Recent Advances in the Surface Functionalization of PLGA-Based Nanomedicines
Abstract
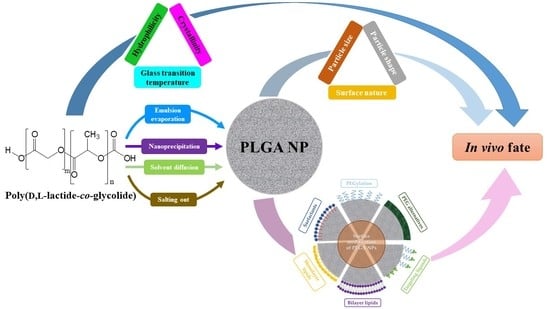
:1. Introduction
2. Physicochemical Properties of PLGA
3. Biodegradation of the PLGA Copolymer
4. Preparation of PLGA NPs
4.1. Emulsion Evaporation Method
4.2. Nanoprecipitation Method
4.3. Solvent Diffusion Method
4.4. Salting Out Method
4.5. Emerging Production Methods
5. Biological Fate of PLGA NPs
6. Surface Modification Strategies
6.1. Surfactants
6.2. PEGylation of PLGA
6.3. PEG Alternatives
6.4. Lipids
6.5. Surface Functionalization with Targeting Ligands
6.5.1. Antibodies
6.5.2. Biotin
6.5.3. Bisphosphonate
6.5.4. Folate
6.5.5. Lectins
6.5.6. Mannan
6.5.7. Nucleotides
6.5.8. Peptides
6.5.9. Sialic Aid
6.5.10. Transferrin
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, properties, and regulatory issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Systemic review of biodegradable nanomaterials in nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Arias, J.L. Advanced Engineering Approaches in the Development of PLGA-Based Nanomedicines. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer International Publishing AG: Cham, Switzerland, 2016; Chapter 39; pp. 1009–1039. [Google Scholar] [CrossRef]
- Sharma, S.; Parmar, A.; Kori, S.; Sandhir, R. PLGA-based nanoparticles: A new paradigm in biomedical applications. TrAC Trends Anal. Chem. 2016, 80, 30–40. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-based nanoparticles in cancer treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [Green Version]
- Roointan, A.; Kianpour, K.; Memari, F.; Gandomani, M.; Hayat, S.M.G.; Mohammadi-Samani, S. Poly(lactic-co-glycolic acid): The most ardent and flexible candidate in biomedicine! Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 1028–1049. [Google Scholar] [CrossRef]
- Swider, E.; Koshkina, O.; Tel, J.; Cruz, L.J.; de Vries, I.J.M.; Srinivas, M. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018, 73, 38–51. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Essa, D.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. The design of poly(lactide-co-glycolide) nanocarriers for medical applications. Front. Bioeng. Biotechnol. 2020, 8, 48. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Small-Howard, A.L.; Fernández-Arévalo, M.; Martín-Banderas, L. Development of enhanced drug delivery vehicles for three cannabis-based terpenes using poly(lactic-co-glycolic acid) based nanoparticles. Ind. Crop. Prod. 2021, 164, 113345. [Google Scholar] [CrossRef]
- Mares, A.G.; Pacassoni, G.; Marti, J.S.; Pujals, S.; Albertazzi, L. Formulation of tunable size PLGA-PEG nanoparticles for drug delivery using microfluidic technology. PLoS ONE 2021, 16, e0251821. [Google Scholar] [CrossRef]
- Jimenez-Lopez, J.; El-Hammadi, M.M.; Ortiz, R.; Cayero-Otero, M.D.; Cabeza, L.; Perazzoli, G.; Martin-Banderas, L.; Baeyens, J.M.; Prados, J.; Melguizo, C. A novel nanoformulation of PLGA with high non-ionic surfactant content improves in vitro and in vivo PTX activity against lung cancer. Pharmacol. Res. 2019, 141, 451–465. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Delgado, A.V.; Melguizo, C.; Prados, J.C.; Arias, J.L. Folic acid-decorated and PEGylated PLGA nanoparticles for improving the antitumour activity of 5-fluorouracil. Int. J. Pharm. 2017, 516, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Roces, C.B.; Christensen, D.; Perrie, Y. Translating the fabrication of protein-loaded poly(lactic-co-glycolic acid) nanoparticles from bench to scale-independent production using microfluidics. Drug Deliv. Transl. Res. 2020, 10, 582–593. [Google Scholar] [CrossRef] [Green Version]
- Dinarvand, R.; Sepehri, N.; Manoochehri, S.; Rouhani, H.; Atyabi, F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int. J. Nanomed. 2011, 6, 877–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, C.S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug-polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef] [PubMed]
- Mittal, G.; Sahana, D.K.; Bhardwaj, V.; Ravi Kumar, M.N. Estradiol loaded PLGA nanoparticles for oral administration: Effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J. Control. Release 2007, 119, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Kumskova, N.; Ermolenko, Y.; Osipova, N.; Semyonkin, A.; Kildeeva, N.; Gorshkova, M.; Kovalskii, A.; Kovshova, T.; Tarasov, V.; Kreuter, J.; et al. How subtle differences in polymer molecular weight affect doxorubicin-loaded PLGA nanoparticles degradation and drug release. J. Microencapsul. 2020, 37, 283–295. [Google Scholar] [CrossRef]
- Fasehee, H.; Dinarvand, R.; Ghavamzadeh, A.; Esfandyari-Manesh, M.; Moradian, H.; Faghihi, S.; Ghaffari, S.H. Delivery of disulfiram into breast cancer cells using folate-receptor-targeted PLGA-PEG nanoparticles: In vitro and in vivo investigations. J. Nanobiotechnol. 2016, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Locatelli, E.; Comes Franchini, M. Biodegradable PLGA-b-PEG polymeric nanoparticles: Synthesis, properties, and nanomedical applications as drug delivery system. J. Nanopart. Res. 2012, 14, 1316. [Google Scholar] [CrossRef]
- Grama, C.N.; Ankola, D.D.; Ravi Kumar, M.N.V. Poly(lactide-co-glycolide) nanoparticles for peroral delivery of bioactives. Curr. Opin. Colloid Interface Sci. 2011, 16, 238–245. [Google Scholar] [CrossRef]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar] [CrossRef]
- Sah, E.; Sah, H. Recent trends in preparation of poly(lactide-co-glycolide) nanoparticles by mixing polymeric organic solution with antisolvent. J. Nanomater. 2015, 2015, 794601. [Google Scholar] [CrossRef] [Green Version]
- Ding, D.; Zhu, Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 1041–1060. [Google Scholar] [CrossRef]
- Derman, S. Caffeic acid phenethyl ester loaded PLGA nanoparticles: Effect of various process parameters on reaction yield, encapsulation efficiency, and particle size. J. Nanomater. 2015, 2015, 341848. [Google Scholar] [CrossRef] [Green Version]
- Halayqa, M.; Domanska, U. PLGA biodegradable nanoparticles containing perphenazine or chlorpromazine hydrochloride: Effect of formulation and release. Int. J. Mol. Sci. 2014, 15, 23909–23923. [Google Scholar] [CrossRef]
- Adebileje, T.; Valizadeh, A.; Amani, A. Effect of formulation parameters on the size of PLGA nanoparticles encapsulating bovine serum albumin: A response surface methodology. J. Contemp. Med. Sci. 2017, 3, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Bohrey, S.; Chourasiya, V.; Pandey, A. Polymeric nanoparticles containing diazepam: Preparation, optimization, characterization, in-vitro drug release and release kinetic study. Nano Converg. 2016, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Contado, C.; Vighi, E.; Dalpiaz, A.; Leo, E. Influence of secondary preparative parameters and aging effects on PLGA particle size distribution: A sedimentation field flow fractionation investigation. Anal. Bioanal. Chem. 2013, 405, 703–711. [Google Scholar] [CrossRef]
- Sahin, A.; Esendagli, G.; Yerlikaya, F.; Caban-Toktas, S.; Yoyen-Ermis, D.; Horzum, U.; Aktas, Y.; Khan, M.; Couvreur, P.; Capan, Y. A small variation in average particle size of PLGA nanoparticles prepared by nanoprecipitation leads to considerable change in nanoparticles’ characteristics and efficacy of intracellular delivery. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1657–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Zhang, C. Tuning the size of poly(lactic-co-glycolic acid) (PLGA) nanoparticles fabricated by nanoprecipitation. Biotechnol. J. 2018, 13, 1700203. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Boyd, B.J.; McIntosh, M.P.; Pouton, C.W.; Kaminskas, L.M.; Whittaker, M. Suggested procedures for the reproducible synthesis of poly(d,l-lactide-co-glycolide) nanoparticles using the emulsification solvent diffusion platform. Curr. Nanosci. 2018, 14, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Abd-Elgawad, A.E.; Soliman, O.A.; Jablonski, M.M. Nanoparticle-based topical ophthalmic formulations for sustained celecoxib release. J. Pharm. Sci. 2013, 102, 1036–1053. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, W.; Wang, D.-T.; Chen, C.-L.; Zhuang, Q.-Y.; Kong, X.-D. A modified spontaneous emulsification solvent diffusion method for the preparation of curcumin-loaded PLGA nanoparticles with enhanced in vitro anti-tumor activity. Front. Mater. Sci. 2014, 8, 332–342. [Google Scholar] [CrossRef]
