Organic-inorganic hybrid halide perovskite solar cells (PeSCs) have greatly impacted the field of solar cells and have received a lot of attention from researchers. The high PCE of up to 25.6% is derived from the suitable and adjustable optical band gap, high light absorption coefficient, low exciton binding energy, direct band gap, long carrier diffusion length, high defect tolerance of perovskite materials and so on. Researchers have done much work and proposed various strategies, especially for interface modification to improve the performance of PeSCs [
1,
2,
3]. These efforts promoted the device performance with an internal quantum efficiency (IQE) approaching 100%, which means that the electrical performance of the device is almost impossible to improve [
4,
5,
6]. However, there is still a gap between the current device PCE and the theoretical limit (31% according to the detailed balance model) [
7]. An analysis by a revised detailed balance model pointed out that optical loss can account for 40% of total energy loss [
8]. Therefore, reducing the optical loss while improving the electrical properties of the device at the same time could provide an alternative strategy to further enhance the PCE of PeSCs, where little attention has been paid.
Cesium carbonate (Cs
2CO
3) is an excellent interface modification material widely used in organic light emitting diodes (OLED), organic solar cells (OSCs), organic field-effect transistors (OFET) and other devices. Cs
2CO
3 can also be used as an interfacial passivation layer for ETL/perovskite to improve the efficiency of perovskite solar cells by reducing the recombination rate at the interface [
9]. We have used Cs
2CO
3 to electrically modify the electron transport layer (ETL) of p-i-n type PeSCs and improved device efficiency [
10]. On the other hand, due to the different refractive index of Cs
2CO
3 from indium tin oxide (ITO) and tin oxide (SnO
2), it is possible to use Cs
2CO
3 to manage optical properties of SnO
2-based PeSCs while utilizing its electrical advantages. In this work, Cs
2CO
3 is inserted between the ITO substrate and SnO
2 ETL to modify both electrical and optical properties of n-i-p type PeSCs. Thanks to the Cs
2CO
3 modification, all the short-circuit current density (J
sc), open-circuit voltage (V
oc) and fill factor (FF) are improved, leading to a high PCE of 22.9%. The greatly improved J
sc and then external quantum efficiency (EQE) are ascribed to the Cs
2CO
3 modification induced by electrical and optical improvements. Moreover, the better electrical properties (the higher carrier extraction capability) with Cs
2CO
3 modification in the device is considered to be the reason for the improvement of V
oc and FF. The better optical properties induced by Cs
2CO
3 modification is the main reason for the enhanced J
sc.
Here, the n-i-p type PeSCs with a structure of ITO/SnO
2/perovskite (FA
0.9MA
0.1PbI
3)/Spiro-OMeTAD/Ag is selected as a reference device (see
Supplementary Material Note 1 for details) [
11]. As mentioned above, a Cs
2CO
3 buffer layer is inserted between ITO and SnO
2 to get a final structure of ITO/Cs
2CO
3/SnO
2/perovskite/Spiro-OMeTAD/Ag. To obtain the best performance in the Cs
2CO
3-modification device, an optimization process has been conducted by changing the concentration of Cs
2CO
3 solution, as shown in
Figure S1. Considering the performance and solubility, a 40 mg/mL solution of Cs
2CO
3 in ethanol is finally selected in this work. The champion Cs
2CO
3-modification device is compared with the reference device in
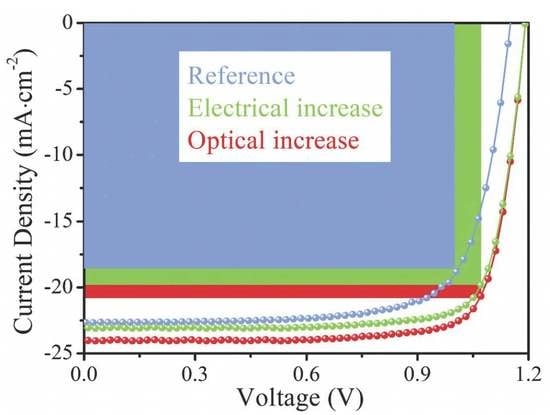
Figure 1 (with corresponding EQE results shown in
Figure S2). The Cs
2CO
3-modification device exhibits a
PCE of 22.9%, a
VOC of 1.19 V, a
JSC of 24.49 mA·cm
−2 and a
FF of 0.78. In comparison, a
PCE of 19.8% is observed in the reference device with a
VOC of 1.15 V, a
JSC of 22.83 mA·cm
−2 and a
FF of 0.75. Obviously, the PCE is significantly improved due to all parameter (
VOC,
JSC and
FF) enhancements in the Cs
2CO
3-modification device compared to the reference device with the greatest contribution from the
JSC (from 22.83 mA·cm
−2 of the reference device to 24.49 mA·cm
−2, with ~7.2% enhancement). The stabilized photocurrent measurement at the maximum power point (MPP) and hysteresis test are performed and shown in
Figures S3 and S4, respectively.
