Green Removal of DUV-Polarity-Modified PMMA for Wet Transfer of CVD Graphene
Abstract
:1. Introduction
2. Materials and Methods
3. Results
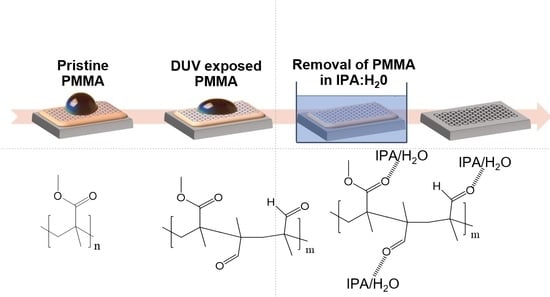
3.1. Mechanism of Solvation of Exposed PMMA
3.2. DUV-Assisted Wet Transfer of Graphene
3.3. Raman Spectroscopy of Graphene after PMMA Removal
3.4. Electrical Properties of Graphene after PMMA Removal
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S.; Falko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K.; Ferrari, A.C.; Bonaccorso, F.; Falko, V.I.; et al. Science and Technology Roadmap for Graphene, Related Two-Dimensional Crystals, and Hybrid Systems. Nature 2012, 7, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Falko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A Roadmap for Graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh Electron Mobility in Suspended Graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Morozov, S.V.; Novoselov, K.S.; Katsnelson, M.I.; Schedin, F.; Elias, D.C.; Jaszczak, J.A.; Geim, A.K. Giant Intrinsic Carrier Mobilities in Graphene and Its Bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef] [Green Version]
- Ni, Z.H.; Ponomarenko, L.A.; Nair, R.R.; Yang, R.; Anissimova, S.; Grigorieva, I.V.; Schedin, F.; Blake, P.; Shen, Z.X.; Hill, E.H.; et al. On Resonant Scatterers as a Factor Limiting Carrier Mobility in Graphene. Nano Lett. 2010, 10, 3868–3872. [Google Scholar] [CrossRef] [Green Version]
- Backes, C.; Abdelkader, A.M.; Alonso, C.; Andrieux-Ledier, A.; Arenal, R.; Azpeitia, J.; Balakrishnan, N.; Banszerus, L.; Barjon, J.; Bartali, R.; et al. Production and Processing of Graphene and Related Materials. 2D Mater. 2020, 7, 022001. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Cai, W.; Borysiak, M.; Han, B.; Chen, D.; Piner, R.D.; Colombo, L.; Ruoff, R.S. Transfer of Large-Area Graphene Films for High-Performance Transparent Conductive Electrodes. Nano Lett. 2009, 9, 4359–4363. [Google Scholar] [CrossRef]
- Ullah, S.; Yang, X.; Ta, H.Q.; Hasan, M.; Bachmatiuk, A.; Tokarska, K.; Trzebicka, B.; Fu, L.; Rummeli, M.H. Graphene Transfer Methods: A Review. Nano Res. 2021, 14, 3756–3772. [Google Scholar] [CrossRef]
- Sun, H.; Chen, D.; Wu, Y.; Yuan, Q.; Guo, L.; Dai, D.; Xu, Y.; Zhao, P.; Jiang, N.; Lin, C.-T. High Quality Graphene Films with a Clean Surface Prepared by an UV/Ozone Assisted Transfer Process. J. Mater. Chem. C 2017, 5, 1880–1884. [Google Scholar] [CrossRef]
- Qi, P.; Huang, Y.; Yao, Y.; Li, Q.; Lian, Y.; Lin, L.; Wang, X.; Gu, Y.; Li, L.; Deng, Z.; et al. Wax-Assisted Crack-Free Transfer of Monolayer CVD Graphene: Extending from Standalone to Supported Copper Substrates. Appl. Surf. Sci. 2019, 493, 81–86. [Google Scholar] [CrossRef]
- de Castro, R.K.; Araujo, J.R.; Valaski, R.; Costa, L.O.O.; Archanjo, B.S.; Fragneaud, B.; Cremona, M.; Achete, C.A. New Transfer Method of CVD-Grown Graphene Using a Flexible, Transparent and Conductive Polyaniline-Rubber Thin Film for Organic Electronic Applications. Chem. Eng. J. 2015, 273, 509–518. [Google Scholar] [CrossRef]