- Ven, H.V.; Vandervoort, J.; Weyenberg, W.; Apers, S.; Ludwig, A. Mixture designs in the optimisation of PLGA nanoparticles: Influence of organic phase composition on beta-aescin encapsulation. J. Microencapsul. 2012, 29, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Van de Ven, H.; Vermeersch, M.; Vandenbroucke, R.E.; Matheeussen, A.; Apers, S.; Weyenberg, W.; De Smedt, S.C.; Cos, P.; Maes, L.; Ludwig, A. Intracellular drug delivery in Leishmania-infected macrophages: Evaluation of saponin-loaded PLGA nanoparticles. J. Drug Target. 2012, 20, 142–154. [Google Scholar] [CrossRef]
- Sengel-Türk, C.T.; Bayram, B. Development and in-vitro evaluation of chitosan chloride decorated PLGA based polymeric nanoparticles of nimesulide. J. Res. Pharm. 2021, 25, 379–387. [Google Scholar] [CrossRef]
- Sengel-Turk, C.T.; Hascicek, C.; Dogan, A.L.; Esendagli, G.; Guc, D.; Gonul, N. Preparation and in vitro evaluation of meloxicam-loaded PLGA nanoparticles on HT-29 human colon adenocarcinoma cells. Drug Dev. Ind. Pharm. 2012, 38, 1107–1116. [Google Scholar] [CrossRef]
- Nava-Arzaluz, M.G.; Piñón-Segundo, E.; Ganem-Rondero, A.; Lechuga-Ballesteros, D. Single emulsion-solvent evaporation technique and modifications for the preparation of pharmaceutical polymeric nanoparticles. Recent Pat. Drug Deliv. Formul. 2012, 6, 209–223. [Google Scholar] [CrossRef]
- Hernández-Giottonini, K.Y.; Rodríguez-Córdova, R.J.; Gutiérrez-Valenzuela, C.A.; Peñuñuri-Miranda, O.; Zavala-Rivera, P.; Guerrero-Germán, P.; Lucero-Acuña, A. PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: Effects of formulation parameters. RSC Adv. 2020, 10, 4218–4231. [Google Scholar] [CrossRef] [Green Version]
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Beck-Broichsitter, M.; Nicolas, J.; Couvreur, P. Solvent selection causes remarkable shifts of the “Ouzo region” for poly(lactide-co-glycolide) nanoparticles prepared by nanoprecipitation. Nanoscale 2015, 7, 9215–9221. [Google Scholar] [CrossRef]
- Almoustafa, H.A.; Alshawsh, M.A.; Chik, Z. Technical aspects of preparing PEG-PLGA nanoparticles as carrier for chemotherapeutic agents by nanoprecipitation method. Int. J. Pharm. 2017, 533, 275–284. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; Rehman, A.U. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef]
- Zabihi, F.; Yang, M.; Leng, Y.; Zhao, Y. PLGA–HPMC nanoparticles prepared by a modified supercritical anti-solvent technique for the controlled release of insulin. J. Supercrit. Fluids 2015, 99, 15–22. [Google Scholar] [CrossRef]
- Albisa, A.; Piacentini, E.; Sebastian, V.; Arruebo, M.; Santamaria, J.; Giorno, L. Preparation of drug-loaded PLGA-PEG nanoparticles by membrane-assisted nanoprecipitation. Pharm. Res. 2017, 34, 1296–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enlow, E.M.; Luft, J.C.; Napier, M.E.; DeSimone, J.M. Potent engineered PLGA nanoparticles by virtue of exceptionally high chemotherapeutic loadings. Nano Lett. 2011, 11, 808–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowerman, C.J.; Byrne, J.D.; Chu, K.S.; Schorzman, A.N.; Keeler, A.W.; Sherwood, C.A.; Perry, J.L.; Luft, J.C.; Darr, D.B.; Deal, A.M.; et al. Docetaxel-loaded PLGA nanoparticles improve efficacy in taxane-resistant triple-negative breast cancer. Nano Lett. 2017, 17, 242–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, K.S.; Hasan, W.; Rawal, S.; Walsh, M.D.; Enlow, E.M.; Luft, J.C.; Bridges, A.S.; Kuijer, J.L.; Napier, M.E.; Zamboni, W.C.; et al. Plasma, tumor and tissue pharmacokinetics of Docetaxel delivered via nanoparticles of different sizes and shapes in mice bearing SKOV-3 human ovarian carcinoma xenograft. Nanomedicine 2013, 9, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wong, D.H.; Byrne, J.D.; Chen, K.; Bowerman, C.; DeSimone, J.M. Future of the particle replication in nonwetting templates (PRINT) technology. Angew. Chem. Int. Ed. Engl. 2013, 52, 6580–6589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoyav, B.; Benny, O. Controlled and tunable polymer particles’ production using a single microfluidic device. Appl. Nanosci. 2018, 8, 905–914. [Google Scholar] [CrossRef] [Green Version]
- Lallana, E.; Donno, R.; Magri, D.; Barker, K.; Nazir, Z.; Treacher, K.; Lawrence, M.J.; Ashford, M.; Tirelli, N. Microfluidic-assisted nanoprecipitation of (PEGylated) poly (d,l-lactic acid-co-caprolactone): Effect of macromolecular and microfluidic parameters on particle size and paclitaxel encapsulation. Int. J. Pharm. 2018, 548, 530–539. [Google Scholar] [CrossRef]
- Bourguignon, T.; Torrano, A.A.; Houel-Renault, L.; Machelart, A.; Brodin, P.; Gref, R. An original methodology to study polymeric nanoparticle-macrophage interactions: Nanoparticle tracking analysis in cell culture media and quantification of the internalized objects. Int. J. Pharm. 2021, 610, 121202. [Google Scholar] [CrossRef]
- Rezaei, G.; Daghighi, S.M.; Raoufi, M.; Esfandyari-Manesh, M.; Rahimifard, M.; Mobarakeh, V.I.; Kamalzare, S.; Ghahremani, M.H.; Atyabi, F.; Abdollahi, M.; et al. Synthetic and biological identities of polymeric nanoparticles influencing the cellular delivery: An immunological link. J. Colloid Interface Sci. 2019, 556, 476–491. [Google Scholar] [CrossRef]
- Ndumiso, M.; Buchtová, N.; Husselmann, L.; Mohamed, G.; Klein, A.; Aucamp, M.; Canevet, D.; D’Souza, S.; Maphasa, R.E.; Boury, F.; et al. Comparative whole corona fingerprinting and protein adsorption thermodynamics of PLGA and PCL nanoparticles in human serum. Colloids Surf. B Biointerfaces 2020, 188, 110816. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, F.; Ghahremani, M.H.; Esmaeili, B.; Khoshayand, M.R.; Atyabi, F.; Dinarvand, R. PLGA nanoparticles of different surface properties: Preparation and evaluation of their body distribution. Int. J. Pharm. 2008, 349, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.W.; Chambers, E.; Mitragotri, S. Factors that control the circulation time of nanoparticles in blood: Challenges, solutions and future prospects. Curr. Pharm. Des. 2010, 16, 2298–2307. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Kutnyanszky, E.; Bertoti, I. Modification of poly(lactic/glycolic acid) surface by chemical attachment of poly(ethylene glycol). Langmuir 2010, 26, 1440–1444. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Hickey, J.W.; Santos, J.L.; Williford, J.M.; Mao, H.Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, S.M.; Arumugam, M.; Wilberforce, S.; Enea, D.; Rushton, N.; Zhang, X.C.; Best, S.M.; Cameron, R.E.; Brooks, R.A. The effect of particle size on the in vivo degradation of poly(d,l-lactide-co-glycolide)/alpha-tricalcium phosphate micro- and nanocomposites. Acta Biomater. 2016, 45, 340–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsewedy, H.S.; Dhubiab, B.E.A.; Mahdy, M.A.; Elnahas, H.M. Development, optimization, and evaluation of PEGylated brucine-loaded PLGA nanoparticles. Drug Deliv. 2020, 27, 1134–1146. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Vaughan, H.J.; Zamboni, C.G.; Sunshine, J.C.; Green, J.J. High-throughput evaluation of polymeric nanoparticles for tissue-targeted gene expression using barcoded plasmid DNA. J. Control. Release 2021, 337, 105–116. [Google Scholar] [CrossRef]
