. Nanomater. 2017, 2017, 1591504. [Google Scholar] [CrossRef]
- Zhang, J.; Elder, T.J.; Pu, Y.; Ragauskas, A.J. Facile synthesis of spherical cellulose nanoparticles. Carbohydr. Polym. 2007, 69, 607–611. [Google Scholar] [CrossRef]
- Dai, J.; Chae, M.; Beyene, D.; Danumah, C.; Tosto, F.; Bressler, D.C. Co-Production of Cellulose Nanocrystals and Fermentable Sugars Assisted by Endoglucanase Treatment of Wood Pulp. Materials 2018, 11, 1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, F.; Troncoso, O.; López, D.; Grande, C.; Gómez, C.M. Reversible stress softening and stress recovery of cellulose networks. Soft Matter 2009, 5, 4185–4190. [Google Scholar] [CrossRef]
- Mittal, N.; Ansari, F.; Gowda Krishne, V.; Brouzet, C.; Chen, P.; Larsson, P.T.; Roth, S.V.; Lundell, F.; Wågberg, L.; Kotov, N.A.; et al. Multiscale Control of Nanocellulose Assembly: Transferring Remarkable Nanoscale Fibril Mechanics to Macroscale Fibers. ACS Nano 2018, 12, 6378–6388. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, K.; Sundberg, J.; Gatenholm, P.; Renneckar, S. Electrospun nanofibrous cellulose scaffolds with controlled microarchitecture. Carbohydr. Polym. 2014, 100, 143–149. [Google Scholar] [CrossRef]
- Rege, A.; Preibisch, I.; Schestakow, M.; Ganesan, K.; Gurikov, P.; Milow, B.; Smirnova, I.; Itskov, M. Correlating Synthesis Parameters to Morphological Entities: Predictive Modeling of Biopolymer Aerogels. Materials 2018, 11, 1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siró, I.; Plackett, D.; Hedenqvist, M.; Ankerfors, M.; Lindström, T. Highly transparent films from carboxymethylated microfibrillated cellulose: The effect of multiple homogenization steps on key properties. J. Appl. Polym. Sci. 2011, 119, 2652–2660. [Google Scholar] [CrossRef]
- Lindström, T. Aspects on nanofibrillated cellulose (NFC) processing, rheology and NFC-film properties. Curr. Opin. Colloid Interface Sci. 2017, 29, 68–75. [Google Scholar] [CrossRef]
- Lin, C.; Ma, Q.; Su, Q.; Bian, H.; Zhu, J.Y. Facile Synthesis of Highly Hydrophobic Cellulose Nanoparticles through Post-Esterification Microfluidization. Fibers 2018, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Kalidhasan, S.; Rajesh, V.; Rajesh, N. Application of Cellulose-Clay Composite Biosorbent toward the Effective Adsorption and Removal of Chromium from Industrial Wastewater. Ind. Eng. Chem. Res. 2012, 51, 58–69. [Google Scholar] [CrossRef]
- Sakthivel, T.; Reid, D.L.; Goldstein, I.; Hench, L.; Seal, S. Hydrophobic High Surface Area Zeolites Derived from Fly Ash for Oil Spill Remediation. Environ. Sci. Technol. 2013, 47, 5843–5850. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.; Ye, J.; Dai, W.; Yan, X.; Hu, J.; Hu, X.; Li, S.; Huang, H. Adsorptive Removal of Methyl Orange and Methylene Blue from Aqueous Solution with Finger-Citron-Residue-Based Activated Carbon. Ind. Eng. Chem. Res. 2013, 52, 14297–14303. [Google Scholar] [CrossRef]
- Monier, M.; Abdel-Latif, D.A. Synthesis and characterization of ion-imprinted resin based on carboxymethyl cellulose for selective removal of UO22+. Carbohydr. Polym. 2013, 97, 743–752. [Google Scholar] [CrossRef]
- Salama, A.; Hesemann, P. Synthesis of N-Guanidinium-Chitosan/Silica Hybrid Composites: Efficient Adsorbents for Anionic Pollutants. J. Polym. Environ. 2018, 26, 1986–1997. [Google Scholar] [CrossRef]
- Salama, A.; Hesemann, P. New N-guanidinium chitosan/silica ionic microhybrids as efficient adsorbent for dye removal from waste water. Int. J. Biol. Macromol. 2018, 111, 762–768. [Google Scholar] [CrossRef]
- Hassan, H.; Salama, A.; El-Ziaty, A.K.; El-Sakhawy, M. New chitosan/silica/zinc oxide nanocomposite as adsorbent for dye removal. Int. J. Biol. Macromol. 2019, 131, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Fabryanty, R.; Valencia, C.; Soetaredjo, F.E.; Putro, J.; Santoso, S.P.; Kurniawan, A.; Ju, Y.-H.; Ismadji, S. Removal of crystal violet dye by adsorption using bentonite–alginate composite. J. Environ. Chem. Eng. 2017, 5, 5677–5687. [Google Scholar] [CrossRef] [Green Version]
- Naseer, A.; Jamshaid, A.; Hamid, A.; Muhammad, N.; Ghauri, M.; Iqbal, J.; Rafiq, S.; Khuram, S.; Shah, N.S. Lignin and Lignin Based Materials for the Removal of Heavy Metals from Waste Water-An Overview. Z. Phys. Chem. 2019, 233, 315–345. [Google Scholar] [CrossRef]
- Barhoum, A.; Samyn, P.; Öhlund, T.; Dufresne, A. Review of recent research on flexible multifunctional nanopapers. Nanoscale 2017, 9, 15181–15205. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, R.E.; Khiari, R.; Beneventi, D.; Dufresne, A. Biomimetic Mineralization of Three-Dimensional Printed Alginate/TEMPO-Oxidized Cellulose Nanofibril Scaffolds for Bone Tissue Engineering. Biomacromolecules 2018, 19, 4442–4452. [Google Scholar] [CrossRef]
- Xu, Q.; Poggi, G.; Resta, C.; Baglioni, M. Grafted nanocellulose and alkaline nanoparticles for the strengthening and deacidification of cellulosic artworks. J. Colloid Interface Sci. 2020, 576, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Rusli, R.; Eichhorn, S. Determination of the stiffness of cellulose nanowhiskers and the fiber-matrix interface in a nanocomposite using Raman spectroscopy. Appl. Phys. Lett. 2008, 93, 033111. [Google Scholar] [CrossRef]
- Tanpichai, S.; Quero, F.; Nogi, M.; Yano, H.; Young, R.J.; Lindström, T.; Sampson, W.W.; Eichhorn, S.J. Effective Young’s Modulus of Bacterial and Microfibrillated Cellulose Fibrils in Fibrous Networks. Biomacromolecules 2012, 13, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of Water Treatment Membranes: A Review of the Underlying Causes, Monitoring Techniques and Control Measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelmouleh, M.; Boufi, S.; Belgacem, M.; Duarte, A.P.; Ben Salah, A.; Gandini, A. Modification of cellulosic fibres with functionalised silanes: Development of surface properties. Int. J. Adhes. Adhes. 2004, 24, 43–54. [Google Scholar] [CrossRef]
- Tan, K.X.; Barhoum, A.; Pan, S.; Danquah, M.K. Risks and toxicity of nanoparticles and nanostructured materials. In Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–139. ISBN 9780128135167. [Google Scholar]
- Wołowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepa, G.; Bajda, T. Removal of Heavy Metals and Metalloids from Water Using Drinking Water Treatment Residuals as Adsorbents: A Review. Minerals 2019, 9, 487. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, M.E. Nanoadsorbents for water and wastewater remediation. Sci. Total Environ. 2020, 739, 139903. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, R.E.; Khiari, R.; El-Wakil, N.; Dufresne, A. Current State and New Trends in the Use of Cellulose Nanomaterials for Wastewater Treatment. Biomacromolecules 2019, 20, 573–597. [Google Scholar] [CrossRef]
- Ma, H.; Hsiao, B.S.; Chu, B. Ultrafine Cellulose Nanofibers as Efficient Adsorbents for Removal of UO22+ in Water. ACS Macro Lett. 2012, 1, 213–216. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Ukkola, J.; Liimatainen, H. Direct sulfation of cellulose fibers using a reactive deep eutectic solvent to produce highly charged cellulose nanofibers. Cellulose 2019, 26, 2303–2316. [Google Scholar] [CrossRef] [Green Version]
- Sehaqui, H.; De Larraya, U.P.; Liu, P.; Pfenninger, N.; Mathew, A.P.; Zimmermann, T.; Tingaut, P. Enhancing adsorption of heavy metal ions onto biobased nanofibers from waste pulp residues for application in wastewater treatment. Cellulose 2014, 21, 2831–2844. [Google Scholar] [CrossRef]
- Suopajärvi, T.; Liimatainen, H.; Karjalainen, M.; Upola, H.; Niinimäki, J. Lead adsorption with sulfonated wheat pulp nanocelluloses. J. Water Process. Eng. 2015, 5, 136–142. [Google Scholar] [CrossRef]
- Liu, P.; Sehaqui, H.; Tingaut, P.; Wichser, A.; Oksman, K.; Mathew, A.P. Cellulose and chitin nanomaterials for capturing silver ions (Ag+) from water via surface adsorption. Cellulose 2014, 21, 449–461. [Google Scholar] [CrossRef]
- Liu, P.; Borrell, P.F.; Božič, M.; Kokol, V.; Oksman, K.; Mathew, A.P. Nanocelluloses and their phosphorylated derivatives for selective adsorption of Ag+, Cu2+ and Fe3+ from industrial effluents. J. Hazard. Mater. 2015, 294, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tong, S.; Ge, M.; Wu, L.; Zuo, J.; Cao, C.; Song, W. Adsorption of heavy metal ions from aqueous solution by carboxylated cellulose nanocrystals. J. Environ. Sci. 2013, 25, 933–943. [Google Scholar] [CrossRef]
- Kardam, A.; Raj, K.R.; Srivastava, S.; Srivastava, M.M. Nanocellulose fibers for biosorption of cadmium, nickel, and lead ions from aqueous solution. Clean Technol. Environ. Policy 2014, 16, 385–393. [Google Scholar] [CrossRef]
- Hamid, H.A.; Jenidi, Y.; Thielemans, W.; Somerfield, C.; Gomes, R.L. Predicting the capability of carboxylated cellulose nanowhiskers for the remediation of copper from water using response surface methodology (RSM) and artificial neural network (ANN) models. Ind. Crop. Prod. 2016, 93, 108–120. [Google Scholar] [CrossRef]
- Singh, K.; Arora, J.K.; Sinha, T.J.M.; Srivastava, S. Functionalization of nanocrystalline cellulose for decontamination of Cr(III) and Cr(VI) from aqueous system: Computational modeling approach. Clean Technol. Environ. Policy 2014, 16, 1179–1191. [Google Scholar] [CrossRef]
- Singh, K.; Sinha, T.; Srivastava, S. Functionalized nanocrystalline cellulose: Smart biosorbent for decontamination of arsenic. Int. J. Miner. Process. 2015, 139, 51–63. [Google Scholar] [CrossRef]
- Anirudhan, T.; Deepa, J.; Christa, J. Nanocellulose/nanobentonite composite anchored with multi-carboxyl functional groups as an adsorbent for the effective removal of Cobalt(II) from nuclear industry wastewater samples. J. Colloid Interface Sci. 2016, 467, 307–320. [Google Scholar] [CrossRef]
