Emerging Separation Applications of Surface Superwettability
Abstract
:1. Introduction
2. Theoretical Basis about Wettability (Typical Wetting States)
3. Solid/Liquid Separation: Removal of Liquids Away from a Solid Material
3.1. Superhydrophobicity
3.2. Superoleophobicity
3.3. Liquid Repellence
3.4. Connotation of Liquid Repellence in Liquid/Solid Separation
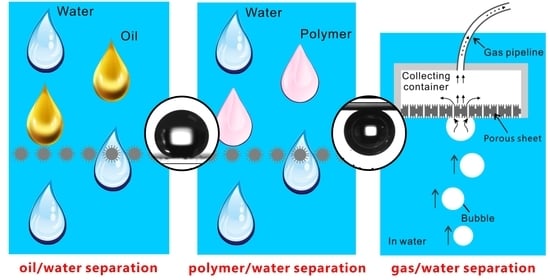
4. Oil/Water Separation Based on Superhydrophobic or Underwater Superoleophobic Materials
4.1. Superhydrophobic Porous Mesh/Membranes
4.2. Underwater Superoleophobic Porous Mesh/Membranes
4.3. Superhydrophobic 3D Porous Materials for Oil Absorption
5. Polymer/Water Separation Based on the Underwater Superpolymphobic Materials
5.1. Underwater Superpolymphobicity
5.2. Polymer/Water Separation
6. Liquid/Gas Separation by Underwater Superaerophobic and Superaerophilic Materials
6.1. Underwater Superaerophobicity and Superaerophilicity
6.2. Selective Passage of the Bubbles
6.3. Collection of Gas Bubbles in Liquid
6.4. Removal of Bubbles from Water
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic Super-Lyophobic and Super-Lyophilic Materials Applied for Oil/Water Separation: A New Strategy beyond Nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef]
- Hyman, A.-A.; Weber, C.-A.; Juelicher, F. Liquid-Liquid Phase Separation in Biology. Annu. Rev. Cell Dev. Biol. 2014, 30, 39–58. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Kuppler, R.-J.; Zhou, H.-C. Selective Gas Adsorption and Separation in Metal-Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Xue, Z.; Cao, Y.; Liu, N.; Feng, L.; Jiang, L. Special Wettable Materials for Oil/Water Separation. J. Mater. Chem. A 2014, 2, 2445–2460. [Google Scholar] [CrossRef]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal-Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Jiang, D.-E.; Cooper, V.-R.; Dai, S. Porous Graphene as the Ultimate Membrane for Gas Separation. Nano Lett. 2009, 9, 4019–4024. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Xue, M.; Zhu, G. Metal-Organic Framework Membranes: From Synthesis to Separation Application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B. Enabling Graphene Oxide Nanosheets as Water Separation Membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef]
- Yong, J.; Huo, J.; Chen, F.; Yang, Q.; Hou, X. Oil/Water Separation based on Natural Materials with Super-Wettability: Recent Advances. Phys. Chem. Chem. Phys. 2018, 20, 25140–25163. [Google Scholar] [CrossRef]
- Britt, D.; Furukawa, H.; Wang, B.; Glover, T.-G.; Yaghi, O.-M. Highly Efficient Separation of Carbon Dioxide by a Metal-Organic Framework Replete with Open Metal Sites. Proc. Natl. Acad. Sci. USA 2009, 106, 20637–20640. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.-S.; Snurr, R.-Q. Development and Evaluation of Porous Materials for Carbon Dioxide Separation and Capture. Angew. Chem. Int. Ed. 2011, 50, 11586–11596. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Ma, Y.; McCarthy, M.-C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon Dioxide Capture-Related Gas adsorption and Separation in Metal-Organic Frameworks. Coordin. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Baker, R.-W.; Low, B.-T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Huo, J.; Hou, X.; Chen, F. Superwettability-based Separation: From Oil/Water Separation to Polymer/Water Separation and Bubble/Water Separation. Nano Select 2021, 2, 1580–1588. [Google Scholar] [CrossRef]