- Platel, A.; Carpentier, R.; Becart, E.; Mordacq, G.; Betbeder, D.; Nesslany, F. Influence of the surface charge of PLGA nanoparticles on their in vitro genotoxicity, cytotoxicity, ROS production and endocytosis. J. Appl. Toxicol. 2016, 36, 434–444. [Google Scholar] [CrossRef]
- Arnida, M.M.; Ray, A.; Peterson, C.M.; Ghandehari, H. Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur. J. Pharm. Biopharm. 2011, 77, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Florez, L.; Herrmann, C.; Cramer, J.M.; Hauser, C.P.; Koynov, K.; Landfester, K.; Crespy, D.; Mailander, V. How shape influences uptake: Interactions of anisotropic polymer nanoparticles and human mesenchymal stem cells. Small 2012, 8, 2222–2230. [Google Scholar] [CrossRef]
- Kolhar, P.; Doshi, N.; Mitragotri, S. Polymer nanoneedle-mediated intracellular drug delivery. Small 2011, 7, 2094–2100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sai Lung, P.; Zhao, S.; Chu, Z.; Chrzanowski, W.; Li, Q. Shape dependent cytotoxicity of PLGA-PEG nanoparticles on human cells. Sci. Rep. 2017, 7, 7315. [Google Scholar] [CrossRef]
- Sah, H.; Thoma, L.A.; Desu, H.R.; Sah, E.; Wood, G.C. Concepts and practices used to develop functional PLGA-based nanoparticulate systems. Int. J. Nanomed. 2013, 8, 747–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Frohlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [Green Version]
- Holgado, M.A.; Alvarez-Fuentes, J.; Fernandez-Arevalo, M.; Arias, J.L. Possibilities of poly(D,L-lactide-co-glycolide) in the formulation of nanomedicines against cancer. Curr. Drug Targets 2011, 12, 1096–1111. [Google Scholar] [CrossRef]
- Holgado, M.A.; Martin-Banderas, L.; Alvarez-Fuentes, J.; Fernandez-Arevalo, M.; Arias, J.L. Drug targeting to cancer by nanoparticles surface functionalized with special biomolecules. Curr. Med. Chem. 2012, 19, 3188–3195. [Google Scholar] [CrossRef] [PubMed]
- Menon, J.U.; Kona, S.; Wadajkar, A.S.; Desai, F.; Vadla, A.; Nguyen, K.T. Effects of surfactants on the properties of PLGA nanoparticles. J. Biomed. Mater. Res. A 2012, 100, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Heinz, H.; Pramanik, C.; Heinz, O.; Ding, Y.; Mishra, R.K.; Marchon, D.; Flatt, R.J.; Estrela-Lopis, I.; Llop, J.; Moya, S.; et al. Nanoparticle decoration with surfactants: Molecular interactions, assembly, and applications. Surf. Sci. Rep. 2017, 72, 1–58. [Google Scholar] [CrossRef]
- Mehanny, M.; Hathout, R.M.; Geneidi, A.S.; Mansour, S. Studying the effect of physically-adsorbed coating polymers on the cytotoxic activity of optimized bisdemethoxycurcumin loaded-PLGA nanoparticles. J. Biomed. Mater. Res. A 2017, 105, 1433–1445. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. Engl. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Martin-Banderas, L.; Munoz-Rubio, I.; Prados, J.; Alvarez-Fuentes, J.; Calderon-Montano, J.M.; Lopez-Lazaro, M.; Arias, J.L.; Leiva, M.C.; Holgado, M.A.; Fernandez-Arevalo, M. In vitro and in vivo evaluation of Delta(9)-tetrahidrocannabinol/PLGA nanoparticles for cancer chemotherapy. Int. J. Pharm. 2015, 487, 205–212. [Google Scholar] [CrossRef]
- Martin-Banderas, L.; Munoz-Rubio, I.; Alvarez-Fuentes, J.; Duran-Lobato, M.; Arias, J.L.; Holgado, M.A.; Fernandez-Arevalo, M. Engineering of Δ⁹-tetrahydrocannabinol delivery systems based on surface modified-PLGA nanoplatforms. Colloids Surf. B Biointerfaces 2014, 123, 114–122. [Google Scholar] [CrossRef]
- Huang, N.; Lu, S.; Liu, X.-G.; Zhu, J.; Wang, Y.-J.; Liu, R.-T. PLGA nanoparticles modified with a BBB-penetrating peptide co-delivering Aβ generation inhibitor and curcumin attenuate memory deficits and neuropathology in Alzheimer’s disease mice. Oncotarget 2017, 8, 81001–81013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, L.J.; Tacken, P.J.; Fokkink, R.; Figdor, C.G. The influence of PEG chain length and targeting moiety on antibody-mediated delivery of nanoparticle vaccines to human dendritic cells. Biomaterials 2011, 32, 6791–6803. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Y.Y.; Yang, M.; Schneider, C.; Zhong, W.; Pulicare, S.; Choi, W.J.; Mert, O.; Fu, J.; Lai, S.K.; et al. Biodegradable mucus-penetrating nanoparticles composed of diblock copolymers of polyethylene glycol and poly(lactic-co-glycolic acid). Drug Deliv. Transl. Res. 2012, 2, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Ensign, L.M.; Boylan, N.J.; Schon, A.; Gong, X.; Yang, J.C.; Lamb, N.W.; Cai, S.; Yu, T.; Freire, E.; et al. Impact of surface polyethylene glycol (PEG) density on biodegradable nanoparticle transport in mucus ex vivo and distribution in vivo. ACS Nano 2015, 9, 9217–9227. [Google Scholar] [CrossRef] [Green Version]
- Kirby, B.P.; Pabari, R.; Chen, C.N.; Al Baharna, M.; Walsh, J.; Ramtoola, Z. Comparative evaluation of the degree of pegylation of poly(lactic-co-glycolic acid) nanoparticles in enhancing central nervous system delivery of loperamide. J. Pharm. Pharmacol. 2013, 65, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Saadati, R.; Dadashzadeh, S.; Abbasian, Z.; Soleimanjahi, H. Accelerated blood clearance of PEGylated PLGA nanoparticles following repeated injections: Effects of polymer dose, PEG coating, and encapsulated anticancer drug. Pharm. Res. 2013, 30, 985–995. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The importance of poly(ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Xue, J.; Gao, S.; Lu, A.; Yang, D.; Jiang, H.; He, Y.; Shi, K. Cleavable PEGylation: A strategy for overcoming the “PEG dilemma” in efficient drug delivery. Drug Deliv. 2017, 24, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorson, T.; Lubtow, M.M.; Wegener, E.; Haider, M.S.; Borova, S.; Nahm, D.; Jordan, R.; Sokolski-Papkov, M.; Kabanov, A.V.; Luxenhofer, R. Poly(2-oxazoline)s based biomaterials: A comprehensive and critical update. Biomaterials 2018, 178, 204–280. [Google Scholar] [CrossRef]
- Bauer, M.; Lautenschlaeger, C.; Kempe, K.; Tauhardt, L.; Schubert, U.S.; Fischer, D. Poly(2-ethyl-2-oxazoline) as alternative for the stealth polymer poly(ethylene glycol): Comparison of in vitro cytotoxicity and hemocompatibility. Macromol. Biosci. 2012, 12, 986–998. [Google Scholar] [CrossRef]
- Dirauf, M.; Grune, C.; Weber, C.; Schubert, U.S.; Fischer, D. Poly(ethylene glycol) or poly(2-ethyl-2-oxazoline)—A systematic comparison of PLGA nanoparticles from the bottom up. Eur. Polym. J. 2020, 134, 109801. [Google Scholar] [CrossRef]
- Lima, I.A.; Khalil, N.M.; Tominaga, T.T.; Lechanteur, A.; Sarmento, B.; Mainardes, R.M. Mucoadhesive chitosan-coated PLGA nanoparticles for oral delivery of ferulic acid. Artif. Cells Nanomed. Biotechnol. 2018, 46, 993–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taghavi, S.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. Chitosan-modified PLGA nanoparticles tagged with 5TR1 aptamer for in vivo tumor-targeted drug delivery. Cancer Lett. 2017, 400, 1–8. [Google Scholar] [CrossRef]
- Fernández-Álvarez, F.; Caro, C.; García-García, G.; García-Martín, M.L.; Arias, J.L. Engineering of stealth (maghemite/PLGA)/chitosan (core/shell)/shell nanocomposites with potential applications for combined MRI and hyperthermia against cancer. J. Mater. Chem. B 2021, 9, 4963–4980. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Massimi, M.; Giardi, M.F.; Cametti, C.; Devirgiliis, L.C.; Dentini, M.; Palocci, C. Chitosan-coated PLGA nanoparticles: A sustained drug release strategy for cell cultures. Colloids Surf. B Biointerfaces 2013, 103, 310–317. [Google Scholar] [CrossRef]
- Lu, B.; Lv, X.; Le, Y. Chitosan-modified PLGA nanoparticles for control-released drug delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Álvarez, F.; García-García, G.; Arias, J.L. A tri-stimuli responsive (maghemite/PLGA)/chitosan nanostructure with promising applications in lung cancer. Pharmaceutics 2021, 13, 1232. [Google Scholar] [CrossRef]