- Pillai, S.S.; Deepa, B.; Abraham, E.; Girija, N.; Geetha, P.; Jacob, L.; Koshy, M. Biosorption of Cd(II) from aqueous solution using xanthated nano banana cellulose: Equilibrium and kinetic studies. Ecotoxicol. Environ. Saf. 2013, 98, 352–360. [Google Scholar] [CrossRef]
- Sheikhi, A.; Safari, S.; Yang, H.; van de Ven, T.G.M. Copper Removal Using Electrosterically Stabilized Nanocrystalline Cellulose. ACS Appl. Mater. Interfaces 2015, 7, 11301–11308. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.; Shainy, F. Effective removal of mercury(II) ions from chlor-alkali industrial wastewater using 2-mercaptobenzamide modified itaconic acid-grafted-magnetite nanocellulose composite. J. Colloid Interface Sci. 2015, 456, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.R.; Chattopadhyay, A.; Zhan, C.; Sharma, S.K.; Geng, L.; Hsiao, B.S. Lead removal from water using carboxycellulose nanofibers prepared by nitro-oxidation method. Cellulose 2018, 25, 1961–1973. [Google Scholar] [CrossRef]
- Zhang, N.; Zang, G.-L.; Shi, C.; Yu, H.-Q.; Sheng, G.-P. A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu(II) removal. J. Hazard. Mater. 2016, 316, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, J.; Cheng, L.; Lu, C.; Wang, Y.; He, X.; Zhang, W. Acrylic acid grafted and acrylic acid/sodium humate grafted bamboo cellulose nanofibers for Cu2+ adsorption. RSC Adv. 2014, 4, 55195–55201. [Google Scholar] [CrossRef]
- Srivastava, S.; Kardam, A.; Raj, K.R. Nanotech Reinforcement onto Cellulosic Fibers: Green Remediation of Toxic Metals. Int. J. Green Nanotechnol. Biomed. 2012, 4, 46–53. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, S.; Wan, T.; Jia, Y.; Yang, H.; Li, J.; Yan, L.; Zhong, C. Biosynthesis of spherical Fe3O4/bacterial cellulose nanocomposites as adsorbents for heavy metal ions. Carbohydr. Polym. 2011, 86, 1558–1564. [Google Scholar] [CrossRef]
- Hokkanen, S.; Repo, E.; Sillanpää, M. Removal of heavy metals from aqueous solutions by succinic anhydride modified mercerized nanocellulose. Chem. Eng. J. 2013, 223, 40–47. [Google Scholar] [CrossRef]
- Hokkanen, S.; Repo, E.; Suopajärvi, T.; Liimatainen, H.; Niinimaa, J.; Sillanpää, M. Adsorption of Ni(II), Cu(II) and Cd(II) from aqueous solutions by amino modified nanostructured microfibrillated cellulose. Cellulose 2014, 21, 1471–1487. [Google Scholar] [CrossRef]
- Geng, B.; Wang, H.; Wu, S.; Ru, J.; Tong, C.; Chen, Y.; Liu, H.; Wu, S.; Liu, X. Surface-Tailored Nanocellulose Aerogels with Thiol-Functional Moieties for Highly Efficient and Selective Removal of Hg(II) Ions from Water. ACS Sustain. Chem. Eng. 2017, 5, 11715–11726. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, P.; Mathew, A.P. Self-Assembled TEMPO Cellulose Nanofibers: Graphene Oxide-Based Biohybrids for Water Purification. ACS Appl. Mater. Interfaces 2017, 9, 21048–21058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mautner, A.; Lee, K.-Y.; Lahtinen, P.; Hakalahti, M.; Tammelin, T.; Li, K.; Bismarck, A. Nanopapers for organic solvent nanofiltration. Chem. Commun. 2014, 50, 5778–5781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maatar, W.; Boufi, S. Poly(methacylic acid-co-maleic acid) grafted nanofibrillated cellulose as a reusable novel heavy metal ions adsorbent. Carbohydr. Polym. 2015, 126, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Sirviö, J.A.; Hasa, T.; Leiviskä, T.; Liimatainen, H.; Hormi, O. Bisphosphonate nanocellulose in the removal of vanadium(V) from water. Cellulose 2016, 23, 689–697. [Google Scholar] [CrossRef]
- Abou-Zeid, R.E.; Dacrory, S.; Ali, K.A.; Kamel, S. Novel method of preparation of tricarboxylic cellulose nanofiber for efficient removal of heavy metal ions from aqueous solution. Int. J. Biol. Macromol. 2018, 119, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zou, Y.; Yan, Z.; Shen, W.; Shi, S.; Zhang, X.; Wang, H. Carboxymethylated-bacterial cellulose for copper and lead ion removal. J. Hazard. Mater. 2009, 161, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Polyethyleneimine-bacterial cellulose bioadsorbent for effective removal of copper and lead ions from aqueous solution. Bioresour. Technol. 2017, 244, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Kondo, K.; Ohto, K.; Inoue, K.; Baba, Y. Preparation of phosphorylated bacterial cellulose as an adsorbent for metal ions. React. Funct. Polym. 2008, 68, 376–383. [Google Scholar] [CrossRef]
- Shen, W.; Chen, S.; Shi, S.; Li, X.; Zhang, X.; Hu, W.; Wang, H. Adsorption of Cu(II) and Pb(II) onto diethylenetriamine-bacterial cellulose. Carbohydr. Polym. 2009, 75, 110–114. [Google Scholar] [CrossRef]
- Stephen, M.; Catherine, N.; Brenda, M.; Andrew, K.; Leslie, P.; Corrine, G. Oxolane-2,5-dione modified electrospun cellulose nanofibers for heavy metals adsorption. J. Hazard. Mater. 2011, 192, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Júnior, O.K.; Gurgel, L.V.A.; de Freitas, R.P.; Gil, L.F. Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by mercerized cellulose and mercerized sugarcane bagasse chemically modified with EDTA dianhydride (EDTAD). Carbohydr. Polym. 2009, 77, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Salama, A.; Abou-Zeid, R.E. Ionic chitosan/silica nanocomposite as efficient adsorbent for organic dyes. Int. J. Biol. Macromol. 2021, 188, 404–410. [Google Scholar] [CrossRef]
- Zhang, X.; Elsayed, I.; Navarathna, C.; Schueneman, G.T.; Hassan, E.B. Biohybrid Hydrogel and Aerogel from Self-Assembled Nanocellulose and Nanochitin as a High-Efficiency Adsorbent for Water Purification. ACS Appl. Mater. Interfaces 2019, 11, 46714–46725. [Google Scholar] [CrossRef]
- Pandey, S.; Do, J.Y.; Kim, J.; Kang, M. Fast and highly efficient removal of dye from aqueous solution using natural locust bean gum based hydrogels as adsorbent. Int. J. Biol. Macromol. 2020, 143, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.R.; Sumi, K.; Matsumura, M. Improved metal affinity of chelating adsorbents through graft polymerization. Water Res. 1999, 33, 2037–2044. [Google Scholar] [CrossRef]
- Hong, H.-J.; Lim, J.S.; Hwang, J.Y.; Kim, M.; Jeong, H.S.; Park, M.S. Carboxymethlyated cellulose nanofibrils(CMCNFs) embedded in polyurethane foam as a modular adsorbent of heavy metal ions. Carbohydr. Polym. 2018, 195, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Salama, A. Cellulose/silk fibroin assisted calcium phosphate growth: Novel biocomposite for dye adsorption. Int. J. Biol. Macromol. 2020, 165, 1970–1977. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, R.E.; Salama, A.; Al-Ahmed, Z.A.; Awwad, N.S.; Youssef, M.A. Carboxylated cellulose nanofibers as a novel efficient adsorbent for water purification. Cellul. Chem. Technol. 2020, 54, 237–245. [Google Scholar] [CrossRef]
- Manatunga, D.C.; de Silva, R.M.; de Silva, K.M.N.; Ratnaweera, R. Natural polysaccharides leading to super adsorbent hydroxyapatite nanoparticles for the removal of heavy metals and dyes from aqueous solutions. RSC Adv. 2016, 6, 105618–105630. [Google Scholar] [CrossRef]
- Cai, J.; Lei, M.; Zhang, Q.; He, J.-R.; Chen, T.; Liu, S.; Fu, S.-H.; Li, T.-T.; Liu, G.; Fei, P. Electrospun composite nanofiber mats of Cellulose@Organically modified montmorillonite for heavy metal ion removal: Design, characterization, evaluation of absorption performance. Compos. Part A: Appl. Sci. Manuf. 2017, 92, 10–16. [Google Scholar] [CrossRef]
- Yao, M.; Wang, Z.; Liu, Y.; Yang, G.; Chen, J. Preparation of dialdehyde cellulose graftead graphene oxide composite and its adsorption behavior for heavy metals from aqueous solution. Carbohydr. Polym. 2019, 212, 345–351. [Google Scholar] [CrossRef]
- Hao, Y.; Cui, Y.; Peng, J.; Zhao, N.; Li, S.; Zhai, M. Preparation of graphene oxide/cellulose composites in ionic liquid for Ce (III) removal. Carbohydr. Polym. 2019, 208, 269–275. [Google Scholar] [CrossRef]
- Salama, A.; Hesemann, P. Recent Trends in Elaboration, Processing, and Derivatization of Cellulosic Materials Using Ionic Liquids. ACS Sustain. Chem. Eng. 2020, 8, 17893–17907. [Google Scholar] [CrossRef]
- Ambashta, R.D.; Sillanpää, M. Water purification using magnetic assistance: A review. J. Hazard. Mater. 2010, 180, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Nata, I.F.; Sureshkumar, M.; Lee, C.-K. One-pot preparation of amine-rich magnetite/bacterial cellulose nanocomposite and its application for arsenate removal. RSC Adv. 2011, 1, 625–631. [Google Scholar] [CrossRef]
- Stoica-Guzun, A.; Stroescu, M.; Jinga, S.I.; Mihalache, N.; Botez, A.; Matei, C.; Berger, D.; Damian, C.M.; Ionita, V. Box-Behnken experimental design for chromium(VI) ions removal by bacterial cellulose-magnetite composites. Int. J. Biol. Macromol. 2016, 91, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Salama, A. Preparation of CMC-g-P(SPMA) super adsorbent hydrogels: Exploring their capacity for MB removal from waste water. Int. J. Biol. Macromol. 2018, 106, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; Favre, T.; Sayegh, S.; Tanos, F.; Coy, E.; Iatsunskyi, I.; Razzouk, A.; Cretin, M.; Bechelany, M. 3D Self-Supported Nitrogen-Doped Carbon Nanofiber Electrodes Incorporated Co/CoOx Nanoparticles: Application to Dyes Degradation by Electro-Fenton-Based Process. Nanomaterials 2021, 11, 2686. [Google Scholar] [CrossRef]
- Alila, S.; Boufi, S. Removal of organic pollutants from water by modified cellulose fibres. Ind. Crop. Prod. 2009, 30, 93–104. [Google Scholar] [CrossRef]
- Mautner, A.; Kobkeatthawin, T.; Bismarck, A. Efficient continuous removal of nitrates from water with cationic cellulose nanopaper membranes. Resour. Technol. 2017, 3, 22–28. [Google Scholar] [CrossRef]
- Bauli, C.R.; Lima, G.F.; de Souza, A.G.; Ferreira, R.R.; Rosa, D.S. Eco-friendly carboxymethyl cellulose hydrogels filled with nanocellulose or nanoclays for agriculture applications as soil conditioning and nutrient carrier and their impact on cucumber growing. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126771. [Google Scholar] [CrossRef]