- Chen, M.; Stekovic, D.; Li, W.; Arkook, B.; Haddon, R.C.; Bekyarova, E. Sublimation-Assisted Graphene Transfer Technique Based on Small Polyaromatic Hydrocarbons. Nanotechnology 2017, 28, 255701. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Doidge, G.P.; Carrion, E.A.; Koepke, J.C.; Kaitz, J.A.; Datye, I.; Behnam, A.; Hewaparakrama, J.; Aruin, B.; Chen, Y.; et al. Annealing Free, Clean Graphene Transfer Using Alternative Polymer Scaffolds. Nanotechnology 2015, 26, 055302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.H.; Suk, J.W.; Lee, J.; Hao, Y.; Park, J.; Yang, J.W.; Ha, H.-W.; Murali, S.; Chou, H.; Akinwande, D.; et al. Simultaneous Transfer and Doping of CVD-Grown Graphene by Fluoropolymer for Transparent Conductive Films on Plastic. ACS Nano 2012, 6, 1284–1290. [Google Scholar] [CrossRef]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Jiao, L.; Fan, B.; Xian, X.; Wu, Z.; Zhang, J.; Liu, Z. Creation of Nanostructures with Poly(Methyl Methacrylate)-Mediated Nanotransfer Printing. J. Am. Chem. Soc. 2008, 130, 12612–12613. [Google Scholar] [CrossRef]
- Nasir, T.; Kim, B.J.; Kim, K.-W.; Lee, S.H.; Lim, H.K.; Lee, D.K.; Jeong, B.J.; Kim, H.C.; Yu, H.K.; Choi, J.-Y. Design of Softened Polystyrene for Crack- and Contamination-Free Large-Area Graphene Transfer. Nanoscale 2018, 10, 21865–21870. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lu, C.-C.; Yeh, C.-H.; Jin, C.; Suenaga, K.; Chiu, P.-W. Graphene Annealing: How Clean Can It Be? Nano Lett. 2012, 12, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yan, M. Removing Contaminants from Transferred CVD Graphene. Nano Res. 2020, 13, 599–610. [Google Scholar] [CrossRef]
- Barin, G.B.; Song, Y.; de Fátima Gimenez, I.; Souza Filho, A.G.; Barreto, L.S.; Kong, J. Optimized Graphene Transfer: Influence of Polymethylmethacrylate (PMMA) Layer Concentration and Baking Time on Graphene Final Performance. Carbon N. Y. 2015, 84, 82–90. [Google Scholar] [CrossRef]
- Park, H.; Lim, C.; Lee, C.-J.; Kang, J.; Kim, J.; Choi, M.; Park, H. Optimized Poly(Methyl Methacrylate)-Mediated Graphene-Transfer Process for Fabrication of High-Quality Graphene Layer. Nanotechnology 2018, 29, 415303. [Google Scholar] [CrossRef]
- Suhail, A.; Islam, K.; Li, B.; Jenkins, D.; Pan, G. Reduction of Polymer Residue on Wet–Transferred CVD Graphene Surface by Deep UV Exposure. Appl. Phys. Lett. 2017, 110, 183103. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhou, Q.; Wang, C.; Li, Q.; Wang, C.; Fang, Y. Toward Intrinsic Graphene Surfaces: A Systematic Study on Thermal Annealing and Wet-Chemical Treatment of SiO2-Supported Graphene Devices. Nano Lett. 2011, 11, 767–771. [Google Scholar] [CrossRef]
- Pettes, M.T.; Jo, I.; Yao, Z.; Shi, L. Influence of Polymeric Residue on the Thermal Conductivity of Suspended Bilayer Graphene. Nano Lett. 2011, 11, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.S.; Wang, H.; Yeo, J.; Martin-Martinez, F.J.; Zubair, A.; Shen, P.-C.; Mao, Y.; Palacios, T.; Buehler, M.J.; Hong, J.-Y.; et al. Paraffin-Enabled Graphene Transfer. Nat. Commun. 2019, 10, 867. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Gong, X.; Gai, J. Progress and Challenges in Transfer of Large-Area Graphene Films. Adv. Sci. 2016, 3, 1500343. [Google Scholar] [CrossRef]