- Liang, Y.; Kiick, K.L. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater. 2014, 10, 1588–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, Y.I.; Kim, J.C.; Kim, Y.H.; Tae, G.; Lee, S.Y.; Kim, K.; Kwon, I.C. The effect of surface functionalization of PLGA nanoparticles by heparin- or chitosan-conjugated Pluronic on tumor targeting. J. Control. Release 2010, 143, 374–382. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Kumari, M.; Pahuja, R.; Pant, A.B.; Shukla, Y.; Kumar, P.; Gupta, K.C. Hyaluronic acid-grafted PLGA nanoparticles for the sustained delivery of berberine chloride for an efficient suppression of Ehrlich ascites tumors. Drug Deliv. Transl. Res. 2018, 8, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Huang, Y.; Feng, X.; Zhu, C.; Yin, S.; Wang, X.; Bai, X.; Pan, X.; Wu, C. TPGS/hyaluronic acid dual-functionalized PLGA nanoparticles delivered through dissolving microneedles for markedly improved chemo-photothermal combined therapy of superficial tumor. Acta Pharm. Sin. B 2020, 11, 3297–3309. [Google Scholar] [CrossRef] [PubMed]
- Kosinski, A.M.; Brugnano, J.L.; Seal, B.L.; Knight, F.C.; Panitch, A. Synthesis and characterization of a poly(lactic-co-glycolic acid) core + poly(N-isopropylacrylamide) shell nanoparticle system. Biomatter 2012, 2, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Salehi, R.; Davaran, S.; Rashidi, M.R.; Entezami, A.A. Thermosensitive nanoparticles prepared from poly(N-isopropylacrylamide-acrylamide-vinilpyrrolidone) and its blend with poly(lactide-co-glycolide) for efficient drug delivery system. J. Appl. Polym. Sci. 2009, 111, 1905–1910. [Google Scholar] [CrossRef]
- Bose, R.J.; Lee, S.H.; Park, H. Lipid-based surface engineering of PLGA nanoparticles for drug and gene delivery applications. Biomater. Res. 2016, 20, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campani, V.; Giarra, S.; De Rosa, G. Lipid-based core-shell nanoparticles: Evolution and potentialities in drug delivery. OpenNano 2018, 3, 5–17. [Google Scholar] [CrossRef]
- Baek, J.S.; Tan, C.H.; Ng, N.K.J.; Yeo, Y.P.; Rice, S.A.; Loo, S.C.J. A programmable lipid-polymer hybrid nanoparticle system for localized, sustained antibiotic delivery to Gram-positive and Gram-negative bacterial biofilms. Nanoscale Horiz. 2018, 3, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, C.; Manzano, M.; Vallet-Regí, M. Nanoparticles coated with cell membranes for biomedical applications. Biology 2020, 9, 406. [Google Scholar] [CrossRef]
- Hu, C.M.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [Green Version]
- Aryal, S.; Hu, C.M.; Fang, R.H.; Dehaini, D.; Carpenter, C.; Zhang, D.E.; Zhang, L. Erythrocyte membrane-cloaked polymeric nanoparticles for controlled drug loading and release. Nanomedicine 2013, 8, 1271–1280. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.M.; Fang, R.H.; Wang, K.C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526, 118–121. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Geng, J.; Glinka, M.; White, K.; Kanczler, J.; Evans, N.D.; Oreffo, R.O.C.; Bradley, M. Combinatorial delivery of bioactive molecules by a nanoparticle-decorated and functionalized biodegradable scaffold. J. Mater. Chem. B 2018, 6, 4437–4445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucci, A.; Locatelli, E.; Ponti, J.; Uboldi, C.; Molinari, V.; Comes Franchini, M. Click chemistry on the surface of PLGA-b-PEG polymeric nanoparticles: A novel targetable fluorescent imaging nanocarrier. J. Nanopart. Res. 2013, 15, 1818. [Google Scholar] [CrossRef] [Green Version]
- Esfandyari-Manesh, M.; Abdi, M.; Talasaz, A.H.; Ebrahimi, S.M.; Atyabi, F.; Dinarvand, R. S2P peptide-conjugated PLGA-Maleimide-PEG nanoparticles containing Imatinib for targeting drug delivery to atherosclerotic plaques. Daru 2020, 28, 131–138. [Google Scholar] [CrossRef]
- Xin, L.; Zhang, H.T.; Yang, W.F.; Li, Y.F.; Liu, C. Evaluation of METase-pemetrexed-loaded PEG-PLGA nanoparticles modified with anti-CD133-scFV for treatment of gastric carcinoma. Biosci. Rep. 2018, 38, BSR20171001. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Sun, J.; Liu, Y. Enhanced targeting of prostate cancer-initiating cells by salinomycin-encapsulated lipid-PLGA nanoparticles linked with CD44 antibodies. Oncol. Lett. 2019, 17, 4024–4033. [Google Scholar] [CrossRef] [Green Version]
- Venugopal Venugopal, V.; Krishnan, S.; Palanimuthu, V.R.; Sankarankutty, S.; Kalaimani, J.K.; Karupiah, S.; Kit, N.S.; Hock, T.T. Anti-EGFR anchored paclitaxel loaded PLGA nanoparticles for the treatment of triple negative breast cancer. In-vitro and in-vivo anticancer activities. PLoS ONE 2018, 13, e0206109. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Amrutiya, J.; Bhatt, P.; Javia, A.; Jain, M.; Misra, A. Targeted delivery of monoclonal antibody conjugated docetaxel loaded PLGA nanoparticles into EGFR overexpressed lung tumour cells. J. Microencapsul. 2018, 35, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Park, C.G.; Hartl, C.A.; Subedi, N.; Cartwright, A.N.; Puerto, R.B.; Zheng, Y.; Maiarana, J.; Freeman, G.J.; Wucherpfennig, K.W.; et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat. Commun. 2017, 8, 1747. [Google Scholar] [CrossRef] [PubMed]
- Hariri, W.; Sudha, T.; Bharali, D.J.; Cui, H.; Mousa, S.A. Nano-targeted delivery of toremifene, an estrogen receptor-α blocker in prostate cancer. Pharm. Res. 2015, 32, 2764–2774. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Ríos, R.; Sánchez-Ramírez, D.R.; Ruiz-Saray, K.; Oceguera-Basurto, P.E.; Almada, M.; Juárez, J.; Zepeda-Moreno, A.; Del Toro-Arreola, A.; Topete, A.; Daneri-Navarro, A. Cisplatin-loaded PLGA nanoparticles for HER2 targeted ovarian cancer therapy. Colloids Surf. B Biointerfaces 2019, 178, 199–207. [Google Scholar] [CrossRef]
- Zhou, Z.; Badkas, A.; Stevenson, M.; Lee, J.Y.; Leung, Y.K. Herceptin conjugated PLGA-PHis-PEG pH sensitive nanoparticles for targeted and controlled drug delivery. Int. J. Pharm. 2015, 487, 81–90. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Li, X.; Li, F.; Lee, R.J.; Sun, F.; Li, Y.; Liu, Z.; Teng, L. Trastuzumab-coated nanoparticles loaded with docetaxel for breast cancer therapy. Dose Response 2019, 17, 1559325819872583. [Google Scholar] [CrossRef]
- Safdari, Y.; Ahmadzadeh, V.; Khalili, M.; Jaliani, H.Z.; Zarei, V.; Erfani-Moghadam, V. Use of single chain antibody derivatives for targeted drug delivery. Mol. Med. 2016, 22, 258–270. [Google Scholar] [CrossRef]
- Arias, J.L.; Unciti-Broceta, J.D.; Maceira, J.; Del Castillo, T.; Hernández-Quero, J.; Magez, S.; Soriano, M.; García-Salcedo, J.A. Nanobody conjugated PLGA nanoparticles for active targeting of African Trypanosomiasis. J. Control. Release 2015, 197, 190–198. [Google Scholar] [CrossRef] [Green Version]
- RenRen, W.X.; Han, J.; Uhm, S.; Jang, Y.J.; Kang, C.; Kim, J.H.; Kim, J.S. Recent development of biotin conjugation in biological imaging, sensing, and target delivery. Chem. Commun. 2015, 51, 10403–10418. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Rouhani, H.; Sepehri, N.; Varshochian, R.; Ghahremani, M.H.; Amini, M.; Gharghabi, M.; Ostad, S.N.; Atyabi, F.; Baharian, A.; et al. Biotin decorated PLGA nanoparticles containing SN-38 designed for cancer therapy. Artif. Cells Nanomed. Biotechnol. 2017, 45, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Meng, X.; Su, J.; Ma, H.; Wang, W.; Fang, L.; Zheng, H.; Qin, Y.; Chen, T. Biotin-modified polylactic-co-glycolic acid nanoparticles with improved antiproliferative activity of 15,16-dihydrotanshinone I in human cervical cancer cells. J. Agric. Food Chem. 2018, 66, 9219–9230. [Google Scholar] [CrossRef]
- Chen, H.; Nan, W.; Wei, X.; Wang, Y.; Lv, F.; Tang, H.; Li, Y.; Zhou, C.; Lin, J.; Zhu, W.; et al. Toxicity, pharmacokinetics, and in vivo efficacy of biotinylated chitosan surface-modified PLGA nanoparticles for tumor therapy. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1115–1122. [Google Scholar] [CrossRef] [Green Version]