- Elfeky, A.S.; Salem, S.S.; Elzaref, A.; Owda, M.E.; Eladawy, H.A.; Saeed, A.; Awad, M.A.; Abou-Zeid, R.E.; Fouda, A. Multifunctional cellulose nanocrystal /metal oxide hybrid, photo-degradation, antibacterial and larvicidal activities. Carbohydr. Polym. 2020, 230, 115711. [Google Scholar] [CrossRef] [PubMed]
- Assem, Y.; Ali, K.; Abu-Zeid, R.; Kame, S. Synthesis of Acrylate-modified Cellulose via RAFT Polymerization and its Application as Efficient Metal Ions Adsorbent. Egypt. J. Chem. 2019, 62, 85–96. [Google Scholar] [CrossRef]
- Salama, A.; Shukry, N.; El-Sakhawy, M. Carboxymethyl cellulose-g-poly(2-(dimethylamino) ethyl methacrylate) hydrogel as adsorbent for dye removal. Int. J. Biol. Macromol. 2015, 73, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Shoueir, K.R.; Aljohani, H.A. Preparation of sustainable nanocomposite as new adsorbent for dyes removal. Fibers Polym. 2017, 18, 1825–1830. [Google Scholar] [CrossRef]
- Salama, A.; Aljohani, H.A.; Shoueir, K.R. Oxidized cellulose reinforced silica gel: New hybrid for dye adsorption. Mater. Lett. 2018, 230, 293–296. [Google Scholar] [CrossRef]
- Abou-Zeid, R.E.; Awwad, N.S.; Nabil, S.; Salama, A.; Youssef, M.A. Oxidized alginate/gelatin decorated silver nanoparticles as new nanocomposite for dye adsorption. Int. J. Biol. Macromol. 2019, 141, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.J.; Gnansounou, E.; Pandey, A. Comprehensive review on toxicity of persistent organic pollutants from petroleum refinery waste and their degradation by microorganisms. Chemosphere 2017, 188, 280–291. [Google Scholar] [CrossRef]
- Owda, M.E.; Elfeky, A.S.; Abouzeid, R.E.; Saleh, A.K.; Awad, M.A.; Abdellatif, H.A.; Ahmed, F.M.; Elzaref, A.S. Enhancement of photocatalytic and biological activities of chitosan/activated carbon incorporated with TiO2 nanoparticles. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Zhou, Y.; Yu, F.; Wang, E.; Min, Y.; Huang, Q.; Pang, L.; Ma, T. Effective removal of cationic dyes using carboxylate-functionalized cellulose nanocrystals. Chemosphere 2015, 141, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Salama, A. New sustainable hybrid material as adsorbent for dye removal from aqueous solutions. J. Colloid Interface Sci. 2017, 487, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Etri, S.; Mohamed, S.A.; El-Sakhawy, M. Carboxymethyl cellulose prepared from mesquite tree: New source for promising nanocomposite materials. Carbohydr. Polym. 2018, 189, 138–144. [Google Scholar] [CrossRef]
- Salama, A. Functionalized hybrid materials assisted organic dyes removal from aqueous solutions. Environ. Nanotechnol. Monit. Manag. 2016, 6, 159–163. [Google Scholar] [CrossRef]
- Mousa, H.M.; Fahmy, H.S.; Abouzeid, R.; Abdel-Jaber, G.; Ali, W. Polyvinylidene fluoride-cellulose nanocrystals hybrid nanofiber membrane for energy harvesting and oil-water separation applications. Mater. Lett. 2022, 306, 130965. [Google Scholar] [CrossRef]
- Abou-Zeid, R.E.; Ali, K.A.; Gawad, R.M.A.; Kamal, K.H.; Kamel, S.; Khiari, R. Removal of Cu(II), Pb(II), Mg(II), and Fe(II) by Adsorption onto Alginate/Nanocellulose Beads as Bio-Sorbent. J. Renew. Mater. 2021, 9, 601–613. [Google Scholar] [CrossRef]
- Shahnaz, T.; Priyan, V.V.; Pandian, S.; Narayanasamy, S. Use of Nanocellulose extracted from grass for adsorption abatement of Ciprofloxacin and Diclofenac removal with phyto, and fish toxicity studies. Environ. Pollut. 2021, 268, 115494. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, X.; Wang, L.; Pei, Y.; An, M.; Liu, J.; Zheng, X.; Tang, K. Highly efficient and selective removal of anionic dyes from water using a cellulose nanofibril/chitosan sponge prepared by dehydrothermal treatment. J. Environ. Chem. Eng. 2021, 9, 105745. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, C.; DiChiara, A.B.; Jiang, W.; Gu, J. Cellulose Nanofibril/Carbon Nanomaterial Hybrid Aerogels for Adsorption Removal of Cationic and Anionic Organic Dyes. Nanomaterials 2020, 10, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batmaz, R.; Mohammed, N.; Zaman, M.; Minhas, G.; Berry, R.M.; Tam, K.C. Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose 2014, 21, 1655–1665. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, D.-Z.; Lu, F.-F.; Yao, J. New Approach for Single-Step Extraction of Carboxylated Cellulose Nanocrystals for Their Use as Adsorbents and Flocculants. ACS Sustain. Chem. Eng. 2016, 4, 2632–2643. [Google Scholar] [CrossRef]
- He, X.; Male, K.B.; Nesterenko, P.N.; Brabazon, D.; Paull, B.; Luong, J.H. Adsorption and Desorption of Methylene Blue on Porous Carbon Monoliths and Nanocrystalline Cellulose. ACS Appl. Mater. Interfaces 2013, 5, 8796–8804. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Sun, Q.; Xu, Q.; Xu, Y. Adsorptive removal of anionic dyes from aqueous solutions using microgel based on nanocellulose and polyvinylamine. Bioresour. Technol. 2015, 197, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A.P. Nanocellulose-based materials for water purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Nanofibrous Microfiltration Membrane Based on Cellulose Nanowhiskers. Biomacromolecules 2012, 13, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Xu, H.; Xu, L.; Xie, K.; Yang, Y. Cellulose nanocrystal-reinforced keratin bioadsorbent for effective removal of dyes from aqueous solution. Bioresour. Technol. 2017, 232, 254–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, N.; Grishkewich, N.; Berry, R.M.; Tam, K.C. Cellulose nanocrystal–alginate hydrogel beads as novel adsorbents for organic dyes in aqueous solutions. Cellulose 2015, 22, 3725–3738. [Google Scholar] [CrossRef]
- Zhou, C.; Lee, S.; Dooley, K.; Wu, Q. A facile approach to fabricate porous nanocomposite gels based on partially hydrolyzed polyacrylamide and cellulose nanocrystals for adsorbing methylene blue at low concentrations. J. Hazard. Mater. 2013, 263, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Melo, B.C.; Paulino, F.A.; Cardoso, V.A.; Pereira, A.G.; Fajardo, A.R.; Rodrigues, F.H. Cellulose nanowhiskers improve the methylene blue adsorption capacity of chitosan-g-poly(acrylic acid) hydrogel. Carbohydr. Polym. 2018, 181, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Eyley, S.; Thielemans, W. Imidazolium grafted cellulose nanocrystals for ion exchange applications. Chem. Commun. 2011, 47, 4177–4179. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Chia, C.H.; Zakaria, S.; Sajab, M.S.; Chin, S.X. Cellulose nanofibrils: A rapid adsorbent for the removal of methylene blue. RSC Adv. 2015, 5, 18204–18212. [Google Scholar] [CrossRef]
- Pei, A.; Butchosa, N.; Berglund, L.A.; Zhou, Q. Surface quaternized cellulose nanofibrils with high water absorbency and adsorption capacity for anionic dyes. Soft Matter 2013, 9, 2047–2055. [Google Scholar] [CrossRef]
- Salama, A. Soy protein acid hydrolysate/silica hybrid material as novel adsorbent for methylene blue. Compos. Commun. 2019, 12, 101–105. [Google Scholar] [CrossRef]
- Salama, A. Cellulose/calcium phosphate hybrids: New materials for biomedical and environmental applications. Int. J. Biol. Macromol. 2019, 127, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, G.; Yang, Y.; Yu, Y. A review on in-vitro oral bioaccessibility of organic pollutants and its application in human exposure assessment. Sci. Total Environ. 2021, 752, 142001. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ma, C.; Tao, J.; Li, J.; Du, K.; Wei, Z.; Chen, C.; Wang, Z.; Zhao, C.; Ma, M. Optimization of polyvinylamine-modified nanocellulose for chlorpyrifos adsorption by central composite design. Carbohydr. Polym. 2020, 245, 116542. [Google Scholar] [CrossRef]
- Khalaf, B.; Hamed, O.; Jodeh, S.; Hanbali, G.; Bol, R.; Dagdag, O.; Samhan, S. Novel, Environment-Friendly Cellulose-Based Derivatives for Tetraconazole Removal from Aqueous Solution. Polymers 2021, 13, 450. [Google Scholar] [CrossRef]
- Ma, C.; Yi, L.; Yang, J.; Tao, J.; Li, J. Nanocellulose–organic montmorillonite nanocomposite adsorbent for diuron removal from aqueous solution: Optimization using response surface methodology. RSC Adv. 2020, 10, 30734–30745. [Google Scholar] [CrossRef]
- Nikou, M.; Samadi-Maybodi, A.; Yasrebi, K.; Sedighi-Pashaki, E. Simultaneous monitoring of the adsorption process of two organophosphorus pesticides by employing GO/ZIF-8 composite as an adsorbent. Environ. Technol. Innov. 2021, 23, 101590. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, W.; Liu, F.; Chen, R. Removal of difenoconazole and nitenpyram by composite calcium alginate beads during apple juice clarification. Chemosphere 2021, 286, 131813. [Google Scholar] [CrossRef] [PubMed]
- Meneguin, A.; Pacheco, G.; Silva, J.M.; de Araujo, F.P.; Silva-Filho, E.C.; Bertolino, L.C.; Barud, H.D.S. Nanocellulose/palygorskite biocomposite membranes for controlled release of metronidazole. Int. J. Biol. Macromol. 2021, 188, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, S.; Hosseinzadeh, H.; Pashaei, S. Fabrication of nanocellulose loaded poly(AA-co-HEMA) hydrogels for ceftriaxone controlled delivery and crystal violet adsorption. Polym. Compos. 2019, 40, E559–E569. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, Y.; Li, M.; Liu, K.; Hu, C.; Yan, K.; Sun, G.; Wang, D. Activable carboxylic acid functionalized crystalline nanocellulose/PVA-co-PE composite nanofibrous membrane with enhanced adsorption for heavy metal ions. Sep. Purif. Technol. 2017, 186, 70–77. [Google Scholar] [CrossRef]