- Li, H.; Song, Z.; Zhang, X.; Huang, Y.; Li, S.; Mao, Y.; Ploehn, H.J.; Bao, Y.; Yu, M. Ultrathin, Molecular-Sieving Graphene Oxide Membranes for Selective Hydrogen Separation. Science 2013, 342, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/Water Separation with Selective Superantiwetting/Superwetting Surface Materials. Angew. Chem. Int. Ed. 2015, 54, 2328–2338. [Google Scholar] [CrossRef]
- Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Cairns, A.J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L.; et al. Porous Materials with Optimal Adsorption Thermodynamics and Kinetics for CO2 Separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Guo, C.; Chen, F.; Hou, X. A Review of Femtosecond Laser-Structured Superhydrophobic or Underwater Superoleophobic Porous Surfaces/Materials for Efficient Oil/Water Separation. RSC Adv. 2019, 9, 12470–12495. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, J.; Li, S.; Ge, M.; Teng, L.; Chen, Z.; Lai, Y. Advanced Materials with Special Wettability toward Intelligent Oily Wastewater Remediation. ACS Appl. Mater. Interfaces 2021, 13, 67–87. [Google Scholar] [CrossRef]
- Gupta, R.-K.; Dunderdale, G.-J.; England, M.-W.; Hozumi, A. Oil/Water Separation Techniques: A Review of Recent Progresses and Future Directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Sanders, D.-E.; Smith, Z.-P.; Guo, R.; Robeson, L.-M.; McGrath, J.-E.; Paul, D.-R.; Freeman, B.-D. Energy-Efficient Polymeric Gas Separation Membranes for Sustainable Future: A Review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef] [Green Version]
- Yampolskii, Y. Polymeric Gas Separation Membranes. Macromolecules 2012, 45, 3298–3311. [Google Scholar] [CrossRef]
- Bloch, E.-D.; Queen, W.-L.; Krishna, R.; Zadrozny, J.-M.; Brown, C.-M.; Long, J.-R. Hydrocarbon Separations in a Metal-Organic Framework with Open Iron(II) Coordination Sites. Science 2012, 335, 1606–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Zhang, M.; Li, C.; Shi, G. Graphene-Based Membranes for Molecular Separation. J. Phys. Chem. Lett. 2015, 6, 2806–2815. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, S.; Jiang, L. Nature-Inspired Superwettability Systems. Nat. Rev. Mater. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Su, B.; Tian, Y.; Jiang, L. Bioinspired Interfaces with Superwettability: From Materials to Chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. [Google Scholar] [CrossRef]
- Liu, K.; Yao, X.; Lei, L. Recent Developments in Bio-Inspired Special Wettability. Chem. Soc. Rev. 2010, 39, 3240–3255. [Google Scholar] [CrossRef]
- Bellanger, H.; Darmanin, T.; Givenchy, E.-T.; Guittard, F. Chemical and Physical Pathways for the Preparation of Superoleophobic Surfaces and Related Wetting Theories. Chem. Rev. 2014, 114, 2694–2716. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Yang, Q.; Jiang, Z.; Hou, X. A Review of Femtosecond-Laser-Induced Underwater Superoleophobic Surfaces. Adv. Mater. Interfaces 2018, 5, 1701370. [Google Scholar] [CrossRef]