- Bolla, P.K.; Gote, V.; Singh, M.; Patel, M.; Clark, B.A.; Renukuntla, J. Lutein-loaded, biotin-decorated polymeric nanoparticles enhance lutein uptake in retinal cells. Pharmaceutics 2020, 12, 798. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Thompson, K.; Gordon, S.; Rogers, M.J. Molecular mechanisms of action of bisphosphonates: Current status. Clin. Cancer Res. 2006, 12, 6222s–6230s. [Google Scholar] [CrossRef] [Green Version]
- Cenni, E.; Avnet, S.; Granchi, D.; Fotia, C.; Salerno, M.; Micieli, D.; Sarpietro, M.G.; Pignatello, R.; Castelli, F.; Baldini, N. The effect of poly(d,l-lactide-co-glycolide)-alendronate conjugate nanoparticles on human osteoclast precursors. J. Biomater. Sci. Polym. Ed. 2012, 23, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, R.; Sarpietro, M.G.; Castelli, F. Synthesis and biological evaluation of a new polymeric conjugate and nanocarrier with osteotropic properties. J. Funct. Biomater. 2012, 3, 79–99. [Google Scholar] [CrossRef] [Green Version]
- Thamake, S.I.; Raut, S.L.; Gryczynski, Z.; Ranjan, A.P.; Vishwanatha, J.K. Alendronate coated poly-lactic-co-glycolic acid (PLGA) nanoparticles for active targeting of metastatic breast cancer. Biomaterials 2012, 33, 7164–7173. [Google Scholar] [CrossRef]
- Ramanlal Chaudhari, K.; Kumar, A.; Megraj Khandelwal, V.K.; Ukawala, M.; Manjappa, A.S.; Mishra, A.K.; Monkkonen, J.; Ramachandra Murthy, R.S. Bone metastasis targeting: A novel approach to reach bone using Zoledronate anchored PLGA nanoparticle as carrier system loaded with Docetaxel. J. Control. Release 2012, 158, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Song, J.X.; Zhang, M.N.; Yuan, B.S. A multiple drug loaded, functionalized pH-sensitive nanocarrier as therapeutic and epigenetic modulator for osteosarcoma. Sci. Rep. 2020, 10, 15497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Deng, T.; Yao, P.; Song, H.; Zhou, S.; Yan, W. Development of drug loaded nanoparticles binding to hydroxyapatite based on a bisphosphonate modified nonionic surfactant. J. Nanomater. 2015, 2015, 393968. [Google Scholar] [CrossRef]
- Zwicke, G.L.; Mansoori, G.A.; Jeffery, C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012, 3, 18496. [Google Scholar] [CrossRef]
- Pieroth, R.; Paver, S.; Day, S.; Lammersfeld, C. Folate and its impact on cancer risk. Curr. Nutr. Rep. 2018, 7, 70–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, J.; Yang, S.M.; Yi, G.; Roh, Y.J.; Park, H.; Park, J.M.; Choi, M.G.; Koo, H. Folate-modified PLGA nanoparticles for tumor-targeted delivery of pheophorbide a in vivo. Biochem. Biophys. Res. Commun. 2018, 498, 523–528. [Google Scholar] [CrossRef]
- Poltavets, Y.I.; Zhirnik, A.S.; Zavarzina, V.V.; Semochkina, Y.P.; Shuvatova, V.G.; Krasheninnikova, A.A.; Aleshin, S.V.; Dronov, D.O.; Vorontsov, E.A.; Balabanyan, V.Y.; et al. In vitro anticancer activity of folate-modified docetaxel-loaded PLGA nanoparticles against drug-sensitive and multidrug-resistant cancer cells. Cancer Nanotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Oliveira, A.L.C.S.L.; Zerillo, L.; Cruz, L.J.; Schomann, T.; Chan, A.B.; de Carvalho, T.G.; Souza, S.V.P.; Araújo, A.A.; de Geus-Oei, L.F.; de Araújo, R.F., Jr. Maximizing the potency of oxaliplatin coated nanoparticles with folic acid for modulating tumor progression in colorectal cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111678. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sadoqi, M.; Shao, J. Biodistribution of indocyanine green-loaded nanoparticles with surface modifications of PEG and folic acid. Int. J. Pharm. 2012, 436, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zheng, M.; Gong, P.; Jia, D.; Zhang, P.; Shi, B.; Sheng, Z.; Ma, Y.; Cai, L. Indocyanine green-loaded biodegradable tumor targeting nanoprobes for in vitro and in vivo imaging. Biomaterials 2012, 33, 5603–5609. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, X.; Huang, J.; Wu, Y.; Huang, X.; Chen, J.; Xia, J.; Jiang, H.; Ma, J.; Wu, J. Immune activity and biodistribution of polypeptide K237 and folic acid conjugated amphiphilic PEG-PLGA copolymer nanoparticles radiolabeled with 99mTc. Oncotarget 2016, 7, 76635–76646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruschi, M.L. Lectins and nanostructured drug delivery systems. Curr. Drug Deliv. 2019, 16, 268–269. [Google Scholar] [CrossRef]
- Song, Q.; Yao, L.; Huang, M.; Hu, Q.; Lu, Q.; Wu, B.; Qi, H.; Rong, Z.; Jiang, X.; Gao, X.; et al. Mechanisms of transcellular transport of wheat germ agglutinin-functionalized polymeric nanoparticles in Caco-2 cells. Biomaterials 2012, 33, 6769–6782. [Google Scholar] [CrossRef]
- Wen, Z.; Yan, Z.; Hu, K.; Pang, Z.; Cheng, X.; Guo, L.; Zhang, Q.; Jiang, X.; Fang, L.; Lai, R. Odorranalectin-conjugated nanoparticles: Preparation, brain delivery and pharmacodynamic study on Parkinson’s disease following intranasal administration. J. Control. Release 2011, 151, 131–138. [Google Scholar] [CrossRef]
- Wen Wen, Z.; Yan, Z.; He, R.; Pang, Z.; Guo, L.; Qian, Y.; Jiang, X.; Fang, L. Brain targeting and toxicity study of odorranalectin-conjugated nanoparticles following intranasal administration. Drug Deliv. 2011, 18, 555–561. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, C.; Liu, Q.; Shao, X.; Feng, C.; Shen, Y.; Zhang, Q.; Jiang, X. Solanum tuberosum lectin-conjugated PLGA nanoparticles for nose-to-brain delivery: In vivo and in vitro evaluations. J. Drug Target. 2012, 20, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Haider, T.; Kumar, A.; Jain, A. Lectin-conjugated clarithromycin and acetohydroxamic acid-loaded PLGA nanoparticles: A novel approach for effective treatment of H. pylori. AAPS PharmSciTech 2016, 17, 1131–1140. [Google Scholar] [CrossRef]
- Wu, G.; Zhou, F.; Ge, L.; Liu, X.; Kong, F. Novel mannan-PEG-PE modified bioadhesive PLGA nanoparticles for targeted gene delivery. J. Nanomater. 2012, 981670. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.; Ge, L.; Liu, X.; Huang, N.; Zhou, F. Mannan-modified PLGA nanoparticles for targeted gene delivery. Int. J. Photoenergy 2012, 2012, 926754. [Google Scholar] [CrossRef] [Green Version]
- Haddadi, A.; Hamdy, S.; Ghotbi, Z.; Samuel, J.; Lavasanifar, A. Immunoadjuvant activity of the nanoparticles’ surface modified with mannan. Nanotechnology 2014, 25, 355101. [Google Scholar] [CrossRef]
- Hamdy, S.; Haddadi, A.; Shayeganpour, A.; Samuel, J.; Lavasanifar, A. Activation of antigen-specific T cell-responses by mannan-decorated PLGA nanoparticles. Pharm. Res. 2011, 28, 2288–2301. [Google Scholar] [CrossRef]
- Ravichandran, G.; Rengan, A.K. Aptamer-mediated nanotheranostics for cancer treatment: A review. ACS Appl. Nano Mater. 2020, 3, 9542–9559. [Google Scholar] [CrossRef]
- Duan, T.; Xu, Z.; Sun, F.; Wang, Y.; Zhang, J.; Luo, C.; Wang, M. HPA aptamer functionalized paclitaxel-loaded PLGA nanoparticles for enhanced anticancer therapy through targeted effects and microenvironment modulation. Biomed. Pharmacother. 2019, 117, 109121. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Xiong, M.; Zhang, X.; Cai, G.; Chen, H.; Zeng, Q.; Yu, Z. Poly(lactic-co-glycolic acid) nanoparticles conjugated with CD133 aptamers for targeted salinomycin delivery to CD133+ osteosarcoma cancer stem cells. Int. J. Nanomed. 2015, 10, 2537–2554. [Google Scholar] [CrossRef] [Green Version]
- Kaur, J.; Tikoo, K. Ets1 identified as a novel molecular target of RNA aptamer selected against metastatic cells for targeted delivery of nano-formulation. Oncogene 2015, 34, 5216–5228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monaco, I.; Camorani, S.; Colecchia, D.; Locatelli, E.; Calandro, P.; Oudin, A.; Niclou, S.; Arra, C.; Chiariello, M.; Cerchia, L.; et al. Aptamer functionalization of nanosystems for glioblastoma targeting through the blood-brain barrier. J. Med. Chem. 2017, 60, 4510–4516. [Google Scholar] [CrossRef]