- Taleb, K.; Markovski, J.; Veličković, Z.; Rusmirović, J.; Rancic, M.; Pavlović, V.; Marinković, A. Arsenic removal by magnetite-loaded amino modified nano/microcellulose adsorbents: Effect of functionalization and media size. Arab. J. Chem. 2019, 12, 4675–4693. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Ma, X.; Zheng, P. Sulfo-functional 3D porous cellulose/graphene oxide composites for highly efficient removal of methylene blue and tetracycline from water. Int. J. Biol. Macromol. 2019, 140, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Sehaqui, H.; Mautner, A.; de Larraya, U.P.; Pfenninger, N.; Tingaut, P.; Zimmermann, T. Cationic cellulose nanofibers from waste pulp residues and their nitrate, fluoride, sulphate and phosphate adsorption properties. Carbohydr. Polym. 2016, 135, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Rethinasabapathy, M.; Kang, S.-M.; Lee, I.; Lee, G.-W.; Hwang, S.K.; Roh, C.; Huh, Y.S. Layer-Structured POSS-Modified Fe-Aminoclay/Carboxymethyl Cellulose Composite as a Superior Adsorbent for the Removal of Radioactive Cesium and Cationic Dyes. Ind. Eng. Chem. Res. 2018, 57, 13731–13741. [Google Scholar] [CrossRef]
- Said, M.M.; Rehan, M.; El-Sheikh, S.M.; Zahran, M.K.; Abdel-Aziz, M.S.; Bechelany, M.; Barhoum, A. Multifunctional Hydroxyapatite/Silver Nanoparticles/Cotton Gauze for Antimicrobial and Biomedical Applications. Nanomaterials 2021, 11, 429. [Google Scholar] [CrossRef]
- Valencia, L.; Kumar, S.; Nomena, E.M.; Salazar-Alvarez, G.; Mathew, A.P. In-Situ Growth of Metal Oxide Nanoparticles on Cellulose Nanofibrils for Dye Removal and Antimicrobial Applications. ACS Appl. Nano Mater. 2020, 3, 7172–7181. [Google Scholar] [CrossRef]
- Hassan, H.S.; Elkady, M.; Farghali, A.; Salem, A.M.; El-Hamid, A.A. Fabrication of novel magnetic zinc oxide cellulose acetate hybrid nano-fiber to be utilized for phenol decontamination. J. Taiwan Inst. Chem. Eng. 2017, 78, 307–316. [Google Scholar] [CrossRef]
- Chen, L.; Berry, R.M.; Tam, K.C. Synthesis of β-Cyclodextrin-Modified Cellulose Nanocrystals (CNCs)@Fe3O4@SiO2 Superparamagnetic Nanorods. ACS Sustain. Chem. Eng. 2014, 2, 951–958. [Google Scholar] [CrossRef]
- Maatar, W.; Alila, S.; Boufi, S. Cellulose based organogel as an adsorbent for dissolved organic compounds. Ind. Crop. Prod. 2013, 49, 33–42. [Google Scholar] [CrossRef]
- Yousefi, N.; Wong, K.K.W.; Hosseinidoust, Z.; Sørensen, H.O.; Bruns, S.; Zheng, Y.; Tufenkji, N. Hierarchically porous, ultra-strong reduced graphene oxide-cellulose nanocrystal sponges for exceptional adsorption of water contaminants. Nanoscale 2018, 10, 7171–7184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Li, Y.; Hu, S.; Sun, J.; Du, Q.; Yang, X.; Ji, Q.; Wang, Z.; Wang, D.; Xia, Y. Removal of methylene blue from water by cellulose/graphene oxide fibres. J. Exp. Nanosci. 2016, 11, 1156–1170. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, S.; Klerks, P.; Nyman, J. Toxicity to freshwater organisms from oils and oil spill chemical treatments in laboratory microcosms. Environ. Pollut. 2003, 122, 205–215. [Google Scholar] [CrossRef]
- Wang, W.; Lin, J.; Cheng, J.; Cui, Z.; Si, J.; Wang, Q.; Peng, X.; Turng, L.-S. Dual super-amphiphilic modified cellulose acetate nanofiber membranes with highly efficient oil/water separation and excellent antifouling properties. J. Hazard. Mater. 2020, 385, 121582. [Google Scholar] [CrossRef]
- Zhang, Z.; Sèbe, G.; Rentsch, D.; Zimmermann, T.; Tingaut, P. Ultralightweight and Flexible Silylated Nanocellulose Sponges for the Selective Removal of Oil from Water. Chem. Mater. 2014, 26, 2659–2668. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Lindström, T.; Hsiao, B.S. Nanocellulose-Enabled Membranes for Water Purification: Perspectives. Adv. Sustain. Syst. 2020, 4, 1900114. [Google Scholar] [CrossRef]
- Zanini, M.; Lavoratti, A.; Lazzari, L.K.; Galiotto, D.; Pagnocelli, M.; Baldasso, C.; Zattera, A.J. Producing aerogels from silanized cellulose nanofiber suspension. Cellulose 2017, 24, 769–779. [Google Scholar] [CrossRef]
- Korhonen, J.T.; Hiekkataipale, P.; Malm, J.; Karppinen, M.; Ikkala, O.; Ras, R.H.A. Inorganic Hollow Nanotube Aerogels by Atomic Layer Deposition onto Native Nanocellulose Templates. ACS Nano 2011, 5, 1967–1974. [Google Scholar] [CrossRef]
- Meng, Y.; Young, T.M.; Liu, P.; Contescu, C.I.; Huang, B.; Wang, S. Ultralight carbon aerogel from nanocellulose as a highly selective oil absorption material. Cellulose 2015, 22, 435–447. [Google Scholar] [CrossRef]
- Sai, H.; Fu, R.; Xing, L.; Xiang, J.; Li, Z.; Li, F.; Zhang, T. Surface Modification of Bacterial Cellulose Aerogels’ Web-like Skeleton for Oil/Water Separation. ACS Appl. Mater. Interfaces 2015, 7, 7373–7381. [Google Scholar] [CrossRef]
- Jeddi, M.K.; Laitinen, O.; Liimatainen, H. Magnetic superabsorbents based on nanocellulose aerobeads for selective removal of oils and organic solvents. Mater. Des. 2019, 183, 108115. [Google Scholar] [CrossRef]
- Gu, H.; Zhou, X.; Lyu, S.; Pan, D.; Dong, M.; Wu, S.; Ding, T.; Wei, X.; Seok, I.; Wei, S.; et al. Magnetic nanocellulose-magnetite aerogel for easy oil adsorption. J. Colloid Interface Sci. 2020, 560, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process. Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Suopajärvi, T.; Liimatainen, H.; Hormi, O.; Niinimäki, J. Coagulation–flocculation treatment of municipal wastewater based on anionized nanocelluloses. Chem. Eng. J. 2013, 231, 59–67. [Google Scholar] [CrossRef]
- Korhonen, M.; Laine, J. Flocculation and retention of fillers with nanocelluloses. Nord. Pulp Pap. Res. J. 2014, 29, 119–128. [Google Scholar] [CrossRef]
- Zhang, L.-W.; Hua, J.-R.; Zhu, W.-J.; Liu, L.; Du, X.-L.; Meng, R.-J.; Yao, J.-M. Flocculation Performance of Hyperbranched Polyethylenimine-Grafted Cellulose in Wastewater Treatment. ACS Sustain. Chem. Eng. 2018, 6, 1592–1601. [Google Scholar] [CrossRef]
- Kemppainen, K.; Suopajärvi, T.; Laitinen, O.; Ämmälä, A.; Liimatainen, H.; Illikainen, M. Flocculation of fine hematite and quartz suspensions with anionic cellulose nanofibers. Chem. Eng. Sci. 2016, 148, 256–266. [Google Scholar] [CrossRef]
- Campano, C.; Lopez-Exposito, P.; Blanco, A.; Negro, C.; van de Ven, T.G. Hairy cationic nanocrystalline cellulose as a novel flocculant of clay. J. Colloid Interface Sci. 2019, 545, 153–161. [Google Scholar] [CrossRef]
- Sun, X.; Danumah, C.; Liu, Y.; Boluk, Y. Flocculation of bacteria by depletion interactions due to rod-shaped cellulose nanocrystals. Chem. Eng. J. 2012, 198–199, 476–481. [Google Scholar] [CrossRef]
- Vandamme, D.; Eyley, S.; Van den Mooter, G.; Muylaert, K.; Thielemans, W. Highly charged cellulose-based nanocrystals as flocculants for harvesting Chlorella vulgaris. Bioresour. Technol. 2015, 194, 270–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhlaghi, S.P.; Zaman, M.; Mohammed, N.; Brinatti, C.; Batmaz, R.; Berry, R.; Loh, W.; Tam, K.C. Synthesis of amine functionalized cellulose nanocrystals: Optimization and characterization. Carbohydr. Res. 2015, 409, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Suopajärvi, T.; Koivuranta, E.; Liimatainen, H.; Niinimäki, J. Flocculation of municipal wastewaters with anionic nanocelluloses: Influence of nanocellulose characteristics on floc morphology and strength. J. Environ. Chem. Eng. 2014, 2, 2005–2012. [Google Scholar] [CrossRef]
- Quinlan, P.J.; Tanvir, A.; Tam, K.C. Application of the central composite design to study the flocculation of an anionic azo dye using quaternized cellulose nanofibrils. Carbohydr. Polym. 2015, 133, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.-L.; Hu, W.-L.; Chen, S.-Y.; Zheng, Y.; Zhou, B.-H.; Wang, H.-P. High photocatalytic properties of zinc oxide nanoparticles with amidoximated bacterial cellulose nanofibers as templates. Chin. J. Polym. Sci. 2014, 32, 169–176. [Google Scholar] [CrossRef]
- Wang, S.-D.; Ma, Q.; Liu, H.; Wang, K.; Ling, L.-Z.; Zhang, K.-Q. Robust electrospinning cellulose acetate@TiO2 ultrafine fibers for dyeing water treatment by photocatalytic reactions. RSC Adv. 2015, 5, 40521–40530. [Google Scholar] [CrossRef]
- Rehan, M.; Barhoum, A.; Khattab, T.; Gätjen, L.; Wilken, R. Colored, photocatalytic, antimicrobial and UV-protected viscose fibers decorated with Ag/Ag2CO3 and Ag/Ag3PO4 nanoparticles. Cellulose 2019, 26, 5437–5453. [Google Scholar] [CrossRef]
- Leong, W.S.; Luo, X.; Li, Y.; Khoo, K.H.; Quek, S.Y.; Thong, J.T.L. Low Resistance Metal Contacts to MoS2 Devices with Nickel-Etched-Graphene Electrodes. ACS Nano 2015, 9, 869–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehan, M.; Barhoum, A.; Van Assche, G.; Dufresne, A.; Gätjen, L.; Wilken, R. Towards multifunctional cellulosic fabric: UV photo-reduction and in-situ synthesis of silver nanoparticles into cellulose fabrics. Int. J. Biol. Macromol. 2017, 98, 877–886. [Google Scholar] [CrossRef]
- Barhoum, A.; Melcher, J.; Van Assche, G.; Rahier, H.; Bechelany, M.; Fleisch, M.; Bahnemann, D.B.D. Synthesis, growth mechanism, and photocatalytic activity of Zinc oxide nanostructures: Porous microparticles versus nonporous nanoparticles. J. Mater. Sci. 2017, 52, 2746–2762. [Google Scholar] [CrossRef]
- Ren, C.; Yang, B.; Wu, M.; Xu, J.; Fu, Z.; Lv, Y.; Guo, T.; Zhao, Y.; Zhu, C. Synthesis of Ag/ZnO nanorods array with enhanced photocatalytic performance. J. Hazard. Mater. 2010, 182, 123–129. [Google Scholar] [CrossRef]
- Lin, Z.; Lu, Y.; Huang, J. A hierarchical Ag2O-nanoparticle/TiO2-nanotube composite derived from natural cellulose substance with enhanced photocatalytic performance. Cellulose 2019, 26, 6683–6700. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, S.A.U.; Luan, H.-Y.; Farid, M.U.; Huang, H. Challenges and opportunities in functional carbon nanotubes for membrane-based water treatment and desalination. Sci. Total Environ. 2019, 646, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.N.; Hou, S.X.; Hao, Z.C.; Cui, G.H. Ultrasonic green synthesis of an Ag/CP nanocomposite for enhanced photodegradation effectiveness. Ultrason. Sonochem. 2018, 40, 1039–1048. [Google Scholar] [CrossRef]
