Nelumbo nucifera Seed–Derived Nitrogen-Doped Hierarchically Porous Carbons as Electrode Materials for High-Performance Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. KOH Activation of Nelumbo Nucifera Precursor
2.2. Characterizations of Nelumbo nucifera–Derived Nanoporous Carbons
2.3. Electrochemical Studies
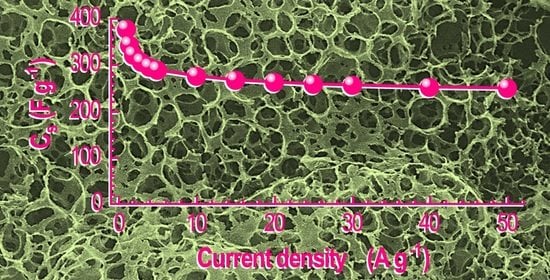
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y. Perspectives for Electrochemical Capacitors and Related Devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef]
- Jakubec, P.; Bartusek, S.; Dvořáček, J.J.; Šedajová, V.; Kupak, V.; Otyepka, M. Flax-Derived Carbon: A Highly Durable Electrode Material for Electrochemical Double-Layer Supercapacitors. Nanomaterials 2021, 11, 2229. [Google Scholar] [CrossRef]
- Zhou, Y.; Qi, H.; Yang, J.; Bo, Z.; Huang, F.; Islam, M.S.; Lu, X.; Dai, L.; Amal, R.; Wang, C.H.; et al. Two-Birds-One-Stone: Multifunctional Supercapacitors Beyond Traditional Energy Storage. Energy Environ. Sci. 2021, 14, 1854–1896. [Google Scholar] [CrossRef]
- Arena, A.; Branca, C.; Ciofi, C.; D’Angelo, G.; Romano, V.; Scandurra, G. Polypyrrole and Graphene Nanoplatelets Inks as Electrodes for Flexible Solid-State Supercapacitor. Nanomaterials 2021, 11, 2589. [Google Scholar] [CrossRef] [PubMed]
- Mirabedini, A.; Lu, Z.; Mostafavian, S.; Foroughi, J. Triaxial Carbon Nanotube/Conducting Polymer Wet-Spun Fibers Supercapacitors for Wearable Electronics. Nanomaterials 2021, 11, 3. [Google Scholar] [CrossRef]
- Wang, T.; Hu, S.; Wu, D.; Zhao, W.; Yu, W.; Wang, M.; Xu, J.; Zhang, J. Boosting the Capacity of Biomass-Based Supercapacitors using Carbon Materials of Wood Derivatives and Redox Molecules from Plant. J. Mater. Chem. A 2021, 9, 11839–11852. [Google Scholar] [CrossRef]
- Huang, T.-F.; Hsieh, T.-H.; Tseng, F.-S.; Wang, L.-Y.; Yang, C.-C.; Yang, C.-C. High-Mass Loading Hierarchically Porous Activated Carbon Electrode for Pouch-Type Supercapacitors with Propylene Carbonate-Based Electrolyte. Nanomaterials 2021, 11, 785. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Thakur, A.K.; Adhikari, A.D.; Zhu, Y.; Wang, N. Current Research of Graphene-Based Nanocomposites and Their Application for Supercapacitors. Nanomaterials 2020, 10, 2046. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.G.; Maji, S.; Shrestha, L.K.; Ariga, K. Nanoarchitectonics of Nanoporous Carbon Materials in Supercapacitors Applications. Nanomaterials 2020, 10, 639. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest Advances in Supercapacitors: From New Electrode Materials to Novel Device Designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef]
- Tagliaferri, S.; Nagaraju, G.; Panagiotopoulos, A.; Och, M.; Cheng, G.; Iacoviello, F.; Mattevi, C. Aqueous Inks of Pristine Graphene for 3D Printed Microsupercapacitors with High Capacitance. ACS Nano 2021, 15, 15342–15353. [Google Scholar] [CrossRef]
- Fite, M.C.; Rao, J.-Y.; Imae, T. Effect of External Magnetic Field on Hybrid Supercapacitors of Nitrogen-Doped Graphene with Magnetic Metal Oxides. Bull. Chem. Soc. Jpn. 2020, 93, 1139–1149. [Google Scholar] [CrossRef]
- Mao, W.; Yue, W.; Xu, Z.; Chang, S.; Hu, Q.; Pei, F.; Huang, Z.; Zhang, J.; Li, D.; Liu, G.; et al. Development of a Synergistic Activation Strategy for the Pilot-Scale Construction of Hierarchical Porous Graphitic Carbon for Energy Storage Applications. ACS Nano 2020, 14, 4741–4754. [Google Scholar] [CrossRef]