- Mehrotra, N.; Kharbanda, S.; Singh, H. Peptide-based combination nanoformulations for cancer therapy. Nanomedicine 2020, 15, 2201–2217. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Sun, J.; Hao, W.; Chen, M.; Wang, Y.; Xu, F.; Gao, C. Dual-target peptide-modified erythrocyte membrane-enveloped PLGA nanoparticles for the treatment of glioma. Front. Oncol. 2020, 10, 563938. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Liu, Y.; Li, M.; Yu, X.; Yuan, W.; Huang, S.; Ren, D.; Wang, Y.; Wang, Y. SP94 peptide-functionalized PEG-PLGA nanoparticle loading with cryptotanshinone for targeting therapy of hepatocellular carcinoma. AAPS PharmSciTech 2020, 21, 124. [Google Scholar] [CrossRef]
- Gessner, I.; Neundorf, I. Nanoparticles modified with cell-penetrating peptides: Conjugation mechanisms, physicochemical properties, and application in cancer diagnosis and therapy. Int. J. Mol. Sci. 2020, 21, 2536. [Google Scholar] [CrossRef] [Green Version]
- Steinbach, J.M.; Seo, Y.E.; Saltzman, W.M. Cell penetrating peptide-modified poly(lactic-co-glycolic acid) nanoparticles with enhanced cell internalization. Acta Biomater. 2016, 30, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feiner-Gracia, N.; Dols-Perez, A.; Royo, M.; Solans, C.; Garcia-Celma, M.J.; Fornaguera, C. Cell penetrating peptide grafting of PLGA nanoparticles to enhance cell uptake. Eur. Polym. J. 2018, 108, 429–438. [Google Scholar] [CrossRef]
- Gonzalez-Pizarro, R.; Parrotta, G.; Vera, R.; Sánchez-López, E.; Galindo, R.; Kjeldsen, F.; Badia, J.; Baldoma, L.; Espina, M.; García, M.L. Ocular penetration of fluorometholone-loaded PEG-PLGA nanoparticles functionalized with cell-penetrating peptides. Nanomedicine 2019, 14, 3089–3104. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Liang, Z.; Huang, W.; Wen, L.; Chen, G. Engineering PLGA nano-based systems through understanding the influence of nanoparticle properties and cell-penetrating peptides for cochlear drug delivery. Int. J. Pharm. 2017, 532, 55–65. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, S.; Gao, Y.; Guo, F.; Li, F.; Xie, B.; Zhou, J.; Zhong, H. Enhanced oral bioavailability of insulin using PLGA nanoparticles co-modified with cell-penetrating peptides and Engrailed secretion peptide (Sec). Drug Deliv. 2016, 23, 1980–1991. [Google Scholar] [CrossRef] [Green Version]
- Hoyos-Ceballos, G.P.; Ruozi, B.; Ottonelli, I.; Da Ros, F.; Vandelli, M.A.; Forni, F.; Daini, E.; Vilella, A.; Zoli, M.; Tosi, G.; et al. PLGA-PEG-ANG-2 nanoparticles for blood-brain barrier crossing: Proof-of-concept study. Pharmaceutics 2020, 12, 72. [Google Scholar] [CrossRef] [Green Version]
- Alipour, M.; Baneshi, M.; Hosseinkhani, S.; Mahmoudi, R.; Jabari Arabzadeh, A.; Akrami, M.; Mehrzad, J.; Bardania, H. Recent progress in biomedical applications of RGD-based ligand: From precise cancer theranostics to biomaterial engineering: A systematic review. J. Biomed. Mater. Res. A 2020, 108, 839–850. [Google Scholar] [CrossRef]
- Martinez-Jothar, L.; Barendrecht, A.D.; de Graaff, A.M.; Oliveira, S.; van Nostrum, C.F.; Schiffelers, R.M.; Hennink, W.E.; Fens, M. Endothelial cell targeting by cRGD-functionalized polymeric nanoparticles under static and flow conditions. Nanomaterials 2020, 10, 1353. [Google Scholar] [CrossRef] [PubMed]
- Rios De La Rosa, J.M.; Spadea, A.; Donno, R.; Lallana, E.; Lu, Y.; Puri, S.; Caswell, P.; Lawrence, M.J.; Ashford, M.; Tirelli, N. Microfluidic-assisted preparation of RGD-decorated nanoparticles: Exploring integrin-facilitated uptake in cancer cell lines. Sci. Rep. 2020, 10, 14505. [Google Scholar] [CrossRef]
- Manthe, R.L.; Muro, S. ICAM-1-targeted nanocarriers attenuate endothelial release of soluble ICAM-1, an inflammatory regulator. Bioeng. Transl. Med. 2017, 2, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Chittasupho, C.; Xie, S.X.; Baoum, A.; Yakovleva, T.; Siahaan, T.J.; Berkland, C.J. ICAM-1 targeting of doxorubicin-loaded PLGA nanoparticles to lung epithelial cells. Eur. J. Pharm. Sci. 2009, 37, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Yang, G.; Guan, F. Biological functions and analytical strategies of sialic acids in tumor. Cells 2020, 9, 273. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; She, Z.; Huang, Z.; Li, J.; Luo, X.; Deng, Y. Application of sialic acid/polysialic acid in the drug delivery systems. Asian J. Pharm. Sci. 2014, 9, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Zou, J.; Xiao, X. Sialic acid-conjugated PLGA nanoparticles enhance the protective effect of lycopene in chemotherapeutic drug-induced kidney injury. IET Nanobiotechnol. 2020, 14, 341–345. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, J.; Gu, X.; Wen, Y. Targeted treatment of CD22-positive non-Hodgkin’s lymphoma with sialic acid–modified chitosan-PLGA hybrid nanoparticles. J. Nanopart. Res. 2019, 21, 154. [Google Scholar] [CrossRef]
- Tosi, G.; Vergoni, A.V.; Ruozi, B.; Bondioli, L.; Badiali, L.; Rivasi, F.; Costantino, L.; Forni, F.; Vandelli, M.A. Sialic acid and glycopeptides conjugated PLGA nanoparticles for central nervous system targeting: In vivo pharmacological evidence and biodistribution. J. Control. Release 2010, 145, 49–57. [Google Scholar] [CrossRef]
- Lee, S.Y.; Nam, S.; Koo, J.S.; Kim, S.; Yang, M.; Jeong, D.I.; Hwang, C.; Park, J.; Cho, H.J. Possible contribution of sialic acid to the enhanced tumor targeting efficiency of nanoparticles engineered with doxorubicin. Sci. Rep. 2020, 10, 19738. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Pandey, M.; Chin, P.X.; Phang, Y.L.; Cheah, J.Y.; Ooi, S.C.; Mak, K.K.; Pichika, M.R.; Kesharwani, P.; Hussain, Z.; et al. Transferrin receptors-targeting nanocarriers for efficient targeted delivery and transcytosis of drugs into the brain tumors: A review of recent advancements and emerging trends. Drug Deliv. Transl. Res. 2018, 8, 1545–1563. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.N.; Xu, Q.X.; Davoodi, P.; Wang, D.P.; Wang, C.H. Enhanced intracellular delivery and controlled drug release of magnetic PLGA nanoparticles modified with transferrin. Acta Pharmacol. Sin. 2017, 38, 943–953. [Google Scholar] [CrossRef] [Green Version]
- Jose, S.; Cinu, T.A.; Sebastian, R.; Shoja, M.H.; Aleykutty, N.A.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B. Transferrin-conjugated docetaxel-PLGA nanoparticles for tumor targeting: Influence on MCF-7 cell cycle. Polymers 2019, 11, 1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frasco, M.F.; Almeida, G.M.; Santos-Silva, F.; Pereira, M.d.C.; Coelho, M.A. Transferrin surface-modified PLGA nanoparticles-mediated delivery of a proteasome inhibitor to human pancreatic cancer cells. J. Biomed. Mater. Res. A 2015, 103, 1476–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Wang, L.; Lv, P.; Zhang, P. Transferrin-conjugated doxorubicin-loaded lipid-coated nanoparticles for the targeting and therapy of lung cancer. Oncol. Lett. 2015, 9, 1065–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, P.; Sarker, S.; Ghosh, A.; Gupta, P.; Das, S.; Ahir, M.; Bhattacharya, S.; Chattopadhyay, S.; Ghosh, S.; Adhikary, A. Transferrin-decorated thymoquinone-loaded PEG-PLGA nanoparticles exhibit anticarcinogenic effect in non-small cell lung carcinoma via the modulation of miR-34a and miR-16. Biomater. Sci. 2019, 7, 4325–4344. [Google Scholar] [CrossRef]
- Mao, J.; Meng, X.; Zhao, C.; Yang, Y.; Liu, G. Development of transferrin-modified poly(lactic-co-glycolic acid) nanoparticles for glioma therapy. Anti-Cancer Drugs 2019, 30, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Paillard, A.; Passirani, C.; Morille, M.; Benoit, J.P.; Betbeder, D.; Garcion, E. Transferrin adsorption onto PLGA nanoparticles governs their interaction with biological systems from blood circulation to brain cancer cells. Pharm. Res. 2012, 29, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xu, Q.; Chow, P.K.; Wang, D.; Wang, C.H. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials 2013, 34, 8511–8520. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, P.; Alata, W.; Tremblay, C.; Paris-Robidas, S.; Calon, F. Transferrin receptor-mediated uptake at the blood-brain barrier is not impaired by Alzheimer’s disease neuropathology. Mol. Pharm. 2019, 16, 583–594. [Google Scholar] [CrossRef] [PubMed]