- Gan, L.; Geng, A.; Xu, L.; Chen, M.; Wang, L.; Liu, J.; Han, S.; Mei, C.; Zhong, Q. The fabrication of bio-renewable and recyclable cellulose based carbon microspheres incorporated by CoFe2O4 and the photocatalytic properties. J. Clean. Prod. 2018, 196, 594–603. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, S.; Cai, J.; Zhang, L. TiO2 Immobilized in Cellulose Matrix for Photocatalytic Degradation of Phenol under Weak UV Light Irradiation. J. Phys. Chem. C 2010, 114, 7806–7811. [Google Scholar] [CrossRef]
- Jiao, Y.; Wan, C.; Bao, W.; Gao, H.; Liang, D.; Li, J. Facile hydrothermal synthesis of Fe3O4@cellulose aerogel nanocomposite and its application in Fenton-like degradation of Rhodamine B. Carbohydr. Polym. 2018, 189, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Yanfen, F.; Yingping, H.; Jing, Y.; Pan, W.; Genwei, C. Unique ability of BiOBr to decarboxylate D-Glu and D-MeAsp in the photocatalytic degradation of microcystin-LR in water. Environ. Sci. Technol. 2011, 45, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, D.; Zhang, P.; Man, P.; Tian, Z.; Li, Y.; Ai, S. The BiOBr/regenerated cellulose composite film as a green catalyst for light degradation of phenol. Colloids Surf. A Physicochem. Eng. Asp. 2016, 501, 132–137. [Google Scholar] [CrossRef]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.Ó.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, H.; Chu, B.; Hsiao, B.S. Fabrication of cellulose nanofiber-based ultrafiltration membranes by spray coating approach. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Azzaoui, K.; Mejdoubi, E.; Lamhamdi, A.; Jodeh, S.; Hamed, O.; Berrabah, M.; Jerdioui, S.; Salghi, R.; Akartasse, N.; Errich, A.; et al. Preparation and characterization of biodegradable nanocomposites derived from carboxymethyl cellulose and hydroxyapatite. Carbohydr. Polym. 2017, 167, 59–69. [Google Scholar] [CrossRef]
- Peng, N.; Hu, D.; Zeng, J.; Li, Y.; Liang, L.; Chang, C. Superabsorbent Cellulose–Clay Nanocomposite Hydrogels for Highly Efficient Removal of Dye in Water. ACS Sustain. Chem. Eng. 2016, 4, 7217–7224. [Google Scholar] [CrossRef]
- Yadav, M.; Rhee, K.Y.; Park, S.-J. Synthesis and characterization of graphene oxide/carboxymethylcellulose/alginate composite blend films. Carbohydr. Polym. 2014, 110, 18–25. [Google Scholar] [CrossRef]
- Wei, X.; Huang, T.; Yang, J.-H.; Zhang, N.; Wang, Y.; Zhou, Z.-W. Green synthesis of hybrid graphene oxide/microcrystalline cellulose aerogels and their use as superabsorbents. J. Hazard. Mater. 2017, 335, 28–38. [Google Scholar] [CrossRef]
- Fang, Q.; Zhou, X.; Deng, W.; Zheng, Z.; Liu, Z. Freestanding bacterial cellulose-graphene oxide composite membranes with high mechanical strength for selective ion permeation. Sci. Rep. 2016, 6, 33185. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Liu, J.; Zhou, W.; Zhang, Y.-K.; Du, K.-F.; Zhao, Z.-M. Fabrication of spherical cellulose/carbon tubes hybrid adsorbent anchored with welan gum polysaccharide and its potential in adsorbing methylene blue. Chem. Eng. J. 2012, 200–202, 452–458. [Google Scholar] [CrossRef]
- Qin, Y.; Qin, Z.; Liu, Y.; Cheng, M.; Qian, P.; Wang, Q.; Zhu, M. Superparamagnetic iron oxide coated on the surface of cellulose nanospheres for the rapid removal of textile dye under mild condition. Appl. Surf. Sci. 2015, 357, 2103–2111. [Google Scholar] [CrossRef]
- Fallah, M.H.; Fallah, S.A.; Zanjanchi, M.A. Synthesis and Characterization of Nano-sized Zinc Oxide Coating on Cellulosic Fibers: Photoactivity and Flame-retardancy Study. Chin. J. Chem. 2011, 29, 1239–1245. [Google Scholar] [CrossRef]
- Ali, K.; Dwivedi, S.; Azam, A.; Saquib, Q.; Al-Said, M.S.; Al-Khedhairy, A.; Musarrat, J. Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolates. J. Colloid Interface Sci. 2016, 472, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Hamad, H.; Bailón-García, E.; Morales-Torres, S.; Carrasco-Marín, F.; Pérez-Cadenas, A.; Maldonado-Hódar, F.J. Physicochemical properties of new cellulose-TiO2 composites for the removal of water pollutants: Developing specific interactions and performances by cellulose functionalization. J. Environ. Chem. Eng. 2018, 6, 5032–5041. [Google Scholar] [CrossRef]
- Nsib, M.F.; Hajji, F.; Mayoufi, A.; Moussa, N.; Rayes, A.; Houas, A. In situ synthesis and characterization of TiO2/HPM cellulose hybrid material for the photocatalytic degradation of 4-NP under visible light. Comptes Rendus Chim. 2014, 17, 839–848. [Google Scholar] [CrossRef]
- Shoukat, A.; Wahid, F.; Khan, T.; Siddique, M.; Nasreen, S.; Yang, G.; Ullah, M.W.; Khan, R. Titanium oxide-bacterial cellulose bioadsorbent for the removal of lead ions from aqueous solution. Int. J. Biol. Macromol. 2019, 129, 965–971. [Google Scholar] [CrossRef]
- Tanzifi, M.; Yaraki, M.T.; Karami, M.; Karimi, S.; Kiadehi, A.D.; Karimipour, K.; Wang, S. Modelling of dye adsorption from aqueous solution on polyaniline/carboxymethyl cellulose/TiO2 nanocomposites. J. Colloid Interface Sci. 2018, 519, 154–173. [Google Scholar] [CrossRef]
- Matsubara, H.; Takada, M.; Koyama, S.; Hashimoto, K.; Fujishima, A. Photoactive TiO2 Containing Paper: Preparation and Its Photocatalytic Activity under Weak UV Light Illumination. Chem. Lett. 1995, 24, 767–768. [Google Scholar] [CrossRef]
- Suman; Kardam, A.; Gera, M.; Jain, V. A novel reusable nanocomposite for complete removal of dyes, heavy metals and microbial load from water based on nanocellulose and silver nano-embedded pebbles. Environ. Technol. 2015, 36, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Dutta, S.D.; Lim, K.-T. Nanocellulose-based polymer hybrids and their emerging applications in biomedical engineering and water purification. RSC Adv. 2019, 9, 19143–19162. [Google Scholar] [CrossRef] [Green Version]
- Patil, K.; Jeong, S.; Lim, H.; Byun, H.-S.; Han, S. Removal of volatile organic compounds from air using activated carbon impregnated cellulose acetate electrospun mats. Environ. Eng. Res. 2019, 24, 600–607. [Google Scholar] [CrossRef]
- Samyn, P.; Barhoum, A. Engineered nanomaterials for papermaking industry. In Fundamentals of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 245–277. [Google Scholar]
- Tavakolian, M.; Jafari, S.M.; van de Ven, T.G.M. A Review on Surface-Functionalized Cellulosic Nanostructures as Biocompatible Antibacterial Materials. Nano-Micro Lett. 2020, 12, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, W.; Yu, J.; Zhang, L.; Liu, L.; Zhou, X.; Huang, C.; Fan, Y. Preparation of nanocellulose/filter paper (NC/FP) composite membranes for high-performance filtration. Cellulose 2019, 26, 1183–1194. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; He, X.; Zhang, X.; Li, Q.; Xia, T.; Zhang, W.; Lu, C.; Deng, Y. One-Step Fabrication of Fe(OH)3@Cellulose Hollow Nanofibers with Superior Capability for Water Purification. ACS Appl. Mater. Interfaces 2017, 9, 25339–25349. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Aubrecht, K.B.; Ma, H.; Wang, R.; Grubbs, R.B.; Hsiao, B.S.; Chu, B. Thiol-modified cellulose nanofibrous composite membranes for chromium (VI) and lead (II) adsorption. Polymer 2014, 55, 1167–1176. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Sun, G.; Wang, M.; Yu, J. Electro-spinning/netting: A strategy for the fabrication of three-dimensional polymer nano-fiber/nets. Prog. Mater. Sci. 2013, 58, 1173–1243. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, J.; Harrisson, S.; Chen, V. Strategies for controlling biofouling in membrane filtration systems: Challenges and opportunities. J. Mater. Chem. 2010, 20, 4567–4586. [Google Scholar] [CrossRef]
- Kong, L.; Yin, X.; Yuan, X.; Zhang, Y.; Liu, X.; Cheng, L.; Zhang, L. Electromagnetic wave absorption properties of graphene modified with carbon nanotube/poly(dimethyl siloxane) composites. Carbon 2014, 73, 185–193. [Google Scholar] [CrossRef]
- Hassan, M.; Berglund, L.; Abou-Zeid, R.; Hassan, E.; Abou-Elseoud, W.; Oksman, K. Nanocomposite Film Based on Cellulose Acetate and Lignin-Rich Rice Straw Nanofibers. Materials 2019, 12, 595. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhou, J.; Jiang, L.; Lubineau, G.; Chen, Y.; Wu, X.-F.; Piere, R. Porous core-shell carbon fibers derived from lignin and cellulose nanofibrils. Mater. Lett. 2013, 109, 175–178. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Highly Permeable Polymer Membranes Containing Directed Channels for Water Purification. ACS Macro Lett. 2012, 1, 723–726. [Google Scholar] [CrossRef]
- Wang, J.-G.; Yang, Y.; Huang, Z.-H.; Kang, F. A high-performance asymmetric supercapacitor based on carbon and carbon–MnO2 nanofiber electrodes. Carbon 2013, 61, 190–199. [Google Scholar] [CrossRef]
- Ferraz, E.R.A.; Oliveira, G.A.R.; Grando, M.D.; Lizier, T.M.; Zanoni, M.V.B.; Oliveira, D.P. Photoelectrocatalysis based on Ti/TiO2 nanotubes removes toxic properties of the azo dyes Disperse Red 1, Disperse Red 13 and Disperse Orange 1 from aqueous chloride samples. J. Environ. Manag. 2013, 124, 108–114. [Google Scholar] [CrossRef]
- Barud, H.S.; Souza, J.L.; Santos, D.B.; Crespi, M.S.; Ribeiro, C.; Messaddeq, Y.; Ribeiro, S. Bacterial cellulose/poly(3-hydroxybutyrate) composite membranes. Carbohydr. Polym. 2011, 83, 1279–1284. [Google Scholar] [CrossRef]
- Asper, M.; Hanrieder, T.; Quellmalz, A.; Mihranyan, A. Removal of xenotropic murine leukemia virus by nanocellulose based filter paper. Biologicals 2015, 43, 452–456. [Google Scholar] [CrossRef]
- Metreveli, G.; Wågberg, L.; Emmoth, E.; Belák, S.; Strømme, M.; Mihranyan, A. A Size-Exclusion Nanocellulose Filter Paper for Virus Removal. Adv. Healthc. Mater. 2014, 3, 1546–1550. [Google Scholar] [CrossRef] [PubMed]