- Pirkle, A.; Chan, J.; Venugopal, A.; Hinojos, D.; Magnuson, C.W.; McDonnell, S.; Colombo, L.; Vogel, E.M.; Ruoff, R.S.; Wallace, R.M. The Effect of Chemical Residues on the Physical and Electrical Properties of Chemical Vapor Deposited Graphene Transferred to SiO2. Appl. Phys. Lett. 2011, 99, 122108. [Google Scholar] [CrossRef]
- Chen, M.; Haddon, R.C.; Yan, R.; Bekyarova, E. Advances in Transferring Chemical Vapour Deposition Graphene: A Review. Mater. Horiz. 2017, 4, 1054–1063. [Google Scholar] [CrossRef]
- Tan, H.; Wang, D.; Guo, Y. Thermal Growth of Graphene: A Review. Coatings 2018, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Lisi, N.; Dikonimos, T.; Buonocore, F.; Pittori, M.; Mazzaro, R.; Rizzoli, R.; Marras, S.; Capasso, A. Contamination-Free Graphene by Chemical Vapor Deposition in Quartz Furnaces. Sci. Rep. 2017, 7, 9927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, B.J.; Hay, J.N. The Thermal Degradation of Poly(Vinyl Alcohol). Polymer 2001, 42, 6775–6783. [Google Scholar] [CrossRef]
- Peterson, J.D.; Vyazovkin, S.; Wight, C.A. Stabilizing Effect of Oxygen on Thermal Degradation of Poly(Methyl Methacrylate). Macromol. Rapid Commun. 1999, 20, 480–483. [Google Scholar] [CrossRef]
- Ahn, Y.; Kim, J.; Ganorkar, S.; Kim, Y.-H.; Kim, S.-I. Thermal Annealing of Graphene to Remove Polymer Residues. Mater. Express 2016, 6, 69–76. [Google Scholar] [CrossRef]
- Liu, B.; Chiu, I.-S.; Lai, C.-S. Improvements on Thermal Stability of Graphene and Top Gate Graphene Transistors by Ar Annealing. Vacuum 2017, 137, 8–13. [Google Scholar] [CrossRef]
- Xie, W.; Weng, L.-T.; Ng, K.M.; Chan, C.K.; Chan, C.-M. Clean Graphene Surface through High Temperature Annealing. Carbon N. Y. 2015, 94, 740–748. [Google Scholar] [CrossRef]
- Zhuang, B.; Li, S.; Li, S.; Yin, J. Ways to Eliminate PMMA Residues on Graphene—Superclean Graphene. Carbon N. Y. 2021, 173, 609–636. [Google Scholar] [CrossRef]
- Peltekis, N.; Kumar, S.; McEvoy, N.; Lee, K.; Weidlich, A.; Duesberg, G.S. The Effect of Downstream Plasma Treatments on Graphene Surfaces. Carbon N. Y. 2012, 50, 395–403. [Google Scholar] [CrossRef]
- Fragalà, M.; Compagnini, G.; Torrisi, L.; Puglisi, O. Ion Beam Assisted Unzipping of PMMA. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1998, 141, 169–173. [Google Scholar] [CrossRef]
- Lehockey, E.M.; Reid, I.; Hill, I. The Radiation Chemistry of Poly(Methyl Methacrylate) Polymer Resists. J. Vac. Sci. Technol. A Vac. Surf. Film. 1988, 6, 2221–2225. [Google Scholar] [CrossRef]
- Tinone, M.C.K.; Tanaka, K.; Ueno, N. Photodecomposition of Poly(Methylmethacrylate) Thin Films by Monochromatic Soft X-ray Radiation. J. Vac. Sci. Technol. A Vac. Surf. Film. 1995, 13, 1885–1892. [Google Scholar] [CrossRef]