- Tian, Y.; Su, B.; Jiang, L. Interfacial Material System Exhibiting Superwettability. Adv. Mater. 2014, 26, 6872–6897. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Chen, F.; Yang, Q.; Hou, X. Femtosecond Laser Controlled Wettability of Solid Surfaces. Soft Matter 2015, 11, 8897–8906. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-N.; Zhang, Y.-L.; Liu, Y.; Zheng, W.; Lee, L.-P.; Sun, H.-B. Recent Developments in Superhydrophobic Graphene and Graphene-Related Materials: From Preparation to Potential Applications. Nanoscale 2015, 7, 7101–7114. [Google Scholar] [CrossRef] [PubMed]
- Teisala, H.; Tuominen, M.; Kuusipalo, J. Superhydrophobic Coatings on Cellulose-Based Materials: Fabrication, Properties, and Applications. Adv. Mater. Interfaces 2014, 1, 1300026. [Google Scholar] [CrossRef]
- Yong, J.; Singh, S.C.; Zhan, Z.; Chen, F.; Guo, C. How to Obtain Six Different Superwettabilities on a Same Microstructured Pattern: Relationship between Various Superwettabilities in Different Solid/Liquid/Gas Systems. Langmuir 2019, 35, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Chen, F.; Yang, Q.; Huo, J.; Hou, X. Superoleophobic Surfaces. Chem. Soc. Rev. 2017, 46, 4168–4217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Wang, D.; Sun, Z.; Song, J.; Deng, X. Robust Superhydrophobicity: Mechanisms and Strategies. Chem. Soc. Rev. 2021, 50, 4031–4061. [Google Scholar] [CrossRef]
- Wen, L.; Tian, Y.; Jiang, L. Bioinspired Super-Wettability from Fundamental Research to Practical Applications. Angew. Chem. Int. Ed. 2015, 54, 3387–3399. [Google Scholar] [CrossRef]
- Yao, X.; Song, Y.; Jiang, L. Applications of Bio-Inspired Special Wettable Surfaces. Adv. Mater. 2011, 23, 719–734. [Google Scholar] [CrossRef]
- Jiang, T.; Guo, Z.; Liu, W. Biomimetic Superoleophobic Surfaces: Focusing on Their Fabrication and Applications. J. Mater. Chem. A 2015, 3, 1811–1827. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the Sacred Lotus, or Escape from Contamination in Biological Surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Z.; Cheng, B.; DeSimone, J.M.; Samulski, E.T. Superhydrophobic Behavior of a Perfluoropolyether Lotus-Lead-like Topography. Langmuir 2006, 22, 8576–8580. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Yang, Q.; Hou, X.; Chen, F. Nature-Inspired Superwettability Achieved by Femtosecond Lasers. Ultrafast Science 2022, 2022, 9895418. [Google Scholar] [CrossRef]
- Zorba, V.; Stratakis, E.; Barberoglou, M.; Spanakis, E.; Tzanetakis, P.; Anastasiadis, S.H.; Fotakis, C. Biomimetic Artificial Surfaces Quantitatively Reproduce the Water Repellency of a Lotus Leaf. Adv. Mater. 2008, 20, 4049–4054. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Fang, Y.; Huo, J.; Yang, Q.; Zhang, J.; Bian, H.; Hou, X. Bioinspired Design of Underwater Superaerophobic and Superaerophilic Surfaces by Femtosecond Laser Ablation for Anti- or Capturing Bubbles. ACS Appl. Mater. Interfaces 2017, 9, 39863–39871. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Chen, F.; Zhang, D.; Du, G.; Si, J.; Yun, F.; Hou, X. A Bioinspired Planar Superhydrophobic Microboat. J. Micromech. Microeng. 2014, 24, 035006. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Wei, Z.; Song, Y.; Jiang, L. Bioinspired Design of a Superoleophobic and Low Adhesive Water/Solid Interface. Adv. Mater. 2009, 21, 665–669. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Huo, J.; Fang, Y.; Yang, Q.; Bian, H.; Li, W.; Wei, Y.; Dai, Y.; Hou, X. Green, Biodegradable, Underwater Superoleophobic Wood Sheet for Efficient Oil/Water Separation. ACS Omega 2018, 3, 1395–1402. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Li, S.; Ge, M.; Wang, L.; Xing, T.; Chen, G.; Liu, X.; Al-Deyab, S.S.; Zhang, K.; Chen, T.; et al. Robust Superhydrophobic TiO2@Fabrics for UV Shielding, Self-Cleaning and Oil-Water Separation. J. Mater. Chem. A 2015, 3, 2825–2832. [Google Scholar] [CrossRef]