- Quellmalz, A.; Mihranyan, A. Citric Acid Cross-Linked Nanocellulose-Based Paper for Size-Exclusion Nanofiltration. ACS Biomater. Sci. Eng. 2015, 1, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Seo, J.Y.; Kim, H.; Beak, K.Y. Structural control of cellulose nanofibrous composite membrane with metal organic framework (ZIF-8) for highly selective removal of cationic dye. Carbohydr. Polym. 2019, 222, 115018. [Google Scholar] [CrossRef] [PubMed]
- Takai, M.; Nonomura, F.; Inukai, T.; Fujiwara, M.; Hayashi, J. Filtration and permeation characteristics of bacterial cellulose composite. Sen’i Gakkaishi 1991, 47, 119–129. [Google Scholar] [CrossRef]
- Mautner, A.; Lee, K.-Y.; Tammelin, T.; Mathew, A.P.; Nedoma, A.; Li, K.; Bismarck, A. Cellulose nanopapers as tight aqueous ultra-filtration membranes. React. Funct. Polym. 2015, 86, 209–214. [Google Scholar] [CrossRef]
- Cheng, Q.; Ye, D.; Chang, C.; Zhang, L. Facile fabrication of superhydrophilic membranes consisted of fibrous tunicate cellulose nanocrystals for highly efficient oil/water separation. J. Membr. Sci. 2017, 525, 1–8. [Google Scholar] [CrossRef]
- Karim, Z.; Claudpierre, S.; Grahn, M.; Oksman, K.; Mathew, A.P. Nanocellulose based functional membranes for water cleaning: Tailoring of mechanical properties, porosity and metal ion capture. J. Membr. Sci. 2016, 514, 418–428. [Google Scholar] [CrossRef]
- Karim, Z.; Mathew, A.P.; Kokol, V.; Wei, J.; Grahn, M. High-flux affinity membranes based on cellulose nanocomposites for removal of heavy metal ions from industrial effluents. RSC Adv. 2016, 6, 20644–20653. [Google Scholar] [CrossRef] [Green Version]
- Karim, Z.; Hakalahti, M.; Tammelin, T.; Mathew, A.P. In situ TEMPO surface functionalization of nanocellulose membranes for enhanced adsorption of metal ions from aqueous medium. RSC Adv. 2017, 7, 5232–5241. [Google Scholar] [CrossRef] [Green Version]
- Karim, Z.; Mathew, A.P.; Grahn, M.; Mouzon, J.; Oksman, K. Nanoporous membranes with cellulose nanocrystals as functional entity in chitosan: Removal of dyes from water. Carbohydr. Polym. 2014, 112, 668–676. [Google Scholar] [CrossRef]
- Goetz, L.A.; Naseri, N.; Nair, S.S.; Karim, Z.; Mathew, A.P. All cellulose electrospun water purification membranes nanotextured using cellulose nanocrystals. Cellulose 2018, 25, 3011–3023. [Google Scholar] [CrossRef] [Green Version]
- Soyekwo, F.; Zhang, Q.G.; Lin, X.C.; Wu, X.M.; Zhu, A.M.; Liu, Q.L. Facile preparation and separation performances of cellulose nanofibrous membranes. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Mautner, A.; Maples, H.A.; Kobkeatthawin, T.; Kokol, V.; Karim, Z.; Li, K.; Bismarck, A. Phosphorylated nanocellulose papers for copper adsorption from aqueous solutions. Int. J. Environ. Sci. Technol. 2016, 13, 1861–1872. [Google Scholar] [CrossRef] [Green Version]
- Varanasi, S.; Low, Z.-X.; Batchelor, W. Cellulose nanofibre composite membranes—Biodegradable and recyclable UF membranes. Chem. Eng. J. 2015, 265, 138–146. [Google Scholar] [CrossRef]
- Wang, R.; Guan, S.; Sato, A.; Wang, X.; Wang, Z.; Yang, R.; Hsiao, B.S.; Chu, B. Nanofibrous microfiltration membranes capable of removing bacteria, viruses and heavy metal ions. J. Membr. Sci. 2013, 446, 376–382. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, C.; Wang, H.; Jin, C.; Sun, Q.; Xu, X. Nano-cellulose hydrogel coated flexible titanate-bismuth oxide membrane for trinity synergistic treatment of super-intricate anion/cation/oily-water. Chem. Eng. J. 2018, 337, 143–151. [Google Scholar] [CrossRef]
- Wanichapichart, P.; Kaewnopparat, S.; Buaking, K.; Puthai, W. Characterization of cellulose membranes produced by Acetobacter xyllinum. Songklanakarin J. Sci. Technol. 2002, 24, 855–862. [Google Scholar]
- Chen, S.; Teng, Q. Quantitative Immobilization of Phthalocyanine onto Bacterial Cellulose for Construction of a High-Performance Catalytic Membrane Reactor. Materials 2017, 10, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, F.; Perales-Perez, O.J.; Hwang, S.; Román, F. Antimicrobial nanomaterials as water disinfectant: Applications, limitations and future perspectives. Sci. Total Environ. 2014, 466–467, 1047–1059. [Google Scholar] [CrossRef]
- Li, J.; Cha, R.; Mou, K.; Zhao, X.; Long, K.; Luo, H.; Zhou, F.; Jiang, X. Nanocellulose-Based Antibacterial Materials. Adv. Healthc. Mater. 2018, 7, e1800334. [Google Scholar] [CrossRef]
- Abitbol, T.; Marway, H.; Cranston, E.D. Surface modification of cellulose nanocrystals with cetyltrimethylammonium bromide. Nord. Pulp Pap. Res. J. 2014, 29, 46–57. [Google Scholar] [CrossRef]
- Fei, P.; Liao, L.; Meng, J.; Cheng, B.; Hu, X.; Song, J. Non-leaching antibacterial cellulose triacetate reverse osmosis membrane via covalent immobilization of quaternary ammonium cations. Carbohydr. Polym. 2018, 181, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Fang, W.; Guo, J.; Jiao, D.; Chen, S.; Ifuku, S.; Wang, H.; Walther, A. Highly Mineralized Biomimetic Polysaccharide Nanofiber Materials Using Enzymatic Mineralization. Biomacromolecules 2020, 21, 2176–2186. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Liu, N.; Guo, Z.; Singh, P.K.; Fu, S. Effect of surface wettability on the antibacterial activity of nanocellulose-based material with quaternary ammonium groups. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 122–128. [Google Scholar] [CrossRef]
- Fu, F.; Gu, J.; Cao, J.; Shen, R.; Liu, H.; Zhang, Y.; Liu, X.; Zhou, J. Reduction of Silver Ions Using an Alkaline Cellulose Dope: Straightforward Access to Ag/ZnO Decorated Cellulose Nanocomposite Film with Enhanced Antibacterial Activities. ACS Sustain. Chem. Eng. 2018, 6, 738–748. [Google Scholar] [CrossRef]
- Nguyen, H.-L.; Jo, Y.K.; Cha, M.; Cha, Y.J.; Yoon, D.K.; Sanandiya, N.D.; Prajatelistia, E.; Oh, D.X.; Hwang, D.S. Mussel-Inspired Anisotropic Nanocellulose and Silver Nanoparticle Composite with Improved Mechanical Properties, Electrical Conductivity and Antibacterial Activity. Polymers 2016, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, A.W.; De Lannoy, C.-F.; Wiesner, M.R. Cellulose Nanomaterials in Water Treatment Technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Neumann, M.; Günter, C.; Taubert, A. Ionic liquid-assisted formation of cellulose/calcium phosphate hybrid materials. Beilstein J. Nanotechnol. 2014, 5, 1553–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salama, A. Dicarboxylic cellulose decorated with silver nanoparticles as sustainable antibacterial nanocomposite material. Environ. Nanotechnol. Monit. Manag. 2017, 8, 228–232. [Google Scholar] [CrossRef]













| Type | Origin | Mean Size | Ref. |
|---|---|---|---|
| Cellulose Microfibers (CMF) | Wood, cotton, hemp, flax, straw | Several microns in diameter and hundred microns in length | [29] |
| Cellulose nanofiber (CNF) | Wood, cotton, hemp, flax, straw | Few microns in dimeter with finely attached nanofibers; several to hundred microns in length | [30] |
| Spherical cellulose nanoparticles (SCNPs) | Wood, cotton, hemp, flax, straw | Diameter: 10–100 nm | [31] |
| Cellulose nanocrystals (CNC) | Wood, cotton, hemp, flax, wheat straw, rice straw, mulberry bark, ramie, Avicel®, tunicin, algae | Width: 5–70 nm Length: 100–250 nm (from plants); 100 nm to several micrometers (from the cellulose of tunicates, algae, and bacteria) | [6] |
| Bacterial nanocellulose (BC) | Low molecular weight sugars and alcohols | Width: 5–70 nm Length: several micrometers | [32] |
| Cellulose nanoyarns (CNY) | Cellulose derivatives | Diameter; 100 to 1000 nm Length: >1 µm | [6] |
| Nanocellulose | Surface Modification | Heavy Metal | pH | Removal Efficiency (mg/g) | Langmuir | Freundlich | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qmax (mg·g−1) | KL (L·mg−1) | R2 | KF | 1/n | R2 | ||||||
| Cellulose nanocrystals | Sulfate (-SO3−) | Ag+ | 6.39 | 34.35 | 1.9 | 0.09 | 0.996 | ˗ | ˗ | ˗ | [60] |
| 3.5–4.5 | 56 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [61] | |||
| Cd2+ | 6.0 | 8 | 1.9 | 0.09 | 0.996 | ˗ | ˗ | ˗ | [62] | ||
| 6.5 | 9.7 | 11.2 | 0.63 | 0.95 | 5.75 | 4.71 | 0.92 | [63] | |||
| Cu2+ | 3.5–4.5 | 19 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [61] | ||
| 6 | 0.59 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [64] | |||
| Pb2+ | 5.5 | 47 | 27.9 | 0.04 | 0.973 | ˗ | ˗ | ˗ | [62] | ||
| 6.5 | 9.42 | 10.20 | 1.81 | 0.98 | 7.06 | 6.89 | 0.94 | [63] | |||
| Fe3+ | 3.5–4.5 | 6.3 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [61] | ||
| Cr3+ | 6.5 | 1.6 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [65] | ||
| Cr4+ | 2.5 | 0.6 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [65] | ||
| Ni2+ | 6.5 | 8.55 | 11.23 | 0.33 | 0.97 | 3.91 | 2.90 | 0.97 | [63] | ||
| As3+ | 7.5 | 2.2 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [66] | ||
| As4+ | 2.5 | 2.3 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [66] | ||
| Phosphorylation (-PO32−) | Ag+ | 3.5–4.5 | 106 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [61] | |
| Cu2+ | 3.5–4.5 | 117 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [61] | ||
| Fe3+ | 3.5–4.5 | 114 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [61] | ||
| Succination (-COO−) | Cd2+ | 6 | 152 | 259.7 | 2.29 | 0.998 | ˗ | ˗ | ˗ | [62] | |
| Pb2+ | 5.5 | 300 | 367.6 | 1.81 | 0.998 | ˗ | ˗ | ˗ | [62] | ||
| Cr3+ | 6.5 | 2.4 | 2.88 | 1.49 | 0.987 | 0.83 | 0.17 | 0.98 | [65] | ||
| Sodium salt succination (-COO−Na+) | Cd2+ | 6 | 167 | 344.8 | 41.88 | 1.000 | ˗ | ˗ | ˗ | [62] | |
| Pb2+ | 5.5 | 300 | 465.1 | 4.13 | 1.000 | ˗ | ˗ | ˗ | [62] | ||
| Poly(itaconic acid/methacrylic acid)-grafted CNC/nanobentonite composite | Co2+ | 6 | - | 241.8 | 0.025 | 0.99 | 16.31 | 0.504 | 0.98 | [67] | |
| Xanthate (-ROCS2−Na+) | Cd2+ | 6.0 | 24.3 | 154.3 | 0.11 | 0.99 | 26.9 | 2.38 | 0.98 | [68] | |
