Label-Free, Rapid and Facile Gold-Nanoparticles-Based Assay as a Potential Spectroscopic Tool for Trastuzumab Quantification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis, Characterization, and Functionalization of AuNPs
2.3. Incorporation of Co2+ Cations and Immobilization of Histidine-Tagged HER2 Protein
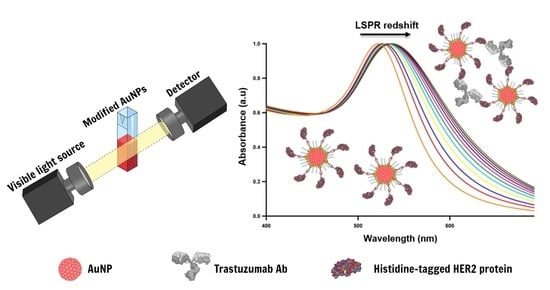
2.4. LSPR-Based Detection of Trastuzumab using His-Tagged HER2 Decorated AuNPs
3. Results and Discussion
3.1. Characterization of Synthesized AuNPs
3.2. Passivation of AuNPs with NTA/TOEG3 SAM
3.3. Incorporation of Co2+ ations and Immobilization of the His-Tagged HER2 Protein
3.4. Detection of Trastuzumab by LSPR in Buffer
3.5. Evaluating the Specificity of the Platform
3.6. Elucidating the Impact of Free His-Tagged HER2 Proteins on LSPR Response
3.7. Evaluating the LSPR Response upon Tuning the Density of the Surface Grafted SAM
3.8. Evaluating Assessment of Trastuzumab detection by LSPR in Human serum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dziawer, Ł.; Majkowska-Pilip, A.; Gaweł, D.; Godlewska, M.; Pruszyński, M.; Jastrzębski, J.; Wąs, B.; Bilewicz, A. Trastuzumab-modified gold nanoparticles labeled with 211At as a prospective tool for local treatment of HER2-positive breast cancer. Nanomaterials 2019, 9, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitri, Z.; Constantine, T.; O’Regan, R. The HER2 receptor in breast cancer: Pathophysiology, clinical use, and new advances in therapy. Chemother. Res. Pract. 2012, 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Casalini, P.; Iorio, M.V.; Galmozzi, E.; Ménard, S. Role of HER receptors family in development and differentiation. J. Cell. Physiol. 2004, 200, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Labidi, S.; Mejri, N.; Lagha, A.; Daoud, N.; El Benna, H.; Afrit, M.; Boussen, H. Targeted therapies in HER2-overexpressing metastatic breast cancer. Breast Care 2016, 11, 418–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudis, C.A. Trastuzumab—Mechanism of action and use in clinical practice. N. Engl. J. Med. 2007, 357, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Orlova, A.; Wållberg, H.; Stone-Elander, S.; Tolmachev, V. On the selection of a tracer for PET imaging of HER2-expressing tumors: Direct comparison of a 124I-labeled affibody molecule and trastuzumab in a murine xenograft model. J. Nucl. Med. 2009, 50, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Nahta, R.; Takahashi, T.; Ueno, N.T.; Hung, M.-C.; Esteva, F.J. P27kip1 down-regulation is associated with trastuzumab resistance in breast cancer cells. Cancer Res. 2004, 64, 3981–3986. [Google Scholar] [CrossRef] [Green Version]
- Ewer, M.S.; Gibbs, H.R.; Swafford, J.; Benjamin, R.S. Cardiotoxicity in patients receiving Transtuzumab (Herceptin): Primary toxicity, synergistic or sequential stress, or surveillance artifact? Semin. Oncol. 1999, 26, 96–101. [Google Scholar]
- Jerusalem, G.; Lancellotti, P.; Kim, S.-B. HER2+ breast cancer treatment and cardiotoxicity: Monitoring and management. Breast Cancer Res. Treat. 2019, 177, 237–250. [Google Scholar] [CrossRef] [Green Version]
- González García, J.; Gutiérrez Nicolás, F.; Ramos Díaz, R.; Nazco Casariego, G.J.; Viña Romero, M.M.; Llabres Martinez, M.; Llanos Munoz, M.; Batista Lopez, J.N.; Jiménez Sosa, A.; Ceballos Lenza, I. Pharmacokinetics of trastuzumab after subcutaneous and intravenous administration in obese patients. Ann. Pharmacother. 2020, 54, 775–779. [Google Scholar] [CrossRef]
- Cosson, V.F.; Ng, V.W.; Lehle, M.; Lum, B.L. Population pharmacokinetics and exposure–response analyses of trastuzumab in patients with advanced gastric or gastroesophageal junction cancer. Cancer Chemother. Pharmacol. 2014, 73, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, B.; Lunardi, G.; Millo, E.; Armirotti, A.; Damonte, G.; Profumo, A.; Gori, S.; Iacono, G.; Levaggi, A.; Del Mastro, L. Trastuzumab quantification in serum: A new, rapid, robust ELISA assay based on a mimetic peptide that specifically recognizes trastuzumab. Anal. Bioanal. Chem. 2014, 406, 4557–4561. [Google Scholar] [CrossRef] [PubMed]