- Kong, L.-H.; Chen, X.-H.; Yu, L.-G.; Wu, Z.-S.; Zhang, P.-Y. Superhydrophobic Cuprous Oxide Nanostructures on Phosphor-Copper Meshes and Their Oil-Water Separation and Oil Spill Cleanup. ACS Appl. Mater. Interfaces 2015, 7, 2616–2625. [Google Scholar] [CrossRef]
- Yu, Z.; Yun, F.; Gong, Z.; Yao, Q.; Dou, S.; Liu, K.; Jiang, L.; Wang, X. A Novel Reusable Superhydrophilic NiO/Ni Mesh Produced by A Facile Fabrication Method for Superior Oil/Water Separation. J. Mater. Chem. A 2017, 5, 10821–10826. [Google Scholar] [CrossRef]
- Su, L.; Wang, H.; Niu, M.; Fan, X.; Ma, M.; Shi, Z.; Guo, S.-W. Ultralight, Recoverable, and High-Temperature-Resistant SiC Nanowire Aerogel. ACS Nano 2018, 12, 3103–3111. [Google Scholar] [CrossRef]
- Tian, D.; Zhang, X.; Tian, Y.; Wu, Y.; Wang, X.; Zhai, J.; Jiang, L. Photo-Induced Water-Oil Separation Based on Switchable Superhydrophobicity-Superhydrophilicity and Underwater Superoleophobicity of the Aligned ZnO Nanorod Array-Coated Mesh Films. J. Mater. Chem. 2012, 22, 19652–19657. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, H.; Liu, Y.; Craig, V.; Li, L.H.; Chen, Y. Superhydrophobic and Superoleophilic Boron Nitride Nanotube-Coated Stainless Steel Meshes for Oil and Water Separation. Adv. Mater. Interfaces 2014, 1, 1300002. [Google Scholar] [CrossRef]
- Dong, Y.; Li, J.; Shi, L.; Wang, X.; Guo, Z.; Liu, W. Underwater Superoleophobic Graphene Oxide Coated Meshes for the Separation of Oil and Water. Chem. Commun. 2014, 50, 5586–5589. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, J.; Lai, H.; Du, Y.; Hou, R.; Li, C.; Zhang, N.Q.; Sun, K.N. pH-Controllable On-Demand Oil/Water Separation on the Switchable Superhydrophobic/Superhydrophilic and Underwater Low-Adhesive Superoleophobic Copper Mesh Film. Langmuir 2015, 31, 1393–1399. [Google Scholar] [CrossRef]
- Kota, A.K.; Kwon, G.; Choi, W.; Mabry, J.M.; Tuteja, A. Hygro-Responsive Membranes for Effective Oil-Water Separation. Nat. Commun. 2012, 3, 1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Lei, S.; Xue, M.; Ou, J.; Li, W. In Situ Separation and Collection of Oil from Water Surface via a Novel Superoleophilic and Superhydrophobic Oil Containment Boom. Langmuir 2014, 30, 1281–1289. [Google Scholar] [CrossRef]
- Liu, Q.; Patel, A.A.; Liu, L. Superhydrophilic and Underwater Superoleophobic Poly (Sulfobetaine Methacrylate)-Grafted Glass Fiber Filters for Oil-Water Separation. ACS Appl. Mater. Interfaces 2014, 6, 8996–9003. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, Z.; Mai, Z.; Ma, Y.; Liu, B.; Jiang, L.; Zhu, D. A Super-Hydrophobic and Super-Oleophilic Coating Mesh Film for the Separation of Oil and Water. Angew. Chem. Int. Ed. 2004, 43, 2012–2014. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wang, S.; Lin, L.; Chen, L.; Liu, M.; Feng, L.; Jiang, L. A Novel Superhydrophilic and Underwater Superoleophobic Hydrogel-Coated Mesh for Oil/Water Separation. Adv. Mater. 2011, 23, 4270–7273. [Google Scholar] [CrossRef]