| TEMPO-oxidation (-COO−) | Cu2+ | 6 | 14.6 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [64] | |
| Periodate/chlorite oxidation | Cu2+ | 4 | 185 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [69] | |
| Itaconic acid-grafted-magnetite modified with 2-mercaptobenzamide | Hg2+ | 8 | 69.7 | ˗ | ˗ | ˗ | 44.05 | 0.36 | 0.991 | [70] | |
| Carboxylation (-COO−) | Pb2+ | 7 | ˗ | 2270 | ˗ | 0.978 | ˗ | ˗ | ˗ | [71] | |
| Amination (NH3+) | Cr6+ | 2.5 | 9.8 | 2.77 | 0.48 | 0.968 | 0.58 | 0.08 | 0.96 | [65] | |
| As3+ | 7.5 | 9.3 | 10.56 | 1.85 | 0.991 | 5.52 | 0.22 | 0.986 | [66] | ||
| As4+ | 2.5 | 9.8 | 12.06 | 5.34 | 0.992 | 6.13 | 0.75 | 0.941 | [66] | ||
| Carboxylated CNC/alginate composite | Pb2+ | 5.2 | 280 | 339.0 | 0.17 | 0.993 | 68.93 | 0.31 | 0.607 | [72] | |
| Cellulose nanofibers | Unmodified | Ag+ | 5.45 | 15.45 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [60] |
| Cu2+ | 6.2 | 13 | - | ˗ | ˗ | ˗ | ˗ | ˗ | [58] | ||
| 4.5 | 17.9 | 24.8 | 0.327 | 0.969 | 0.04 | 0.69 | 0.992 | [73] | |||
| Pb2+ | 5 | ˗ | 16.5 | 49.14 | 0.64 | 2.9 | 1.48 | 0.74 | [59] | ||
| Zn2+ | 6 | 3.5 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [58] | ||
| Cr3+ | 5 | 14.1 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [58] | ||
| Phosphorylation (-PO32−) | Ag+ | 3.5–4.5 | 120 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [61] | |
| Cu2+ | 3.5–4.5 | 114 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [61] | ||
| Fe3+ | 3.5–4.5 | 73 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [61] | ||
| TEMPO-oxidation (-COO−) | Cu2+ | 6 | 112 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [74] | |
| 6.2 | 112 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [74] | |||
| 5 | ˗ | 18.9 | 0.17 | 0.975 | 6.71 | 0.24 | 0.903 | [72] | |||
| 5.7 | 114 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [75] | |||
| Ni2+ | 6 | 8.6 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [74] | ||
| 6 | 49 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [58] | |||
| UO22+ | 6.5 | 167 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [56] | ||
| Pb2+ | 6 | 9.7 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [74] | ||
| Zn2+ | 6.2 | 66.0 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [74] | ||
| 6 | 66 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [58] | |||
| Cr3+ | 6 | 8.9 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [74] | ||
| 5 | 58 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [58] | |||
| Mercerization/Succination (-COO−) | Cd2+ | 5 | 5 | 2.06 | 691 | 0.923 | ˗ | ˗ | ˗ | [76] | |
| Co2+ | 5 | 5 | 1.34 | 3.55 | 0.984 | ˗ | ˗ | ˗ | [76] | ||
| Cu2+ | 5 | 4.7 | 1.90 | 58.89 | 0.849 | ˗ | ˗ | ˗ | [76] | ||
| Ni2+ | 5 | 4.6 | 0.74 | 12.32 | 0.964 | ˗ | ˗ | ˗ | [76] | ||
| Zn2+ | 5 | 5 | 1.61 | 59.21 | 0.902 | ˗ | ˗ | ˗ | [76] | ||
| Amination (NH3+) | Cd2+ | 5 | 58.1 | 405.6 | 61.30 | 0.892 | ˗ | ˗ | ˗ | [77] | |
| Cu2+ | 5 | 50.6 | 195.6 | 4.072 | 0.770 | ˗ | ˗ | ˗ | [77] | ||
| Poly (acrylic acid) grafting | Cu2+ | 4.5 | 45.8 | 57.5 | 0.124 | 0.972 | 0.104 | 0.720 | 0.988 | [73] | |
| Poly (acrylic acid)/sodium humate grafting | Cu2+ | 4.5 | 44.7 | 64.6 | 0.175 | 0.970 | 0.11 | 0.66 | 0.991 | [73] | |
| TEMPO oxidation/polyethyleneimine grafting | Cu2+ | 5 | ˗ | 52.3 | 0.17 | 0.985 | 31.0 | 10.4 | 0.919 | [78] | |
| TEMPO-oxidation/GO nanocomposite | Cu2+ | 5.7 | 63.5 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [79] | |
| TEMPO-oxidation/nanoGO composite | Cu2+ | 5.7 | 68.1 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [79] | |
| TEMPO-oxidation (-COO−)/thiolation (-Si-SH) | Hg2+ | 5–9 | 145 | 729.9 | 0.36 | 0.998 | 112.0 | 0.43 | 0.835 | [78] | |
| Pb2+ | 5.5 | 133 | 137.7 | 0.783 | 0.998 | ˗ | ˗ | ˗ | [80] | ||
| Cr6+ | 4 | 76.5 | 87.5 | 0.308 | 0.997 | ˗ | ˗ | ˗ | [80] | ||
| Poly-methacylic acid-co-maleic acid grafting | Pb2+ | 5 | 165 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [81] | |
| Ni2+ | 5 | 117 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [81] | ||
| Zn2+ | 5 | 138 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [81] | ||
| Cd2+ | 5 | 135 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [81] | ||
| Bisphosphonate (-PO(OH)2)2 | V5+ | 3 | 10.3 | 1.13–1.98 | 1.69–2.92 | 0.97–0.996 | 0.65–1.44 | 0.41–0.48 | 0.87–0.993 | [82] | |
| Sulfonation | Pb2+ | 5 | 123 | 251 | 0.69 | 0.97 | 72.8 | 0.66 | 0.93 | [59] | |
| Periodate/chlorite oxidation/polyamide-amine-epichlorohydrin | Cu2+ | - | 82.19 | - | - | - | - | - | - | [83] | |
| Pb2+ | 76.11 | - | - | - | - | - | - | [83] | |||
| Ca2+ | - | 94.77 | - | - | - | - | - | - | [83] | ||
| Bacterial nanocellulose | Unmodified | Cu2+ | 4.5 | 9.67 | 11.2 | 0.011 | 0.962 | 5.389 | 0.13 | 0.838 | [84] |
| ˗ | ˗ | 90.91 | 0.002 | 0.876 | 1.626 | 0.53 | 0.909 | [85] | |||
| Pb2+ | 4.5 | 22.6 | 24.59 | 0.084 | 0.976 | 5.17 | 0.30 | 0.960 | [84] | ||
| ˗ | ˗ | 100.0 | 0.004 | 0.799 | 1.37 | 0.64 | 0.802 | [85] | |||
| Phosphorylation (-PO32−) | Co2+ | 4.5 | 4.25 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [86] | |
| Cu2+ | 4.5 | 4.77 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [86] | ||
| Mn2+ | 4.5 | 3.92 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [86] | ||
| Zn2+ | 4.5 | 4.36 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [86] | ||
| Fe3+ | 4.5 | 4.19 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [86] | ||
| Ho3+ | 8 | 13.6 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [86] | ||
| La3+ | 8 | 10.9 | 12.6 | ˗ | ˗ | ˗ | ˗ | ˗ | [86] | ||
| Sm3+ | 8 | 12.4 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [86] | ||
| Amination (NH3+) | Pb2+ | 4.5 | 57.2 | 87.41 | 0.003 | 0.993 | 0.995 | 0.62 | 0.982 | [87] | |
| Carboxymethylation (-COO−) | Pb2+ | 4.5 | 60.4 | 65.53 | 0.058 | 0.961 | 5.70 | 0.50 | 0.898 | [84] | |
| Fe3O4/BC hybrid nanocomposite | Pb2+ | 7 | 65 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [75] | |
| Polyethyleneimine grafting | Pb2+ | 5.5 | 28.6 | 125.0 | 0.002 | 0.500 | 1.10 | 0.66 | 0.793 | [85] | |
| Fe3O4/BC hybrid nanocomposite | Cr3+ | 7 | 25 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [75] | |
| Mn2+ | 7 | 33 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [75] | ||
| Nanocellulose | Surface Modification | Dye | pH | Removal Efficiency mg/g | Langmuir | Freundlich | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qmax (mg·g−1) | KL (L·mg−1) | R2 | KF | 1/n | R2 | ||||||
| Cellulose nanocrystals | Sulfate (-SO3−) | Basic fuchsin | 6–9 | 261 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [118] |
| Crystal violet | 6–9 | 178 | 185 | 1.023 | 0.997 | 44.7 | 0.26 | 0.68 | [127] | ||
| Methylene blue | 9 | - | 118 | 0.014 | 0.99 | 18.56 | 0.27 | 0.97 | [118] | ||
| 6–9 | 178 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [118] | |||
| 7 | 107 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [128] | |||
| Malachite green | 6–9 | 92 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [118] | ||
| Carboxylation (-COO−) | Basic fuchsin | 6–9 | 318 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [118] | |
| Crystal violet | 6–9 | 223 | 243.9 | 1.129 | 0.986 | 76.50 | 0.21 | 0.803 | [118] | ||
| Methylene blue | 9 | - | 769 | 0.009 | 0.99 | ˗ | ˗ | ˗ | [128] | ||
| 6–9 | 233 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [118] | |||
| 7 | 79 | 101.2 | 0.019 | 0.974 | ˗ | ˗ | ˗ | [129] | |||
| Malachite green | 6–9 | 148 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [118] | ||
| Amination (NH3+) | Acid red GR | 3.5 | 199.9 | 873.8 | 0.0305 | 0.92 | 120.3 | 0.34 | 0.985 | [130] | |
| Congo red | 4.7 | 199.5 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [131] | ||
| Light yellow K-4G | 4.7 | 183.0 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [131] | ||
| PVAm grafting | Acid red GR | 3.5 | 199.9 | 873.8 | 0.0305 | 0.92 | 120.3 | 0.34 | 0.985 | [130] | |
| Light yellow K-4G | 3.5 | 196.9 | 1211 | 0.046 | 0.80 | 237.1 | 0.26 | 0.974 | [130] | ||
| Congo red | 3.5 | 199.8 | 1619.9 | 0.210 | 0.96 | 267.3 | 0.56 | 0.770 | [130] | ||
| CNC-infused polyacrylonitrile membrane | Crystal violet | 7 | 4 | 68 | ˗ | 0.993 | ˗ | ˗ | ˗ | [132] | |
| Keratin composite | Direct Red 80 | 2 | 485 | 1111 | 0.043 | 0.995 | 186.8 | 0.28 | 0.944 | [133] | |
| Reactive Black 5 | 2 | 475 | 1250 | 0.028 | 0.992 | 156.8 | 0.33 | 0.976 | [133] | ||
| Alginate hydrogel | Methylene blue | 7 | ˗ | 256.4 | 0.002 | 0.998 | 1.931 | 0.649 | 0.988 | [134] | |
| Polyacrylamide composite | Methylene blue | 6.5 | 19 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [135] | |
| Chitosan-g-poly(acrylic acid) composite (5 wt% CNC) | Methylene blue | 6 | 1968 | 2074 | 0.139 | 0.999 | ˗ | ˗ | ˗ | [136] | |
| Imidazolium grafting | Orange II | - | 98 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [137] | |
| UnmodifiedQuaternization | Methylene blue | 9 | 100 | 122.2 | 0.178 | 0.996 | 12.8 | 0.22 | 0.85 | [138] | |
| Crystal violet | - | 664 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [139] | ||
| Cellulose nanofibers | Unmodified | Acid green 25 | - | 683 | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [139] |
| TEMPO-oxidation (-COO−) TEMPO-periodate-chlorite oxidation | Methylene blue | 7 | - | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | [96] | |
| Methylene blue | 7 | - | 502 | ˗ | ˗ | 64 | ˗ | ˗ | [96] | ||
| Cellulosic Material | Inorganic | Preparation Method | Removal Process | Pollutants | Removal Capacity | References |
|---|---|---|---|---|---|---|
| Cellulose-graft-soy protein isolate | Calcium phosphate–rod-like shape hydroxyapatite | Bio mineralization | Adsorption | Methylene blue | 454 mg/g | [161] |
| Carboxymethyl cellulose | Hydroxyapatite | In situ precipitation | Adsorption | Pb2+ acid yellow 220 | 625 mg/g and 200 mg/g | [97] |