- Damen, C.W.; Derissen, E.J.; Schellens, J.H.; Rosing, H.; Beijnen, J.H. The bioanalysis of the monoclonal antibody trastuzumab by high-performance liquid chromatography with fluorescence detection after immuno-affinity purification from human serum. J. Pharm. Biomed. Anal. 2009, 50, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Cetin, A.E.; Coskun, A.F.; Galarreta, B.C.; Huang, M.; Herman, D.; Ozcan, A.; Altug, H. Handheld high-throughput plasmonic biosensor using computational on-chip imaging. Light: Sci. Appl. 2014, 3, e122. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Koduru, J.R.; Desai, M.L.; Park, T.J.; Singhal, R.K.; Basu, H. Recent progress on surface chemistry of plasmonic metal nanoparticles for colorimetric assay of drugs in pharmaceutical and biological samples. TrAC Trends Anal. Chem. 2018, 105, 106–120. [Google Scholar] [CrossRef]
- Tseng, S.-Y.; Li, S.-Y.; Yi, S.-Y.; Sun, A.Y.; Gao, D.-Y.; Wan, D. Food quality monitor: Paper-based plasmonic sensors prepared through reversal nanoimprinting for rapid detection of biogenic amine odorants. ACS Appl. Mater. Interfaces 2017, 9, 17306–17316. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 308–319. [Google Scholar]
- Jin, R.; Wu, G.; Li, Z.; Mirkin, C.A.; Schatz, G.C. What controls the melting properties of DNA-linked gold nanoparticle assemblies? J. Am. Chem. Soc. 2003, 125, 1643–1654. [Google Scholar] [CrossRef]
- Swartz, J.D.; Gulka, C.P.; Haselton, F.R.; Wright, D.W. Development of a histidine-targeted spectrophotometric sensor using Ni (II) NTA-functionalized Au and Ag nanoparticles. Langmuir 2011, 27, 15330–15339. [Google Scholar] [CrossRef]
- Ventura, B.D.; Cennamo, M.; Minopoli, A.; Campanile, R.; Censi, S.B.; Terracciano, D.; Portella, G.; Velotta, R. Colorimetric test for fast detection of SARS-CoV-2 in nasal and throat swabs. ACS Sensors 2020, 5, 3043–3048. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Rusmini, F.; Zhong, Z.; Feijen, J. Protein immobilization strategies for protein biochips. Biomacromolecules 2007, 8, 1775–1789. [Google Scholar] [CrossRef]
- Terpe, K. Overview of tag protein fusions: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2003, 60, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Takahashi, H.K.; Yamaoka, K.; Okamoto, M.; Nishibori, M. High affinity binding of serum histidine-rich glycoprotein to nickel-nitrilotriacetic acid: The application to microquantification. Life Sci. 2003, 73, 93–102. [Google Scholar] [CrossRef]
- Mori, S.; Shinohata, R.; Renbutsu, M.; Takahashi, H.K.; Fang, Y.-I.; Yamaoka, K.; Okamoto, M.; Yamamoto, I.; Nishibori, M. Histidine-rich glycoprotein plus zinc reverses growth inhibition of vascular smooth muscle cells by heparin. Cell Tissue Res. 2003, 312, 353–359. [Google Scholar] [CrossRef]
- Crowe, J.; Masone, B.S.; Ribbe, J. One-step purification of recombinant proteins with the 6xHis tag and Ni-NTA resin. Mol. Biotechnol. 1995, 4, 247–258. [Google Scholar] [CrossRef]
- Chao, A.; Jiang, N.; Yang, Y.; Li, H.; Sun, H. A Ni-NTA-based red fluorescence probe for protein labelling in live cells. J. Mater. Chem. B 2017, 5, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; He, M.; Taussig, M.J. Double-hexahistidine tag with high-affinity binding for protein immobilization, purification, and detection on Ni− nitrilotriacetic acid surfaces. Anal. Chem. 2006, 78, 3072–3079. [Google Scholar] [CrossRef]
- El Alami, A.; Lagarde, F.; Huo, Q.; Zheng, T.; Baitoul, M.; Daniel, P. Acetylcholine and acetylcholinesterase inhibitors detection using gold nanoparticles coupled with dynamic light scattering. Sens. Int. 2020, 1, 100007. [Google Scholar] [CrossRef]
- Arcas, A.S.; Jaramillo, L.; Costa, N.S.; Allil, R.C.S.; Werneck, M.M. Localized surface plasmon resonance-based biosensor on gold nanoparticles for Taenia solium detection. Appl. Opt. 2021, 60, 8137–8144. [Google Scholar] [CrossRef]
