Micro Scalable Graphene Oxide Productions Using Controlled Parameters in Bench Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods and Experimental
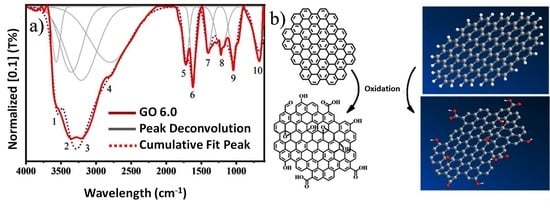
GO Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Benega, M.A.G.; Silva, W.M.; Schnitzler, M.C.; Andrade, R.J.E.; Ribeiro, H. Improvements in thermal and mechanical properties of composites based on epoxy-carbon nanomaterials—A brief landscape. Polym. Test. 2021, 98, 107180. [Google Scholar] [CrossRef]
- Ribeiro, H.; Trigueiro, J.P.C.; Woellner, C.F.; Pedrotti, J.J.; Miquita, D.R.; Silva, W.M.; Lopes, M.C.; Fechine, G.J.M.; Luciano, M.A.; Silva, G.G.; et al. Higher thermal conductivity and mechanical enhancements in hybrid 2D polymer nanocomposites. Polym. Test. 2020, 87, 106510. [Google Scholar]
- Kumar, S.S.A.; Bashir, S.; Ramesh, K.; Ramesh, S. New perspectives on Graphene/Graphene oxide based polymer nanocomposites for corrosion applications: The relevance of the Graphene/Polymer barrier coatings. Prog. Org. Coat. 2021, 154, 106215. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Huang, L.; Li, C.; Shi, G. High-yield preparation of graphene oxide from small graphite flakes via an improved Hummers method with a simple purification process. Carbon 2015, 81, 826–834. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, C.; Hao, R.; Hou, Y. Liquid-phase exfoliation, functionalization and applications of graphene. Nanoscale 2011, 3, 2118–2126. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid Phase Production of Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [Green Version]
- Luciano, M.A.; Ribeiro, H.; Bruch, G.E.; Silva, G.G. Efficiency of capacitive deionization using carbon materials based electrodes for water desalination. J. Electroanal. Chem. 2020, 859, 113840. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Muzyka, R.; Kwoka, M.; Smędowski, Ł.; Díez, N.; Gryglewicz, G. Oxidation of graphite by different modified Hummers methods. New Carbon Mater. 2017, 32, 15–20. [Google Scholar] [CrossRef]
- Yoo, M.J.; Park, H.B. Effect of hydrogen peroxide on properties of graphene oxide in Hummers method. Carbon 2019, 141, 515–522. [Google Scholar] [CrossRef]
- You, S.; Luzan, S.M.; Szabó, T.; Talyzin, A.V. Effect of synthesis method on solvation and exfoliation of graphite oxide. Carbon 2013, 52, 171–180. [Google Scholar] [CrossRef]
- Guerrero-Contreras, J.; Caballero-Briones, F. Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater. Chem. Phys. 2015, 153, 209–220. [Google Scholar] [CrossRef]
- Chen, J.; Chi, F.; Huang, L.; Zhang, M.; Yao, B.; Li, Y.; Li, C.; Shi, G. Synthesis of graphene oxide sheets with controlled sizes from sieved graphite flakes. Carbon 2016, 110, 34–40. [Google Scholar] [CrossRef]
- Godoy, A.P.; Amurim, L.G.; Mendes, A.; Gonçalves, E.S.; Ferreira, A.; de Andrade, C.S.; Kotsilkova, R.; Ivanov, E.; Lavorgna, M.; Saito, L.A.M.; et al. Enhancing the electromagnetic interference shielding of flexible films with reduced graphene oxide-based coatings. Prog. Org. Coat 2021, 158, 106341. [Google Scholar] [CrossRef]
- Moraes, L.R.d.C.; Ribeiro, H.; Cargnin, E.; Andrade, R.J.E.; Naccache, M.F. Rheology of graphene oxide suspended in yield stress fluid. J. Non-Newton. Fluid. 2020, 286, 104426. [Google Scholar] [CrossRef]
- Kulyk, B.; Freitas, M.A.; Santos, N.F.; Mohseni, F.; Carvalho, A.F.; Yasakau, K.; Fernandes, A.J.S.; Bernardes, A.; Figueiredo, B.; Silva, R.; et al. A critical review on the production and application of graphene and graphene-based materials in anti-corrosion coatings. Crit. Rev. Solid State Mater. Sci. 2021, 1–48. [Google Scholar] [CrossRef]