| Carboxymethyl cellulose-graft-polymethacrylic acid | Rod-like hydroxyapatite | Bio mineralization | Adsorption | Methylene blue | 671 mg/g | [121] |
| Carboxymethyl cellulose | Carboxymethyl cellulose, hydroxyapatite and lysine membrane | Conventional casting or double decomposition methods | Adsorption | Bisphenol A | [201] | |
| Cellulose | Montmorillonite | - | Adsorption | Cr (VI) | 22.2 mg/g | [21] |
| Cellulose acetate | Organically modified montmorillonite | - | Adsorption | Cr (VI) | - | [98] |
| Cellulose and carboxymethyl cellulose | Clay | Crosslinking in NaOH/urea aqueous solution | Adsorption | Methylene blue | 98% | [202] |
| Cellulose | GO/cellulose membranes | Pressed membranes | Adsorption | Heavy metals | - | [203] |
| Dialdehyde cellulose | GO | Crosslinked with triethylenetetramine | Adsorption | Cu2+ and Pb2+ | 65.1 and 80.9 mg/g | [102] |
| MCC | GO | GO/microcrystalline cellulose aerogels prepared using lithium bromide | Adsorption | Methylene blue | 2630 mg/g | [204] |
| CNC | rGO | 3D sponge using vitamin C | Adsorption | Methylene blue | 850 mg/g | [160] |
| Cellulose | GO | Wet-spinning technique | Adsorption | Methylene blue | 481mg/g | [161] |
| Bacterial cellulose | GO | Composite membrane | Selective ion permeation | Different inorganic/organic ions | - | [205] |
| Cellulose | GO | Using ionic liquids as solvent | Adsorption | Ce3+ | 109.1 mg/g | [205] |
| Cellulose | CNTs | Grafting Welan gum onto CNTs entrapped cellulose beads | Adsorption | Methylene blue | 300 mg/g | [206] |
| CNCs | Carbon monolith | - | Adsorption | Methylene blue | 127 mg/g | [129] |
| Bacterial cellulose | Fe3O4 | Cellulose-magnetite composites | Adsorption | Cr(VI) | --- | [104] |
| Bacterial cellulose | Fe3O4 | Agitation fermentation | Adsorption | Pb2+, Mn2+ and Cr3+ | 65, 33 and 25 mg/g | [75] |
| Mesquite tree pulp | Fe3O4 | Spherical magnetic NPs through co-precipitation | Adsorption | Methylene blue | 1225 mg/g | [120] |
| Cellulose acetate fibers | Zno NPs with Fe3O4 | Coprecipitation | Adsorption | Phenol | 64% | [157] |
| Carbon microspheres | Magnetic CoFe2O4 | - | Photodegradation | Rhodamine B | - | [194] |
| Cellulose | Fe3O4 | Co-precipitation | Photodegradation | Methylene blue | - | [192] |
| Cellulose | Fe3O4 | Hydrothermal technique | Photodegradation | Rhodamine B | 100% | [196] |
| Carboxyl cellulose | Fe3O4 | Chemical co-precipitation technique | Photodegradation | Navy blue | - | [207] |
| Amidoximated bacterial cellulose | ZnO | In situ polyol method | Photocatalytic property | Methyl orange | 92% | [184] |
| Cellulosic fabric | ZnO | Sol-gel process | Photodegradation | Methylene blue | - | [208] |
| Cellulose | ZnO | Co-precipitation | Photodegradation | Methylene blue | 69.5% | [209] |
| Cellulose | TiO2 | Acids | Photodegradation | - | - | [210] |
| Hydroxypropyl methyl cellulose | TiO2 | Nano-photocatalysts prepared in situ | Photodegradation | 4-nitrophenol | [211] | |
| Bacterial cellulose | TiO2 | - | - | Pb | 90% | [212] |
| CMC | TiO2 | Polyaniline/CMC/TiO2 nanocomposite | Adsorption | Congo red | 94.3 mg/g | [213] |
| Paper | TiO2 | - | Catalytic degradation | Acetaldehyde | - | [214] |
| Regenerated Cellulose | TiO2 | Sol–gel | Photodegradation | Phenol | [195] | |
| Cellulose | BiOBr | - | Photo-degradation | Phenol | 80% | [198] |
| Nanocellulose | Ag | In situ precipitation | Adsorption | Pb2+ Cr3+ and | 99.5%, and 98.3% | [215] |
| Nano Celluloses | Membrane | Pollutants | Thickness (µm) | Porosity (%) | Flux (L.h−1.m−2) | TMP (kPa) | Permeability (L.h−1.m−2.Pa−1) | Efficiency (% or mg·g−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Cellulose nanocrystals | Unmodified CNC | PEG | 20–60 | 35 | - | 200–500 | 1.8 × 10−5–6.1 × 10−5 | 20–75% | [237] |
| Oil | 0.6–10 | - | 1036–1734 | 50 | 2.1 × 10−2–3.5 × 10−2 | 99.9% | [238] | ||
| CNC-coated CNF | Ag+ Cu2+ Fe2+ Fe3+ | 201–210 | 22–23 | 0 | 450 | 0 | 91–94% | [239] | |
| Acetone treated CNC-coated CNF | 201–210 | 29–34 | 8.8–13.9 | 450 | 2.0 × 10−5–3.1 × 10−5 | - | [239] | ||
| Oxidized CNC/gelatin-coated cellulose sludge/CNF | 166–208 | - | - | 450 | 7.7 × 10−5–5.5 × 10−4 | 0.81–0.87 mg·g−1 | [240] | ||
| CNC/gelatin-coated cellulose | 440–448 | 65 | - | 50–150 | 9.5 × 10−3 | 0.33 mg·g−1 | [241] | ||
| Phosphorylated CNC/gelatin-coated cellulose | 440–448 | 65 | - | 50–150 | 2.6 × 10−2 | 0.33 mg·g−1 | [241] | ||
| CNC/chitosan | Methyl violet 2B | 250–270 | - | - | 196 | 6.4 × 10−5 | 90% | [242] | |
| Cross-linked CNC/chitosan | 250–270 | - | - | 196 | 6.4 × 10−5 | 48–91% | [242] | ||
| CNC/chitosan | Rhodamine 6G | 250–270 | - | - | 196 | 6.4 × 10−5 | 69% | [242] | |
| Cross-linked CNC/chitosan | 250–270 | - | - | 196 | 6.4 × 10−5 | 13–70% | [242] | ||
| CNC-impregnated electrospun CA | Victoria blue 2B | 52–116 | 69–73 | 17,838 | 600–800 | 2.069 × 10−2 | 52.6–99.9% | [243] | |
| CNC/chitosan | 250–270 | - | - | 196 | 6.4 × 10−5 | 98% | [242] | ||
| Cross-linked CNC/chitosan | 250–270 | - | - | 196 | 6.4 × 10−5 | 88–98% | [242] | ||
| Oxidized CNC-impregnated electrospun PAN | Phage MS2 | 45 | 80 | 192 | 30 | 6.4 × 10−2 | 99% | [133] | |
| CNC-impregnated electrospun CA | 52–116 | 69–73 | 17,838 | 600–800 | 2.069 × 10−2 | 2.5–25.9% | [243] | ||
| Cellulose nanofibers | Unmodified CNF | Au | 0.496–0.564 | 55.8–68.5 | 0.952–2.2 | - | 1.19 × 10−5–2.75 × 10−5 | 84.6–93.5% | [244] |
| Ferritin (12 nm) | 0.496–0.564 | 55.8–68.5 | 0.952–2.2 | - | 1.19 × 10−5–2.75 × 10−5 | 90.2–94.3% | [244] | ||
| Polyethylene glycol | 25–65 | 35 | - | 200–103 | 10−6–4 × 10−6 | 88–100% | [237] | ||
| 20–70 | 35 | - | 200–103 | 4 × 10−6–2.7 × 10−5 | 88–100% | [80] | |||
| Polystyrene | 20–70 | 35 | - | 200–103 | 4 × 10−6–2.7 × 10−5 | 75–100% | [80] | ||
| Ag+ | 176 | 23–25 | 0–2.9 | 450 | 0–6.4 × 10−6 | 77% | [239] | ||
| Cu2+ | 176 | 23–25 | 0–2.9 | 450 | 0–6.4 × 10−6 | 94% | [239] | ||
| Methylene blue | 0.496–0.564 | 55.8–68.5 | 0.952–2.2 | 1.19 × 10−5–2.75 × 10−5 | 99.22% | [244] | |||
| Oxidized CNF | Polyethylene glycol | 20–70 | 35 | - | 200–103 | 10−6–4 × 10−6 | 58–100% | [237] | |
| Polyethylene glycol Polystyrene | 20–70 | 35 | - | 200–103 | 1.2 × 10−6–5 × 10−6 | 58–95% | [80] | ||
| 20–70 | 35 | - | 200–103 | 1.2 × 10−6–5 × 10−6 | 67–100% | [80] | |||
| Ca2+/SO42− | 20–70 | 35 | - | 200–103 | 10−6–4 × 10−6 | 5–34% | [245] | ||
| Oil | 20–100 | - | 192 | 103 | 1.92 × 10−4 | 92.9–97.9% | [217] | ||
| PAE cross-linked CNF | Polyethylene glycol | 20 | - | 110 | 200 | 5.5 × 10−4 | - | [246] | |
| CNF/silica/PAE | White water effluent | 0.55–0.64 | - | 98–122 | 500–103 | 1.2 × 10−4–2.0 × 10−4 | 93% | [247] | |
| PAE cross-linked oxidized CNF | Ag(I) | 176 | 34–42 | 6.3–8.7 | 450 | 1.4 × 10−5–1.9 × 10−5 | - | [239] | |
| Acetone treated CNF | Cr(VI) | 200 | 78 | - | - | 0.145 | 60% | [222] | |
| Thiol-functionalized CNF- impregnated electrospun PAN | Cr(VI) Cu2+ | 40 | 80 | - | - | 0.189 | 100% | [247] | |
| Aminated CNF-impregnated electrospun PAN/PET | 25 | - | - | 200 | 2.3 × 10−6 | 7.7% | [245] | ||
| Phosphorylated CNF | Cu2+ Pb2+ | 176 | 34–42 | 6.3–8.7 | 450 | 1.4 × 10−5–1.9 × 10−5 | - | [239] | |
| Acetone-treated CNF | 200 | 78 | - | - | 0.145 | 137.7 mg·g−1 | [222] | ||
| Thiol-functionalized CNF- impregnated electrospun PAN | Pb2+ Br− | 40 | 80 | 215 | - | 0.189 | 260 mg·g−1 | [247] | |
| Aminated CNF-impregnated electrospun PAN/PET | 40 - | 80 - | 215 215 | - - | 0.189 - | 260 mg·g−1 89.6 mg·g−1 | [247] [248] | ||
| Oxidized CNF-coated Ti-Bi oxide | |||||||||
| Cs+ | - | - | 215 | - | - | 125.4 mg·g−1 | [248] | ||
| I− | - | - | 215 | - | - | 225.9 mg·g−1 | [248] | ||
| SeO32− | - | - | 215 | - | - | 204.5 mg·g−1 | [248] | ||
| SeO42− | - | - | 215 | - | - | 85.1 mg·g−1 | [248] | ||
| Sr2+ | - | - | 215 | - | - | 81.4 mg·g−1 | [248] | ||
| Polyethylene glycol | 16 | - | 18–54 | 196–392 | 6.9 × 10−5–2.8 × 10−4 | 30–50% | [249] | ||
| Bacterial cellulose (BC) | Unmodified BC | Polyethylene glycol Oil | 15–85 | 35 | - | 200–103 | 5 × 10−6–2 × 10−5 | 60–75% | [237] |
| 20–100 | 95–97 | 441–845 | 103 | 4.41 × 10−4–8.45 × 10−4 | 98.3–99.3% | [7] | |||
| Chlorella sp. | 8–27 | 1.4–2.4 | 3.2–11.7 | 0–220 | 3.2 × 10−5–1.17 × 10−4 | 99.6–99.8% | [249] | ||
| Bovine serum albumin | 8–27 | 1.4–2.4 | 11–81 | 0–220 | 1.1 × 10−4–8.1 × 10−4 | 61.6–99.0% | [249] | ||
| Polyethylene glycol | 16–102 | - | 0.03–6 | 196–392 | 7.6 × 10−8 -1.5 × 10−5 | 90% | [236] | ||
| BC derivative | Polyethylene glycol Inorganic/organic ions | 16–102 | - | 8–15 | 196–392 | 5 × 10−6–5 × 10−5 | 85–90% | [236] | |
| Chitin laminated BC | 11–17 | - | - | - | - | - | [205] | ||
| GO/BC | Reactive red X-3B dye | 14 | - | 1146 | - | - | - | [250] | |
| Cobalt-phthalocyanine immobilized BC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salama, A.; Abouzeid, R.; Leong, W.S.; Jeevanandam, J.; Samyn, P.; Dufresne, A.; Bechelany, M.; Barhoum, A. Nanocellulose-Based Materials for Water Treatment: Adsorption, Photocatalytic Degradation, Disinfection, Antifouling, and Nanofiltration. Nanomaterials 2021, 11, 3008. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11113008
Salama A, Abouzeid R, Leong WS, Jeevanandam J, Samyn P, Dufresne A, Bechelany M, Barhoum A. Nanocellulose-Based Materials for Water Treatment: Adsorption, Photocatalytic Degradation, Disinfection, Antifouling, and Nanofiltration. Nanomaterials. 2021; 11(11):3008. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11113008
Chicago/Turabian StyleSalama, Ahmed, Ragab Abouzeid, Wei Sun Leong, Jaison Jeevanandam, Pieter Samyn, Alain Dufresne, Mikhael Bechelany, and Ahmed Barhoum. 2021. "Nanocellulose-Based Materials for Water Treatment: Adsorption, Photocatalytic Degradation, Disinfection, Antifouling, and Nanofiltration" Nanomaterials 11, no. 11: 3008. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11113008