- Chen, Q.; Tan, X.; Liu, Y.; Liu, S.; Li, M.; Gu, Y.; Zhang, P.; Ye, S.; Yang, Z.; Yang, Y. Biomass-Derived Porous Graphitic Carbon Materials for Energy and Environmental Applications. J. Mater. Chem. A 2020, 8, 5773–5811. [Google Scholar] [CrossRef]
- Singh, G.; Bahadur, R.; Ruban, A.M.; Davidraj, J.M.; Su, D.; Vinu, A. Synthesis of Functionalized Nanoporous Biocarbons with High Surface Area for CO2 Capture and Supercapacitor Applications. Green Chem. 2021, 23, 5571–5583. [Google Scholar] [CrossRef]
- Joseph, S.; Saianand, G.; Benzigar, M.R.; Ramadass, K.; Sing, G.; Gopalan, A.-I.; Yang, J.H.; Mori, T.; Al-Muhtaseb, A.H.; Yi, J.; et al. Recent Advances in Functionalized Nanoporous Carbons Derived from Waste Resources and Their Applications in Energy and Environment. Adv. Sustain. Syst. 2021, 5, 2000169. [Google Scholar] [CrossRef]
- Qian, Q.; Lin, D.; Zhao, X.; Han, F. Vertically Oriented Grid-like Reduced Graphene Oxide for Ultrahigh Power Supercapacitor. Bull. Chem. Soc. Jpn. 2019, 48, 824–827. [Google Scholar] [CrossRef]
- Sahoo, S.; Krishnamoorthy, K.; Pazhamalai, P.; Mariappan, V.K.; Manoharan, S.; Kim, S.-J. High Performance Self-Charging Supercapacitors using a Porous PVDF-Ionic Liquid Electrolyte Sandwiched between Two-Dimensional Graphene Electrodes. J. Mater. Chem. A 2019, 7, 21693–21703. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Nandi, A.K. A Review on the Recent Advances in Hybrid Supercapacitor. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Aken, K.L.V.; Deidaghi, M.; Gogotsi, Y. Formulation of Ionic-Liquid Electrolyte to Expand the Voltage Window of Supercapacitors. Angew. Chem. Int. Ed. 2015, 54, 4806–4809. [Google Scholar] [CrossRef]
- Pan, S.; Yao, M.; Zhang, J.; Li, B.; Xing, C.; Song, X.; Su, P.; Zhang, H. Recognition of Ionic Liquids as High-Voltage Electrolytes for Supercapacitors. Front. Chem. 2020, 8, 261. [Google Scholar] [CrossRef]
- Li, H.; Tao, Y.; Zheng, X.; Luo, J.; Kang, F.; Cheng, H.-M.; Yang, Q.-H. Ultra-Thick Graphene Bulk Supercapacitor Electrode for Compact Energy Storage. Energy Environ. Sci. 2016, 9, 3135–3142. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.-W. Hierarchically Porous Carbon Derived from Polymers and Biomass: Effect of Interconnected Pores on Energy Applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent Advances in Functionalized Micro and Mesoporous Carbon Materials: Synthesis and Applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Su, F.; Xie, L.; Guo, X.; Wang, Z.; Kong, Q.; Sun, G.; Ahmad, A.; Li, X.; Yi, Z.; et al. Effect of Pore Structure and Doping Species on Charge Storage Mechanisms in Porous Carbon-Based Supercapacitors. Mater. Chem. Front. 2020, 4, 2610–2634. [Google Scholar] [CrossRef]
- Lyu, L.; Seong, K.-d.; Ko, D.; Choi, J.; Lee, C.; Hwang, T.; Cho, Y.; Jin, X.; Zhang, W.; Pang, H.; et al. Recent Development of Biomass-Derived Carbons and Composites as Electrode Materials for Supercapacitors. Mater. Chem. Front. 2019, 3, 2543–2570. [Google Scholar] [CrossRef]