- Wollersheim, O.; Zumaqué, H.; Hormes, J.; Kadereit, D.; Langen, J.; Häußling, L.; Hoessel, P.; Hoffmann, G. Quantitative Studies of the Radiation Chemical Behaviour of PMMA and Poly(Lactides). Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1995, 97, 273–278. [Google Scholar] [CrossRef]
- Cao, H.; Yu, Q.; Jauregui, L.A.; Tian, J.; Wu, W.; Liu, Z.; Jalilian, R.; Benjamin, D.K.; Jiang, Z.; Bao, J.; et al. Electronic Transport in Chemical Vapor Deposited Graphene Synthesized on Cu: Quantum Hall Effect and Weak Localization. Appl. Phys. Lett. 2010, 96, 122106. [Google Scholar] [CrossRef] [Green Version]
- Han, C.C.; Corelli, J.C. Mechanism Study of Deep-UV Irradiated Poly(Methyl Methacrylate)-Azide Resist System. Radiat. Eff. Defects Solids 1989, 111–112, 45–58. [Google Scholar] [CrossRef]
- Torikai, A.; Ohno, M.; Fueki, K. Photodegradation of Poly(Methyl Methacrylate) by Monochromatic Light: Quantum Yield, Effect of Wavelengths, and Light Intensity. J. Appl. Polym. Sci. 1990, 41, 1023–1032. [Google Scholar] [CrossRef]
- Johnstone, R.W.; Foulds, I.G.; Parameswaran, M. Deep-UV Exposure of Poly(Methyl Methacrylate) at 254 Nm Using Low-Pressure Mercury Vapor Lamps. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2008, 26, 682. [Google Scholar] [CrossRef]
- Zollner, C.J.; DenBaars, S.P.; Speck, J.S.; Nakamura, S. Germicidal Ultraviolet LEDs: A Review of Applications and Semiconductor Technologies. Semicond. Sci. Technol. 2021, 36, 123001. [Google Scholar] [CrossRef]
- Jeong, H.J.; Kim, H.Y.; Jeong, S.Y.; Han, J.T.; Baeg, K.-J.; Hwang, J.Y.; Lee, G.-W. Improved Transfer of Chemical-Vapor-Deposited Graphene through Modification of Intermolecular Interactions and Solubility of Poly(Methylmethacrylate) Layers. Carbon N. Y. 2014, 66, 612–618. [Google Scholar] [CrossRef]
- Deokar, G.; Avila, J.; Razado-Colambo, I.; Codron, J.-L.; Boyaval, C.; Galopin, E.; Asensio, M.-C.; Vignaud, D. Towards High Quality CVD Graphene Growth and Transfer. Carbon N. Y. 2015, 89, 82–92. [Google Scholar] [CrossRef]
- Dai, B.; Fu, L.; Zou, Z.; Wang, M.; Xu, H.; Wang, S.; Liu, Z. Rational Design of a Binary Metal Alloy for Chemical Vapour Deposition Growth of Uniform Single-Layer Graphene. Nat. Commun. 2011, 2, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobadilla, A.D.; Ocola, L.E.; Sumant, A.V.; Kaminski, M.; Seminario, J.M. PMMA-Assisted Plasma Patterning of Graphene. J. Nanotechnol. 2018, 2018, 8349626. [Google Scholar] [CrossRef] [Green Version]
- Mohsin, M.A.; Cowie, J.M.G. Enhanced Sensitivity in the Electron Beam Resist Poly(Methyl Methacrylate) Using Improved Solvent Developer. Polymer 1988, 29, 2130–2135. [Google Scholar] [CrossRef]
- Yasin, S.; Hasko, D.G.; Ahmed, H. Comparison of MIBK/IPA and Water/IPA as PMMA Developers for Electron Beam Nanolithography. Microelectron. Eng. 2002, 61–62, 745–753. [Google Scholar] [CrossRef]
- Hu, W.; Sarveswaran, K.; Lieberman, M.; Bernstein, G.H. Sub-10 Nm Electron Beam Lithography Using Cold Development of Poly(Methylmethacrylate). J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2004, 22, 1711. [Google Scholar] [CrossRef] [Green Version]