| Property | Effect | Polymer Composition |
|---|---|---|
| Hydrophilicity | ↑ | ↓ lactide content ↓ MW |
| Glass transition temperature (Tg) | ↓ | ↓ lactide content ↓ MW |
| Crystallinity | Amorphous | Poly(glycolide) < 70% Lactide segment: poly(d,l-lactide) |
| Crystalline | Lactide segment: poly(l-lactide) |
| Method | Procedure | Size Range (nm) | Advantages | Disadvantages | Examples |
|---|---|---|---|---|---|
| Emulsion evaporation | A non-water miscible solvent containing PLGA is emulsified with an aqueous solution containing a surfactant using high shear force | ≈50 to 700 | Relatively non-toxic, small particle size, easy to scale up, and can be used to encapsulate both water-soluble and water-insoluble drugs | Drug stability may be affected during high energy mixing, and long solvent removal step | [27,28,29,30] |
| Nanoprecipitation | A water-miscible solvent containing PLGA is dispersed into an aqueous phase using low energy mixing | ≈80 to 700 | Simple, rapid, narrow size distribution, and non-toxic solvents and low energy are used | Low entrapment efficiency of polar drugs, long solvent removal step, and particle size is considerably affected by polymer concentration | [31,32,33] |
| Solvent diffusion | A partially water miscible solvent containing PLGA is emulsified with an aqueous solution of a suitable surfactant | ≈50 to 400 | Toxic solvents and high stress shear are avoided | Large quantities of water and long agitation time are required, polymer concentration notably affect the particle size, and low entrapment efficiency of polar drugs | [34,35,36] |
| Salting out | A water-miscible solvent containing PLGA is emulsified with an aqueous phase containing a high concentration of salts under high shear stress agitation. The resulting o/w emulsion is diluted with water | ≈100 to 500 | Rapid, high concentrations of PLGA can be used, no high stress shear is required, and suitable for heat-sensitive drugs | Purification step is needed, solvents used may be explosive, and not suitable for lipophilic drugs | [37,38,39,40] |
| Coating Material | Examples | Advantages | Disadvantages |
|---|---|---|---|
| Surfactants | PVA, poloxamers, polysorbates, sodium cholate, vitamin E TPGS | Preventing NP aggregation, reduced size and uniform distribution, and sustained drug release | Potential toxicity |
| PEG | – | Stealth effect, prolonged blood circulation time, and enhanced mucus penetration | Compromised drug activity, non-biodegradability with potential accumulation in the body, and potential immunogenicity |
| PEG alternatives | Poly(2-oxazoline)s, glycosaminoglycan, poly(acrylamide)s, and CS | Biodegradable, stealth effect, mucosal adhesiveness (CS), improved cellular uptake, and sustained drug release | Poor solubility (CS), cost, and potential toxicity |
| Phospholipids | Erythrocyte, platelet membranes, nanoghosts, and 1,2-dioleoyl-3-(trimethylammonium) propane (DOTAP) | Biomimetic and biodegradable properties, extended blood circulation, and controlled drug release | Increased cytotoxicity, and induction of immune response |
| Name/Company | Surface Functionality | Drug | Investigated Application | ClinicalTrials.gov Identifier Number/Status |
|---|---|---|---|---|
| BIND-014/BIND Therapeutics | PEG | Docetaxel | Advanced urothelial carcinoma, cervical cancer, cholangiocarcinoma or carcinomas of the biliary tree and squamous cell carcinoma of the head and neck | NCT02479178/Phase II completed (January 2020) |
| v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation positive or squamous cell non-small cell lung cancer (NSCLC) that have progressed after treatment of one prior platinum-containing chemotherapy regimen | NCT02283320/Phase II completed (April 2016) | |||
| Metastatic castration-resistant prostate cancer | NCT01812746/Phase II completed (April 2016) | |||
| Advanced NSCLC | NCT01792479/Phase II completed (April 2016) | |||
| Advanced or metastatic cancer | NCT01300533/Phase I completed (February 2016) | |||
| RECIOUS-01/Radboud University | – | IMM60 and NY-ESO-1 | Advanced solid tumor (immunomodulatory) | NCT04751786/Phase I recruiting (Estimated study completion date: December 2022) |
| Targeting Ligand | Ligand Attachment Technique | Preparation Method | Stabilizer | Other Surface Modifications | Mean Size (nm) | Therapeutic Agent | Application/Experiments Performed | References |
|---|---|---|---|---|---|---|---|---|
| Antibodies | ||||||||
| Anti-CD133 | Carbodiimide chemistry | Double emulsion solvent evaporation | Tween® 20/PVA | PEG | ≈175 | Methioninase/pemetrexed | Gastric carcinoma/In vitro | [119] |
| Anti-CD44 | Maleimide chemistry | Emulsification solvent evaporation | PVA (30 to 70 kDa) | Lipid film (phosphatidylcholine, DSPE, cholesterol)/ PEG | ≈140 | Salinomycin | Prostate cancer cells/In vivo | [120] |
| Anti-EGFR protein | Maleimide chemistry | Nanoprecipitation | – | PEG | ≈335 | Paclitaxel | Breast cancer/In vivo | [121] |
| Cetuximab | Carbodiimide chemistry | Emulsification solvent evaporation | PVA (30 to 50 kDa) | – | ≈130 | Docetaxel | Lung cancer/In vitro and in vivo | [122] |
| Anti-PD-1 | Carbodiimide chemistry | Emulsification solvent evaporation | PVA (30 to 70 kDa) | PEG | ≈270 | SD-208 (inhibitor of TGF-β kinase) | Immunotherapy—CD8+ T cells targeting/In vitro and in vivo | [123] |
| PSMA antibody | Maleimide chemistry | Emulsion solvent diffusion | PVA | PEG | ≈250 | Toremifene | Prostate cancer cells/In vitro and in vivo | [124] |
| Trastuzumab | Carbodiimide chemistry | Nanoprecipitation | PVA (9 to 10 kDa) | CS | ≈125 | Cisplatin | Ovarian cancer/In vitro | [125] |
| Click chemistry | Nanoprecipitation | – | PEG | ≈100 | Doxorubicin | Breast cancer/In vitro | [126] | |
| Physical electrostatic adhesion | Emulsion solvent diffusion | PVA (13 to 23 kDa) | Polyethylenimine/phosphatidylcholine | ≈220 | Docetaxel | Breast cancer/In vitro | [127,128,129,130] | |
| Biotin | Carbodiimide chemistry | Emulsification solvent evaporation | PVA (20 to 30 kDa) | PEG | ≈180 | SN-38 | Breast cancer/In vitro | [131] |
| Carbodiimide chemistry | Emulsification solvent evaporation | PVA | PEG | ≈180 | 15,16-Dihydrotanshinone I | Cervical cancer/In vitro | [132] | |
| Carbodiimide chemistry | Nanoprecipitation | PVA (30 to 70 kDa) | CS | ≈220 | Epirubicin | Breast cancer/In vitro and in vivo | [133] | |
| Not mentioned | Emulsification solvent evaporation | PVA (30 to 70 kDa) | PEG | ≈210 | Lutein | Delivery to the posterior segment of the eye/In vitro | [134,135] | |
| Bisphosphonates | ||||||||
| Alendronate | Carbodiimide chemistry | Nanoprecipitation | Pluronic® F-68 | – | ≈200 | N/A | Osteolytic bone metastases/In vitro | [136] |
| Carbodiimide chemistry | Emulsification solvent evaporation | Pluronic® F-68 | – | ≈245 | doxorubicin | Bone cancer/In vitro and in vivo | [137] | |
| Physical adhesion | Emulsification solvent evaporation | PVA (30 to 70 kDa) | – | ≈235 | Curcumin/bortezomi | Bone cancer/In vitro and in vivo | [138] | |
| Zoledronic acid | Carbodiimide chemistry | Nanoprecipitation | Pluronic® F-68 | PEG | ≈130 | Docetaxel | Bone cancer/In vitro and in vivo | [139] |
| Carbodiimide chemistry | Nanoprecipitation | Pluronic® F-68 | – | ≈190 to 245 | Gemcitabine/epirubicin | Bone cancer/In vitro and in vivo | [140] | |
| Pamidronate | Physical adhesion | Emulsification solvent evaporation | Brij® 78 | – | ≈155 | Curcumin | – | [141,142] |
| Folic acid | Carbodiimide chemistry | Nanoprecipitation | PVA | PEG | ≈190 | 5-FU | Colon and breast cancer/In vitro | [143] |