- Jin, Y.; Tian, K.; Wei, L.; Zhang, X.; Guo, X. Hierarchical Porous Microspheres of Activated Carbon with a High Surface Area from Spores for Electrochemical Double-Layer Capacitors. J. Mater. Chem. A 2016, 4, 15968–15979. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Shrestha, R.G.; Yamauchi, Y.; Hill, J.P.; Nishimura, T.; Miyazawa, K.; Kawai, T.; Okada, S.; Wakabayashi, K.; Ariga, K. Nanoporous Carbon Tubes from Fullerene Crystals as the π-Electron Carbon Source. Angew. Chem. Int. Ed. 2015, 54, 951–955. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Shrestha, R.G.; Hill, J.P.; Tsuruoka, T.; Ji, Q.; Nishimura, T.; Ariga, K. Surfactant-Triggered Nanoarchitectonics of Fullerene C60 Crystals at a Liquid−Liquid Interface. Langmuir 2016, 32, 12511–12519. [Google Scholar] [CrossRef]
- Bairi, P.; Maji, S.; Hill, J.P.; Kim, J.H.; Ariga, K.; Shrestha, L.K. Mesoporous Carbon Cubes Derivied from Fullerene Crystals as a High Rate Performance Electrode Materials for Sueprcapacitors. J. Mater. Chem. A 2019, 7, 12654–12660. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Joseph, S.; Ilbeygi, H.; Park, D.-H.; Sarkar, S.; Chandra, G.; Umapathy, S.; Srinivasan, S.; Talapaneni, S.; Vinu, A. Highly Crystalline Mesoporous C60 with Ordered Pores: A Class of Nanomaterials for Energy Applications. Angew. Chem. Int. Ed. 2018, 57, 569–573. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Joseph, S.; Baskar, A.V.; Park, D.-H.; Chandra, G.; Umapathy, S.; Talapaneni, N.; Vinu, A. Ordered Mesoporous C70 with Highly Crystalline Pore Walls for Energy Applications. Adv. Funct. Mater. 2018, 28, 1803701. [Google Scholar] [CrossRef]
- Lu, Z.; Raad, R.; Safaei, F.; Xi, J.; Liu, Z.; Foroughi, J. Carbon Nanotube Based Fiber Supercapacitor as Wearable Energy Storage. Front. Mater. 2019, 6, 138. [Google Scholar] [CrossRef]
- Sengottaiyan, C.; Jayavel, R.; Shrestha, R.G.; Subramani, T.; Maji, S.; Kim, J.H.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Indium Oxide/Carbon Nanotube/Reduced Graphene Oxide Ternary Nanocomposite with Enhanced Electrochemical Supercapacitance. Bull. Chem. Soc. Jpn. 2019, 92, 521–528. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Kim, J.; Tang, J.; Na, J.; Kang, Y.-M.; Kim, M.; Lim, H.; Bando, Y.; Li, J.; Yamauchi, Y. Large-Scale Synthesis of MOF-Derived Superporous Carbon Aerogels with Extraordinary Adsorption Capacity for Organic Solvents. Angew. Chem. Int. Ed. 2020, 59, 2066–2070. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Salunkhe, R.R.; Zhang, H.; Malgras, V.; Ahamad, T.; Alshehri, S.M.; Kobayashi, N.; Tominaka, S.; Ide, Y.; Kim, J.H.; et al. Bimetallic Metal-Organic Frameworks for Controlled Catalytic Graphitization of Nanoporous Carbons. Sci. Rep. 2016, 6, 30295. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.; Tang, J.; Kim, M.; Lim, H.; Malgras, V.; You, J.; Xu, Q.; Li, J.; Yamauchi, Y. New strategies for novel MOF-derived carbon materials based on nanoarchitectures. Chem 2020, 6, 19–40. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, X.S. Biomass-Derived Carbon Electrode Materials for Supercapacitors. Sustain. Energy Fuels 2017, 1, 1265–1281. [Google Scholar] [CrossRef]
- Lin, T.; Chen, I.-W.; Liu, F.; Yang, C.; Bi, H.; Xu, F.; Huang, F. Nitrogen-Doped Mesoporous Carbon of Extraordinary Capacitance for Electrochemical Energy Storage. Science 2015, 350, 1508–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.; Xu, X.; Zhang, S.; Pan, L.; Wang, Y.; Alshehri, S.M.; Ahamad, T.; Kim, M.; Na, J.; Hossain, M.d.S.A.; et al. High-Performance Capacitive Deionization by Lignocellulose-Derived Eco-Friendly Porous Carbon Materials. Bull. Chem. Soc. Jpn. 2020, 93, 1014–1019. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Z.; Liu, Y.; Fan, L.-Z. Biowaste-Derived 3D Honeycomb-Like Porous Carbon with Binary-Heteroatom Doping for High-Performance Flexible Solid-State Supercapacitors. J. Mater. Chem. A 2018, 6, 160–166. [Google Scholar] [CrossRef]