- Olzierski, A. Development and Molecular-Weight Issues on the Lithographic Performance of Poly (Methyl Methacrylate). Microelectron. Eng. 2004, 73–74, 244–251. [Google Scholar] [CrossRef]
- Meyer, P.; El-Kholi, A.; Schulz, J. Investigations of the Development Rate of Irradiated PMMA Microstructures in Deep X-ray Lithography. Microelectron. Eng. 2002, 63, 319–328. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Becer, C.R.; Guerrero-Sanchez, C.; Hoeppener, S.; Schubert, U.S. Solubility and Thermoresponsiveness of PMMA in Alcohol-Water Solvent Mixtures. Aust. J. Chem. 2010, 63, 1173. [Google Scholar] [CrossRef]
- Jewrajka, S.K.; Chatterjee, U.; Mandal, B.M. Homogeneous Atom Transfer Radical Polymerization of Methyl Methacrylate at Ambient Temperature in Aqueous Ethanol. Macromolecules 2004, 37, 4325–4328. [Google Scholar] [CrossRef]
- European Environment Agency. Chemicals for a Sustainable Future: Report of the EEA Scientific Committee Seminar; Copenhagen, 17 May 2017; EU Publications Office: Luxembourg, 2018. [Google Scholar]
- Persson, L.; Carney Almroth, B.M.; Collins, C.D.; Cornell, S.; de Wit, C.A.; Diamond, M.L.; Fantke, P.; Hassellöv, M.; MacLeod, M.; Ryberg, M.W.; et al. Outside the Safe Operating Space of the Planetary Boundary for Novel Entities. Environ. Sci. Technol. 2022, 56, 1510–1521. [Google Scholar] [CrossRef] [PubMed]
- Ocola, L.E.; Costales, M.; Gosztola, D.J. Development Characteristics of Polymethyl Methacrylate in Alcohol/Water Mixtures: A Lithography and Raman Spectroscopy Study. Nanotechnology 2016, 27, 035302. [Google Scholar] [CrossRef] [PubMed]
- Rene, E.R.; Sethurajan, M.; Kumar Ponnusamy, V.; Kumar, G.; Bao Dung, T.N.; Brindhadevi, K.; Pugazhendhi, A. Electronic Waste Generation, Recycling and Resource Recovery: Technological Perspectives and Trends. J. Hazard. Mater. 2021, 416, 125664. [Google Scholar] [CrossRef]
- Anaman, R.; Peng, C.; Jiang, Z.; Liu, X.; Zhou, Z.; Guo, Z.; Xiao, X. Identifying Sources and Transport Routes of Heavy Metals in Soil with Different Land Uses around a Smelting Site by GIS Based PCA and PMF. Sci. Total Environ. 2022, 823, 153759. [Google Scholar] [CrossRef]
- Suzuki, A.; Eemoto, A.; Shirai, A.; Nagamatsu, K. Ultraviolet Light-Emitting Diode (UV-LED) Sterilization of Citrus Bacterial Canker Disease Targeted for Effective Decontamination of Citrus Sudachi Fruit. Biocontrol Sci. 2022, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shirai, M.; Yamamoto, T.; Tsunooka, M. Ablative Photodegradation of Poly(Methyl Methacrylate) and Its Homologues by 185-Nm Light. Polym. Degrad. Stab. 1999, 63, 481–487. [Google Scholar] [CrossRef]
- Dybal, J.; Krimm, S. Normal-Mode Analysis of Infrared and Raman Spectra of Crystalline Isotactic Poly(Methyl Methacrylate). Macromolecules 1990, 23, 1301–1308. [Google Scholar] [CrossRef]
- Huszank, R.; Szilágyi, E.; Szoboszlai, Z.; Szikszai, Z. Investigation of Chemical Changes in PMMA Induced by 1.6 MeV He+ Irradiation by Ion Beam Analytical Methods (RBS-ERDA) and Infrared Spectroscopy (ATR-FTIR). Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2019, 450, 364–368. [Google Scholar] [CrossRef]