- Ribeiro, H.; Trigueiro, J.P.C.; Owuor, P.S.; Machado, L.D.; Woellner, C.F.; Pedrotti, J.J.; Jaques, Y.M.; Kosolwattana, S.; Chipara, A.; Silva, W.M.; et al. Hybrid 2D nanostructures for mechanical reinforcement and thermal conductivity enhancement in polymer composites. Compos. Sci. Technol. 2018, 159, 103–110. [Google Scholar]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Zhang, Z.; Schniepp, H.C.; Adamson, D.H. Characterization of graphene oxide: Variations in reported approaches. Carbon 2019, 154, 510–521. [Google Scholar] [CrossRef]
- Church, R.B.; Hu, K.; Magnacca, G.; Cerruti, M. Intercalated Species in Multilayer Graphene Oxide: Insights Gained from In Situ FTIR Spectroscopy with Probe Molecule Delivery. J. Phys. Chem. C 2016, 120, 23207–23211. [Google Scholar] [CrossRef]
- Ribeiro, H.; da Silva, W.M.; Neves, J.C.; Calado, H.D.R.; Paniago, R.; Seara, L.M.; Camarano, D.d.M.; Silva, G.G. Multifunctional nanocomposites based on tetraethylenepentamine-modified graphene oxide/epoxy. Polym. Test. 2015, 43, 182–192. [Google Scholar] [CrossRef]
- Lima, M.C.F.S.; Amparo, S.Z.d.; Ribeiro, H.; Soares, A.L.; Viana, M.M.; Seara, L.M.; Paniago, R.M.; Silva, G.G.; Caliman, V. Aqueous suspensions of carbon black with ethylenediamine and polyacrylamide-modified surfaces: Applications for chemically enhanced oil recovery. Carbon 2016, 109, 290–299. [Google Scholar] [CrossRef]
- Da Silva, W.M.; Ribeiro, H.; Neves, J.C.; Sousa, A.R.; Silva, G.G. Improved impact strength of epoxy by the addition of functionalized multiwalled carbon nanotubes and reactive diluent. J. Appl. Polym. Sci. 2015, 132, 42587. [Google Scholar] [CrossRef]
- Acik, M.; Mattevi, C.; Gong, C.; Lee, G.; Cho, K.; Chhowalla, M.; Chabal, Y.J. The Role of Intercalated Water in Multilayered Graphene Oxide. ACS Nano 2010, 4, 5861–5868. [Google Scholar] [CrossRef] [PubMed]
- Batakliev, T.; Georgiev, V.; Kalupgian, C.; Muñoz, P.A.R.; Ribeiro, H.; Fechine, G.J.M.; Andrade, R.J.E.; Ivanov, E.; Kotsilkova, R. Physico-chemical Characterization of PLA-based Composites Holding Carbon Nanofillers. Appl. Compos. Mater. 2021. [Google Scholar] [CrossRef]







| Sample | 2θ | Δ2θ | Distance (nm) |
|---|---|---|---|
| Graphite | 26.4° | 0.0 | 0.319 |
| GO 3.0 g | 10.6° | 15.8 | 0.775 |
| GO 4.5 g | 9.8° | 16.6 | 0.837 |
| GO 6.0 g | 10.1° | 16.3 | 0.813 |
| Sample | D Band λ (cm−1) | D Band Intensity (a.u.) | G Band λ (cm−1) | G Band Intensity (a.u.) | ID/IG |
|---|---|---|---|---|---|
| GO 3.0 g | 1355 | 918 | 1591 | 920 | 0.99 |
| GO 4.5 g | 1350 | 890 | 1589 | 891 | 1.00 |
| GO 6.0 g | 1353 | 911 | 1594 | 909 | 1.00 |
| Sample | Mass Loss % 25–100 °C | Mass Loss % 100–300 °C | Mass Loss % 400–700 °C | Mass Loss % above 700 °C | Residual Mass Loss % |
|---|---|---|---|---|---|
| Graphite | 0 | 0 | 0 | 100.00 | - |
| GO 3.0 g | 15.00 | 40.50 | 42.00 | - | 2.90 |
| GO 4.5 g | 15.00 | 39.00 | 44.40 | - | 1.60 |
| GO 6.0 g | 15.00 | 39.00 | 44.40 | - | 1.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, C.S.; Godoy, A.P.S.; Benega, M.A.G.; Andrade, R.J.E.; Andrade, R.C.; Silva, W.M.; Cremonezzi, J.M.d.O.; Macedo, W.A.d.A.; Gastelois, P.L.; Ribeiro, H.; et al. Micro Scalable Graphene Oxide Productions Using Controlled Parameters in Bench Reactor. Nanomaterials 2021, 11, 1975. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11081975
Andrade CS, Godoy APS, Benega MAG, Andrade RJE, Andrade RC, Silva WM, Cremonezzi JMdO, Macedo WAdA, Gastelois PL, Ribeiro H, et al. Micro Scalable Graphene Oxide Productions Using Controlled Parameters in Bench Reactor. Nanomaterials. 2021; 11(8):1975. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11081975
Chicago/Turabian StyleAndrade, Carolina S., Anna Paula S. Godoy, Marcos Antonio Gimenes Benega, Ricardo J. E. Andrade, Rafael Cardoso Andrade, Wellington Marcos Silva, Josué Marciano de Oliveira Cremonezzi, Waldemar Augusto de Almeida Macedo, Pedro Lana Gastelois, Helio Ribeiro, and et al. 2021. "Micro Scalable Graphene Oxide Productions Using Controlled Parameters in Bench Reactor" Nanomaterials 11, no. 8: 1975. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11081975