- Yao, Y.; Feng, Q.; Huo, B.; Zhou, H.; Huang, Z.; Li, H.; Yan, Z.; Yang, X.; Kuang, Y. Facile Self-Templating Synthesis of Heteroatom-Doped 3D Porous Carbon Materials from Waste Biomass for Supercapacitors. Chem. Commun. 2020, 56, 11689–11692. [Google Scholar] [CrossRef]
- Pang, M.; Jiang, S.; Zhao, J.; Zhang, S.; Wang, R.; Li, N.; Liu, R.; Pan, Q.; Qu, W.; Xing, B. “Water-in-Salt” Electrolyte Enhanced High Voltage Aqueous Supercapacitor with Carbon Electrodes Derived from Biomass Waste-Ground Grain Hulls. RSC Adv. 2020, 10, 35545–35556. [Google Scholar] [CrossRef]
- Shrestha, R.L.; Chaudhary, R.; Shrestha, R.G.; Shrestha, T.; Maji, S.; Ariga, K.; Shrestha, L.K. Washnut Seed–Derived Ultrahigh Surface Area Nanoporous Carbons as High Rate Performance Electrode Material for Supercapacitors. Bull. Chem. Soc. Jpn. 2021, 94, 565–572. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Phuriragpitikhon, J.; Choma, J.; Jaroniec, M. Recent Advances in the Development and Applications of Biomass-Derived Carbons with Uniform Porosity. J. Mater. Chem. A 2020, 8, 18464–18491. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Shrestha, R.G.; Maji, S.; Pokharel, B.P.; Rajbhandari, R.; Shrestha, R.L.; Pradhananga, R.; Hill, J.P.; Ariga, K. High Surface Area Nanoporous Graphitic Carbon Materials Derived from Lapsi Seed with Enhanced Supercapacitance. Nanomaterials 2020, 10, 728. [Google Scholar] [CrossRef] [Green Version]
- Minakshi, M.; Meyrick, D.; Appadoo, D. Maricite (NaMn1/3Ni1/3Co1/3PO4)/Activated Carbon: Hybrid Capacitor. Energy Fuels 2013, 27, 3516–3522. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, M.; Zhang, Y.; Zhao, W. A Biomass-Derived Nitrogen-Doped Porous Carbon for High-Energy Supercapacitor. Carbon 2018, 140, 404–412. [Google Scholar] [CrossRef]
- Wu, F.; Gao, J.; Zhai, X.; Xie, M.; Sun, Y.; Kang, H.; Tian, Q.; Qiu, H. Hierarchical Porous Carbon Microrods Derived from Albizia Flower for High Performance Supercapacitors. Carbon 2019, 147, 242–251. [Google Scholar] [CrossRef]
- Chen, H.; Wang, G.; Chen, L.; Dai, B.; Yu, F. Three-Dimensional Honeycomb-Like Porous Carbon with Both Interconnected Hierarchical Porosity and Nitrogen Self-Doping from Cotton Seed Husk for Supercapacitor Electrode. Nanomaterials 2018, 8, 412. [Google Scholar] [CrossRef] [Green Version]
- Wickramaarachchi, W.A.M.K.P.; Minakshi, M.; Gao, X.; Dabare, R.; Wong, K.W. Hierarchical Porous Carbon from Mango Seed Husk for Electro-Chemical Energy Storage. Chem. Eng. J. Adv. 2021, 8, 100158. [Google Scholar] [CrossRef]
- Shrestha, R.L.; Chaudhary, R.; Shrestha, T.; Tamrakar, B.M.; Shrestha, R.G.; Maji, S.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Nanoarchitectonics of Lotus Seed Derived Nanoporous Carbon Materials for Supercapacitor Applications. Materials 2020, 13, 5434. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and Quantitative Analysis of Lignocellulosic Biomass Using Infrared Techniques: A Mini-Review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active Sites of Nitrogen-Doped Carbon Materials for Oxygen Reduction Reaction Clarified using Model Catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Mehra, P.; Singh, C.; Cherian, I.; Giri, A.; Paul, A. Diciphering the Incredible Supercapacitor Performance Conducting Biordered Ultramicroporous Graphitic Carbon. ACS Appl. Energy Mater. 2021, 4, 4416–4427. [Google Scholar] [CrossRef]