- Szilasi, S.Z.; Huszank, R.; Szikra, D.; Váczi, T.; Rajta, I.; Nagy, I. Chemical Changes in PMMA as a Function of Depth Due to Proton Beam Irradiation. Mater. Chem. Phys. 2011, 130, 702–707. [Google Scholar] [CrossRef]
- Dirlikov, S.; Koenig, J.L. Infrared Spectra of Poly(Methyl Methacrylate) Labeled with Oxygen-18. Appl. Spectrosc. 1979, 33, 551–555. [Google Scholar] [CrossRef]
- Dirlikov, S.K.; Koenig, J.L. Assignment of the Carbon-Hydrogen Stretching and Bending Vibrations of Poly(Methyl Methacrylate) by Selective Deuteration. Appl. Spectrosc. 1979, 33, 555–561. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C.; Buck, S.M.; Chen, Z. Molecular Chemical Structure on Poly(Methyl Methacrylate) (PMMA) Surface Studied by Sum Frequency Generation (SFG) Vibrational Spectroscopy. J. Phys. Chem. B 2001, 105, 12118–12125. [Google Scholar] [CrossRef]
- Trusovas, R.; Račiukaitis, G.; Niaura, G.; Barkauskas, J.; Valušis, G.; Pauliukaite, R. Recent Advances in Laser Utilization in the Chemical Modification of Graphene Oxide and Its Applications. Adv. Opt. Mater. 2016, 4, 37–65. [Google Scholar] [CrossRef]
- Giovambattista, N.; Debenedetti, P.G.; Rossky, P.J. Effect of Surface Polarity on Water Contact Angle and Interfacial Hydration Structure. J. Phys. Chem. B 2007, 111, 9581–9587. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; Mohsin, M.A.; McEwen, I.J. Alcohol-Water Cosolvent Systems for Poly(Methyl Methacrylate). Polymer 1987, 28, 1569–1572. [Google Scholar] [CrossRef]
- Wolf, B.A.; Blaum, G. Measured and Calculated Solubility of Polymers in Mixed Solvents: Monotony and Cosolvency. J. Polym. Sci. Polym. Phys. Ed. 1975, 13, 1115–1132. [Google Scholar] [CrossRef]
- Liang, X.; Sperling, B.A.; Calizo, I.; Cheng, G.; Hacker, C.A.; Zhang, Q.; Obeng, Y.; Yan, K.; Peng, H.; Li, Q.; et al. Toward Clean and Crackless Transfer of Graphene. ACS Nano 2011, 5, 9144–9153. [Google Scholar] [CrossRef]
- Suk, J.W.; Kitt, A.; Magnuson, C.W.; Hao, Y.; Ahmed, S.; An, J.; Swan, A.K.; Goldberg, B.B.; Ruoff, R.S. Transfer of CVD-Grown Monolayer Graphene onto Arbitrary Substrates. ACS Nano 2011, 5, 6916–6924. [Google Scholar] [CrossRef]
- Lee, J.E.; Ahn, G.; Shim, J.; Lee, Y.S.; Ryu, S. Optical Separation of Mechanical Strain from Charge Doping in Graphene. Nat. Commun. 2012, 3, 1024. [Google Scholar] [CrossRef] [Green Version]
- Ding, F.; Ji, H.; Chen, Y.; Herklotz, A.; Dörr, K.; Mei, Y.; Rastelli, A.; Schmidt, O.G. Stretchable Graphene: A Close Look at Fundamental Parameters through Biaxial Straining. Nano Lett. 2010, 10, 3453–3458. [Google Scholar] [CrossRef]
- Melios, C.; Huang, N.; Callegaro, L.; Centeno, A.; Cultrera, A.; Cordon, A.; Panchal, V.; Arnedo, I.; Redo-Sanchez, A.; Etayo, D.; et al. Towards Standardisation of Contact and Contactless Electrical Measurements of CVD Graphene at the Macro-, Micro- and Nano-Scale. Sci. Rep. 2020, 10, 3223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bøggild, P.; Mackenzie, D.M.A.; Whelan, P.R.; Petersen, D.H.; Buron, J.D.; Zurutuza, A.; Gallop, J.; Hao, L.; Jepsen, P.U. Mapping the Electrical Properties of Large-Area Graphene. 2D Mater. 2017, 4, 042003. [Google Scholar] [CrossRef] [Green Version]