The increased V
oc and FF should be attributed to the improvement of carrier extraction of the device brought by Cs
2CO
3 modification, which is verified by transient photocurrent decay (TPC) tests. The TPC test is conducted under short circuit condition by recording the decay of photocurrent caused by a pulsed laser. Thus, the TPC signal reflects the carrier extraction capacity inside the device. In general, the faster the TPC signal decays, the easier it is for carriers in the device to be collected for a given laser intensity [
12]. The normalized TPCs of the two devices are compared in
Figure 2. Clearly, the decay time, defined as the time scale from the maximum current to no current, of the reference and Cs
2CO
3-modification device are ~40 and 10 ns, respectively. Thus, the Cs
2CO
3-modification device shows a faster decay behavior compared to the reference device, which suggests a much higher extraction capability of charge carriers in the Cs
2CO
3-modified device. It should be highlighted that the protrusion in the TPC curve at ~6 ns is attributed to the broadening of the laser itself. The corresponding J-V characteristics displayed in
Figure S5 reflects the improvement of electrical performance of ITO/SnO
2 substrate after Cs
2CO
3 modification, which should be the reason for the improved carrier extraction ability. Two samples with the structure of ITO/(with or without) Cs
2CO
3/SnO
2/PEDOT:PSS/MoO
3/Ag have been designed to verify the electrical advantage of Cs
2CO
3 modification. As evidenced in
Figure S5, the Cs
2CO
3-modification layer increases the current at the positive bias and decreases the current at the negative bias, which suggests a relative high electron extraction capability and a low leakage current in the PeSCs device after Cs
2CO
3 modification. The improved carrier extraction ability may be attributed to the effect of Cs
2CO
3 on the work function of ITO and the smoother and denser SnO
2 layer after Cs
2CO
3 modification. A large number of references have proven that Cs
2CO
3 can reduce the work function of the ITO substrate, so that electrons are more easily transferred from SnO
2 to ITO [
13]. In addition to that, the introduction of Cs
2CO
3 improves the wettability of the ITO substrate to the SnO
2 solution, thus making the prepared SnO
2 film more dense and flat, as shown in
Figures S6 and S7. However, these small changes are not reflected in the morphology and then the properties of perovskite layer on SnO
2. SEM images of perovskite films in
Figure S8 and transient photovoltage decay (TPV) test results in
Figure S9 prove that positive effects of Cs
2CO
3 to the device performance are not realized via changing the perovskite layer. In addition, XPS tests on the surface of the sample ITO/Cs
2CO
3/SnO
2 show that SnO
2 effectively prevents the diffusion of Cs to the perovskite, which prove that the effect of Cs
2CO
3 modification is not caused by the incorporation of Cs elements into perovskite, as shown in
Figure S10.
The improvement of J
sc after Cs
2CO
3 modification is not only related to the improvement of the electrical performance of the device, but also to the increase of the transmittance of the substrate. The transmittance changes of ITO and ITO/SnO
2 substrate after CS
2CO
3 modification is obtained using a Shimadzu UV-2550 Spectrophotometer (Shimadzu, Kyoto, Japan), with the optical path shown in
Figure S11. As shown in
Figure 3, the transmittance ratios of both ITO and ITO/SnO
2 substrates before and after CS
2CO
3 modification are greater than 1 at almost all wavelengths, which means that CS
2CO
3 greatly improves the transmission of the substrate. The matrix optical calculation results of the device confirm this observation. The structure of Air/glass (700,000 nm)/SiO
2 (30 nm)/ITO (135 nm)/SnO
2 or Cs
2CO
3/SnO
2 (SnO
2 for 45 nm and Cs
2CO
3/SnO
2 for 50 nm)/perovskite (600 nm)/Spiro-OMeTAD (135 nm)/Ag (100 nm) is used in the optical simulation, as shown in
Figure S12 (the details of calculation program and parameters are given in the
Supplementary Material Note 2). The thickness of each layer was verified by cross-sectional SEM as shown in
Figure S13. The optical constants of SnO
2 or Cs
2CO
3/SnO
2 layers were obtained by an ellipsometer, and the optical constants of other layers were given in the literature [
14,
15,
16]. All optical constants are shown in the spreadsheet file “Optical Constants” in the
Supplementary Material. After calculation, the light absorptions of the perovskite layer before and after Cs
2CO
3 modification are shown in
Figure 4a.
Figure 4b shows the ratio of light absorption of the perovskite layer after Cs
2CO
3 modification divided by the one before Cs
2CO
3 modification. It can be seen that the light absorption of the perovskite layer in the 300–800 nm wavelength range after Cs
2CO
3 modification has been enhanced to certain degrees. SEM results show that this increase in transmittance may be due to the unique structure of the Cs
2CO
3 layer after being washed by SnO
2 solution. As shown in
Figure S14, a continuous layer with some pinholes is formed on ITO after spin-coating Cs
2CO
3. Then, the same amount of deionized water as the SnO
2 solution used in preparing SnO
2 layer was spin-coated on Cs
2CO
3 to mimic the real experimental condition. It is found that the Cs
2CO
3 layer changes to a discontinuous distribution, which is also confirmed by energy dispersive X-ray spectroscopy (EDS) test, which can be the reason for the increased optical transmission of the substrate, as shown in
Figure S15.
In summary, we have fabricated a high efficiency PeSC device by modifying the ITO substrate with the Cs2CO3 layer. The PCE increases from 19.8% of the reference device to 22.9% of the Cs2CO3-modification device. Through TPV, TPC, AFM, UV-vis, etc. measurement, the roles of the Cs2CO3-modification layer have been addressed: improving the electron extraction capability and adjusting the light-field distribution in the perovskite layer at the same time. The improved electron extraction capability is presumably from the more compact and denser SnO2 layer after Cs2CO3-modification and work function change of ITO substrate, thus contributing a part improvement of Jsc, Voc, and FF in the device. On the other hand, the light field distribution adjustment is beneficial for utilizing more photons in the perovskite layer due to enhanced transmission of the ITO substrate. Undoubtedly, this work provides a new strategy to improve the PCE of PeSCs devices by simultaneously enhancing the electrical and optical properties, which has rarely been reported before.