- Golden, T.C.; Sircar, S. Activated Carbon Adsorbent for PSA Driers. Carbon 1990, 28, 683–690. [Google Scholar] [CrossRef]
- Schneider, P.; Hudec, P.; Solcova, O. Pore-Volume and Surface Area in Microporous–Mesoporous Solids. Microporous Mesoporous Mater. 2008, 115, 491–496. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for Electrochemical Capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Karnan, M.; Subramani, K.; Sudhan, N.; Ilayaraja, N.; Sathish, M. Aloe Vera Derived Activated High-Surface-Area Carbon for Flexible and High-Energy Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 35191–35202. [Google Scholar] [CrossRef]
- Liu, X.; Mi, R.; Yuan, L.; Yang, F.; Fu, Z.; Wang, C.; Tang, Y. Nitrogen-Doped Multi-Scale Porous Carbo for High Voltage Aqueous Supercapacitors. Fornt. Chem. 2018, 6, 475. [Google Scholar]
- Jäckel, N.; Simon, P.; Gogotsi, Y.; Presser, V. Increase in Capacitance by Subnanometer Pores in Carbon. ACS Energy Lett. 2016, 1, 1262–1265. [Google Scholar] [CrossRef] [Green Version]
- Salanne, M.; Rotenberg, B.; Naoi, K.; Kaneko, K.; Taberna, P.L.; Grey, C.P.; Dunn, B.; Simon, P. Efficient Storage Mechanism for Building Better Supercapacitors. Nat. Energy 2016, 1, 16070. [Google Scholar] [CrossRef]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]








| Sample | SSA (m2 g−1) | Smicro (m2 g−1) | Smeso (m2 g−1) | Vp (cm3 g−1) | Vmicro (cm3 g−1) | Wp (nm) | Dp (nm) |
|---|---|---|---|---|---|---|---|
| LTS_800 | 46.1 | 18.8 | 27.3 | 0.102 | 0.044 | - | 3.09 |
| LTSC_K600 | 1059.6 | 824.5 | 235.1 | 0.819 | 0.472 | 0.285 | 3.92 |
| LTSC_K700 | 1878.4 | 1556.1 | 322.3 | 1.232 | 0.775 | 0.286 | 3.93 |
| LTSC_K800 | 2236.6 | 1891.3 | 345.3 | 1.499 | 1.034 | 0.274 | 3.91 |
| LTSC_K900 | 2330.1 | 1905.7 | 424.4 | 1.793 | 1.206 | 0.286 | 3.92 |
| LTSC_K1000 | 2489.3 | 1725.6 | 763.7 | 2.384 | 1.488 | 0.705 | 3.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, L.K.; Shrestha, R.G.; Chaudhary, R.; Pradhananga, R.R.; Tamrakar, B.M.; Shrestha, T.; Maji, S.; Shrestha, R.L.; Ariga, K. Nelumbo nucifera Seed–Derived Nitrogen-Doped Hierarchically Porous Carbons as Electrode Materials for High-Performance Supercapacitors. Nanomaterials 2021, 11, 3175. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123175
Shrestha LK, Shrestha RG, Chaudhary R, Pradhananga RR, Tamrakar BM, Shrestha T, Maji S, Shrestha RL, Ariga K. Nelumbo nucifera Seed–Derived Nitrogen-Doped Hierarchically Porous Carbons as Electrode Materials for High-Performance Supercapacitors. Nanomaterials. 2021; 11(12):3175. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123175
Chicago/Turabian StyleShrestha, Lok Kumar, Rekha Goswami Shrestha, Rashma Chaudhary, Raja Ram Pradhananga, Birendra Man Tamrakar, Timila Shrestha, Subrata Maji, Ram Lal Shrestha, and Katsuhiko Ariga. 2021. "Nelumbo nucifera Seed–Derived Nitrogen-Doped Hierarchically Porous Carbons as Electrode Materials for High-Performance Supercapacitors" Nanomaterials 11, no. 12: 3175. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123175