- Deka, J.; Mojumdar, A.; Parisse, P.; Onesti, S.; Casalis, L. DNA-conjugated gold nanoparticles based colorimetric assay to assess helicase activity: A novel route to screen potential helicase inhibitors. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Xu, K.; Gu, H.; Zhong, X.; Guo, Z.; Zheng, R.; Zhang, X.; Xu, B. Nitrilotriacetic acid-modified magnetic nanoparticles as a general agent to bind histidine-tagged proteins. J. Am. Chem. Soc. 2004, 126, 3392–3393. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Lin, Y.-S.; Tsai, P.-J.; Chen, C.-T.; Chen, W.-Y.; Chen, Y.-C. Nitrilotriacetic acid-coated magnetic nanoparticles as affinity probes for enrichment of histidine-tagged proteins and phosphorylated peptides. Anal. Chem. 2007, 79, 7519–7525. [Google Scholar] [CrossRef] [PubMed]
- Sosibo, N.M.; Tshikhudo, R.T.; Revaprasadu, N. Stable, hydrophilic nitrilotriacetic acid-capped gold monolayer protected clusters. In MRS Online Proceedings Library (OPL); Cambridge University Press: Cambridge, UK, 2007; Volume 1064. [Google Scholar]
- Sanavio, B.; Scaini, D.; Grunwald, C.; Legname, G.; Scoles, G.; Casalis, L. Oriented immobilization of Prion protein demonstrated via precise interfacial nanostructure measurements. ACS Nano 2010, 4, 6607–6616. [Google Scholar] [CrossRef]
- Schollbach, M.; Zhang, F.; Roosen-Runge, F.; Skoda, M.W.; Jacobs, R.M.; Schreiber, F. Gold nanoparticles decorated with oligo (ethylene glycol) thiols: Surface charges and interactions with proteins in solution. J. Colloid Interface Sci. 2014, 426, 31–38. [Google Scholar] [CrossRef]
- Baselga, J.; Carbonell, X.; Castañeda-Soto, N.-J.; Clemens, M.; Green, M.; Harvey, V.; Morales, S.; Barton, C.; Ghahramani, P. Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J. Clin. Oncol. 2005, 23, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape-and size-dependent refractive index sensitivity of gold nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef]
- Cao, C.; Sim, S.J. Preparation of highly stable oligo (ethylene glycol) derivatives-functionalized gold nanoparticles and their application in LSPR-based detection of PSA/ACT complex. J. Nanosci. Nanotechnol. 2007, 7, 3754–3757. [Google Scholar] [CrossRef]
- Han, J.-H.; Li, F.; Gunawan, R.C. Development of homogeneous plasmonic potency assay using gold nanoparticle immunocomplexes. J. Pharm. Biomed. Anal. 2020, 181, 113101. [Google Scholar] [CrossRef]
- Yoshimura, K.; Maeda, M.; Kamiya, N.; Zako, T. Protein-functionalized gold nanoparticles for antibody detection using the darkfield microscopic observation of nanoparticle aggregation. Anal. Sci. 2020, 37, 507–511. [Google Scholar] [CrossRef]
- Duan, Y.; Wu, W.; Zhao, Q.; Liu, S.; Liu, H.; Huang, M.; Wang, T.; Liang, M.; Wang, Z. Enzyme-antibody-modified gold nanoparticle probes for the ultrasensitive detection of nucleocapsid protein in SFTSV. Int. J. Environ. Res. Public Health 2020, 17, 4427. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsadig, A.; Vondracek, H.; Pengo, P.; Pasquato, L.; Posocco, P.; Parisse, P.; Casalis, L. Label-Free, Rapid and Facile Gold-Nanoparticles-Based Assay as a Potential Spectroscopic Tool for Trastuzumab Quantification. Nanomaterials 2021, 11, 3181. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123181
Alsadig A, Vondracek H, Pengo P, Pasquato L, Posocco P, Parisse P, Casalis L. Label-Free, Rapid and Facile Gold-Nanoparticles-Based Assay as a Potential Spectroscopic Tool for Trastuzumab Quantification. Nanomaterials. 2021; 11(12):3181. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123181
Chicago/Turabian StyleAlsadig, Ahmed, Hendrik Vondracek, Paolo Pengo, Lucia Pasquato, Paola Posocco, Pietro Parisse, and Loredana Casalis. 2021. "Label-Free, Rapid and Facile Gold-Nanoparticles-Based Assay as a Potential Spectroscopic Tool for Trastuzumab Quantification" Nanomaterials 11, no. 12: 3181. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123181