| Physical incorporation of DSPE-PEG-FA | Nanoprecipitation | – | Phospholipids/PEG | ≈200 | Pheophorbide | Gastric cancer/In vitro and in vivo | [144] | |
| Physical adhesion using a folic acid–dodecylamine conjugate | Emulsification solvent evaporation | PVA (30 to 70 kDa) | – | ≈230 | Docetaxel | Breast adenocarcinoma/In vitro and in vivo | [145] | |
| Carbodiimide chemistry | Emulsification solvent evaporation | PVA (13 to 23 kDa) | PEG | ≈200 | Oxaliplatin | Colorectal cancer/In vitro and in vivo | [146] | |
| Carbodiimide chemistry | Emulsion solvent diffusion | PVA | PEG | ≈280 | ICG | Breast cancer/In vivo | [147] | |
| Physical incorporation of DSPE-PEG-FA | Nanoprecipitation | – | Lecithin/DSPE-PEG | ≈100 | ICG | Tumor diagnosis and targeted imaging/In vitro and in vivo | [148] | |
| Carbodiimide chemistry | Emulsification solvent evaporation | Pluronic® F-68 | PEG/polypeptide K237 | ≈105 to 130 | Technetium-99 (99mTc, radiolabeled) | Ovarian cancer/In vitro and in vivo | [149] | |
| Lectins | ||||||||
| Wheat germ agglutinin | Maleimide chemistry | Emulsification solvent evaporation | Sodium cholate | PEG | ≈120 to 135 | Curcumin | Enhanced transcellular transport/In vitro | [150,151] |
| Odorranalectin | Maleimide chemistry | Double emulsion solvent evaporation | Sodium cholate | PEG | ≈115 | Urocortin peptide | Nose-to-brain delivery-Parkinson’s disease/In vivo | [152,153] |
| Solanum tuberosum | Maleimide chemistry | Emulsification solvent evaporation | Sodium cholate | PEG | ≈125 | – | Nose-to-brain delivery/In vitro and in vivo | [154] |
| Concanavalin-A | Carbodiimide chemistry | Nanoprecipitation | Pluronic® F-68 | – | ≈550 to 700 | Clarithromycin/acetohydroxamic acid | Helicobacter pylori infection/In vitro and ex vivo bioadhesion | [155] |
| Mannan | Carbodiimide chemistry | Emulsion solvent diffusion | Carbopol® 940 | PEG-PE | ≈215 | Plasmide DNA | Targeted gene delivery/In vitro and in vivo | [156] |
| Carbodiimide chemistry | Nanoprecipitation | Carbopol® 940 | PE | ≈190 | Plasmide DNA | Targeted gene delivery/In vitro and in vivo | [157] | |
| Physical adsorption or carbodiimide chemistry | Double emulsion solvent evaporation | PVA (30 to 50 kDa) | – | ≈300 to 500 | – | Vaccine formulations/In vitro | [158] | |
| Carbodiimide chemistry | Double emulsion solvent evaporation | PVA (30 to 50 kDa) | – | ≈405 | Ovalbumin | Antigen-specific T-cell responses/In vitro and in vivo | [159] | |
| Aptamers | ||||||||
| Heparanase | Carbodiimide chemistry | Nanoprecipitation | TPGS | PEG | ≈145 | Paclitaxel | Breast cancer/In vitro and in vivo | [160,161] |
| CD133 aptamers | Carbodiimide chemistry | Emulsification solvent evaporation | Sodium cholate | PEG | ≈150 | Salinomycin | Osteosarcoma cancer stem cells targeting/In vitro and in vivo | [162] |
| RNA aptamer specific for Ets1 | Carbodiimide chemistry | Emulsification solvent evaporation | PVA | – | Not specified | Gefitinib | Lung cancer/In vitro and in vivo | [163] |
| Gint4.T aptamer (anti-PDGFRβ) | Carbodiimide chemistry | Double emulsion solvent evaporation | Sodium cholate | PEG | ≈50 | Dactolisib | Glioblastoma/In vitro and in vivo | [164] |
| Peptides | ||||||||
| S2P | Maleimide chemistry | Emulsification solvent evaporation | PVA (30 to 70 kDa) | PEG | ≈185 | Imatinib | Atherosclerotic plaques/None | [165] |
| DWSW and NGR | Maleimide chemistry | Nanoprecipitation | - | PEG/erythrocyte membranes | ≈150 | Euphorbia factor L1 | Glioblastoma/In vitro and in vivo | [166] |
| SP94 | Maleimide chemistry | Nanoprecipitation | RH40 | PEG | ≈145 | Cryptotanshinone | Hepatocellular carcinoma/In vitro and in vivo | [167] |
| Penetratin, end-binding protein 1, MPG, and MPGΔNLS CPP | Carbodiimide and maleimide chemistries | Emulsification solvent evaporation | PVA | Avidin-palmitate/ DSPE-PEG | ≈325 to 390 | – | Cellular uptake enhancement/In vitro | [168,169] |
| Tat | Carbodiimide and maleimide chemistries | Emulsification solvent evaporation | Polysorbate 80 | – | ≈60 | – | Cellular uptake enhancement/In vitro | [170] |
| CPPs (Tat, pAntp4, G2) | Physical adhesion | Nanoprecipitation | PVA | PEG | ≈150 to 170 | Fluorometholone | Ocular inflammatory disorders/In vitro and in vivo | [171] |
| CPPs, e.g., Tat, penetratin, and poly(arginine) 8 | Physical adhesion | Emulsification solvent evaporation | PVA (31 kDa) | CS/PEG/Pluronic F127 | ≈150 | – | Inner-ear therapy/In vitro and in vivo | [172] |
| CPPs (R8, Tat, penetratin), and a secretion peptide | Physical adhesion | Emulsification solvent evaporation | Sugar Ester S-1670 | – | ≈115 to 160 | Insulin | Enhanced oral bioavailability of insulin/In vitro and in vivo | [173] |
| Angiopep-2 | Maleimide chemistry | Nanoprecipitation | Pluronic® F-127 | PEG | ≈165 to 180 | – | Brain targeting/In vivo | [174] |
| Cyclic-RGD peptide | Maleimide chemistry | Double emulsion solvent evaporation | Pluronic® F-127 | PEG | ≈310 to 330 | – | Angiogenic endothelium targeting/In vitro | [175,176] |
| RGD peptides | Covalent conjugation to Pluronic® F-127 via vinylsulfone-tiol reaction, and surface adhesion | Microfluidics-based nanoprecipitation | Pluronic® F-127 | PEG | ≈140 to 160 | – | Ovarian carcinoma and glioma/In vitro | [177] |
| Cyclo-(1,12)- PenITDGEATDSGC | Carbodiimide chemistry | Solvent displacement | Pluronic® F-127 | – | ≈285 to 305 | Doxorubicin | Lung cancer/In vitro | [178] |
| Sialic acid | Carbodiimide chemistry | Nanoprecipitation | Pluronic® F-68 | – | ≈125 | Lycopene | Kidney injury/In vitro | [179,180,181,182] |
| Electrostatic adsorption | Emulsification solvent evaporation | PVA | CS | ≈195 | Doxorubicin | Non-Hodgkin’s lymphoma/In vitro and in vivo | [183] | |
| Carbodiimide chemistry | Nanoprecipitation | Pluronic® F-68 | Similopioid peptide (BBB-penetrating peptide) | ≈180 | Loperamide | Central nervous system targeting/In vitro and in vivo | [184] | |
| Binds to sialic acid | Emulsification solvent evaporation | PVA (30 to 70 kDa) | Doxorubicin/Phloretin | – | [185] | |||
| Transferrin | Physical adsorption | Emulsification solvent evaporation | PVA (30 to 70 kDa) | – | ≈465 | Paclitaxel/Superparamagnetic NP | Breast cancer, brain glioma/In vitro | [186,187] |
| Carbodiimide chemistry | Emulsification solvent evaporation | PVA | – | ≈210 | Docetaxel | Breast cancer/In vitro | [188] | |
| Physical adsorption | Emulsification solvent evaporation | Pluronic® F-127 | – | ≈200 | Bortezomib | Pancreatic cancer/In vitro | [189] | |
| Physical adsorption | Double emulsion solvent evaporation | PVA | Lipid coat (lecithin/DSPE-PEG) | ≈110 | Doxorubicin | Lung cancer/In vitro and in vivo | [190] | |
| Carbodiimide chemistry | Nanoprecipitation | PVA | PEG | ≈110 | Thymoquinone | Lung cancer/In vitro and in vivo | [191] | |
| Carbodiimide chemistry | Emulsification solvent evaporation | PVA | PEG | ≈150 | Temozolomide | Brain glioma/In vitro and in vivo | [192] | |
| Physical adsorption | Nanoprecipitation | – | – | ≈90 | – | Brain glioma/In vitro and in vivo | [193] | |
| Carbodiimide chemistry | Double emulsion solvent evaporation | – | – | ≈150 | Doxorubicin/paclitaxel | Brain glioma/In vitro and in vivo | [194] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Hammadi, M.M.; Arias, J.L. Recent Advances in the Surface Functionalization of PLGA-Based Nanomedicines. Nanomaterials 2022, 12, 354. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12030354
El-Hammadi MM, Arias JL. Recent Advances in the Surface Functionalization of PLGA-Based Nanomedicines. Nanomaterials. 2022; 12(3):354. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12030354
Chicago/Turabian StyleEl-Hammadi, Mazen M., and José L. Arias. 2022. "Recent Advances in the Surface Functionalization of PLGA-Based Nanomedicines" Nanomaterials 12, no. 3: 354. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12030354