- Buron, J.D.; Pizzocchero, F.; Jessen, B.S.; Booth, T.J.; Nielsen, P.F.; Hansen, O.; Hilke, M.; Whiteway, E.; Jepsen, P.U.; Bøggild, P.; et al. Electrically Continuous Graphene from Single Crystal Copper Verified by Terahertz Conductance Spectroscopy and Micro Four-Point Probe. Nano Lett. 2014, 14, 6348–6355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelan, P.R.; Shen, Q.; Luo, D.; Wang, M.; Ruoff, R.S.; Jepsen, P.U.; Bøggild, P.; Zhou, B. Reference-Free THz-TDS Conductivity Analysis of Thin Conducting Films. Opt. Express 2020, 28, 28819. [Google Scholar] [CrossRef]
- Jorudas, J.; Pashnev, D.; Alexeeva, N.; Ignatjev, I.; Urbanowicz, A.; Kasalynas, I. Characterization of Graphene Drude Conductivity by Terahertz and Infrared Spectroscopy Methods. In Proceedings of the 2021 46th International Conference on Infrared, Millimeter and Terahertz Waves (IRMMW-THz), Chengdu, China, 29 August–3 September 2021; IEEE: Manhattan, NY, USA, 2021; pp. 1–2. [Google Scholar]
- Pashnev, D.; Korotyeyev, V.V.; Jorudas, J.; Urbanowicz, A.; Prystawko, P.; Janonis, V.; Kasalynas, I. Investigation of Electron Effective Mass in AlGaN/GaN Heterostructures by THz Spectroscopy of Drude Conductivity. IEEE Trans. Electron Devices 2022, 69, 3636–3640. [Google Scholar] [CrossRef]








| Sample | I2D/IG | FWHM (cm−1) | Processing Run |
|---|---|---|---|
| DUV1 | 2.74 ± 0.31 | 30.2 ± 1.3 | #1 |
| DUV2 | 3.00 ± 0.41 | 27.8 ± 1.0 | #1 |
| DUV3 | 2.90 ± 0.22 | 27.9 ± 1.3 | #2 |
| Chloroform | 2.45 ± 0.35 | 29.5 ± 2.2 | #2 |
| Acetone | 3.13 ± 0.36 | 31.4 ± 1.2 | #2 |
| Sample | Sheet Resistance RS (Ω/sq.) | Sheet Carrier Density NS (×1012 cm−2) | Carrier Mobility µ (cm2/(V∙s)) | Carrier Type | Processing Run |
|---|---|---|---|---|---|
| DUV1 | 670 | 2.5 | 3720 | p | #1 |
| DUV2 | 840 | 2.0 | 3650 | p | #1 |
| DUV3 | 1110 | 0.8 | 6910 | n | #2 |
| Chloroform | 850 | 1.3 | 5890 | n | #2 |
| Acetone | 850 | 3.1 | 2410 | p | #2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorudas, J.; Pashnev, D.; Kašalynas, I.; Ignatjev, I.; Niaura, G.; Selskis, A.; Astachov, V.; Alexeeva, N. Green Removal of DUV-Polarity-Modified PMMA for Wet Transfer of CVD Graphene. Nanomaterials 2022, 12, 4017. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12224017
Jorudas J, Pashnev D, Kašalynas I, Ignatjev I, Niaura G, Selskis A, Astachov V, Alexeeva N. Green Removal of DUV-Polarity-Modified PMMA for Wet Transfer of CVD Graphene. Nanomaterials. 2022; 12(22):4017. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12224017
Chicago/Turabian StyleJorudas, Justinas, Daniil Pashnev, Irmantas Kašalynas, Ilja Ignatjev, Gediminas Niaura, Algirdas Selskis, Vladimir Astachov, and Natalia Alexeeva. 2022. "Green Removal of DUV-Polarity-Modified PMMA for Wet Transfer of CVD Graphene" Nanomaterials 12, no. 22: 4017. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12224017