- Cao, C.; Ge, M.; Huang, J.; Li, S.; Deng, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. Robust Fluorine-Free Superhydrophobic PDMS-Ormosil@Fabrics for Highly Effective Self-Cleaning and Efficient Oil-Water Separation. J. Mater. Chem. A 2016, 4, 12179–12187. [Google Scholar] [CrossRef]
- Cheng, Z.; Lai, H.; Du, Y.; Fu, K.; Hou, R.; Zhang, N.; Sun, K. Underwater Superoleophilic to Superoleophobic Wetting Control on the Nanostructured Copper Substrates. ACS Appl. Mater. Interfaces 2013, 5, 11363–11370. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lai, H.; Du, Y.; Fu, K.; Hou, R.; Li, C.; Zhang, N.; Sun, K. pH-Induced Reversible Wetting Transition between the Underwater Superoleophilicity and Superoleophobicity. ACS Appl. Mater. Interfaces 2014, 6, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, Y.; Liu, X.; Wang, D.; Li, J.; Jiang, L.; Jin, J. Salt-Induced Fabrication of Superhydrophilic and Underwater Superoleophobic PAA-g-PVDF Membranes for Effective Separation of Oil-in-Water Emulsions. Angew. Chem. Int. Ed. 2015, 53, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, L.; Li, H.; Li, W.; Zha, F.; Lei, Z. Underwater Superoleophobic Palygorskite Coated Meshes for Efficient Oil/Water Separation. J. Mater. Chem. A 2015, 3, 14696–14702. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, X.; Tao, L.; Li, K.; Xue, Z.; Feng, L.; Wei, Y. Mussel-Inspired Chemistry and Michael Addition Reaction for Efficient Oil/Water Separation. ACS Appl. Mater. Interfaces 2013, 5, 4438–4442. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-F.; Lin, S.-J. Robust Superhydrophobic/Superoleophilic Sponge for Effective Continuous Absorption and Expulsion of Oil Pollutants from Water. ACS Appl. Mater. Interface 2013, 5, 8861–8864. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, F.; Wang, D.; Pei, X.; Zhang, W.; Jin, J. A Novel Zwitterionic Polyelectrolyte Grafted PVDF Membrane for Thoroughly Separating Oil from Water with Ultrahigh Efficiency. J. Mater. Chem. A 2013, 1, 5758–5765. [Google Scholar] [CrossRef]
- Gui, X.; Wei, J.; Wang, K.; Cao, A.; Zhu, H.; Jia, Y.; Shu, Q.; Wu, D. Carbon Nanotube Sponges. Adv. Mater. 2010, 22, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yao, T.; Wu, J.; Ma, C.; Fan, Z.; Wang, Z.; Cheng, Y.; Lin, Q.; Yang, B. Facile Approach in Fabricating Superhydrophobic and Superoleophilic Surface for Water and Oil Mixture Separation. ACS Appl. Mater. Interfaces 2009, 11, 2613–2617. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Moni, P.; Gleason, K.K. Ultrathin Zwitterionic Coatings for Roughness-Independent Underwater Superoleophobicity and Gravity-Driven Oil-Water Separation. Adv. Mater. Interfaces 2014, 1, 1400489. [Google Scholar] [CrossRef]
- Li, J.; Xu, C.; Zhang, Y.; Wang, R.; Zha, F.; She, H. Robust Superhydrophobic Attapulgite Coated Polyurethane Sponge for Efficient Immiscible Oil/Water Mixture and Emulsion Separation. J. Mater. Chem. A 2016, 4, 15546–15553. [Google Scholar] [CrossRef]
- Zhu, Q.; Chu, Y.; Wang, Z.; Chen, N.; Lin, L.; Li, F.; Pan, Q. Robust Superhydrophobic Polyurethane Sponge as A Highly Reusable Oil-Absorption Material. J. Mater. Chem. A 2013, 1, 5386–5393. [Google Scholar] [CrossRef]
- Bian, H.; Yong, J.; Yang, Q.; Hou, X.; Chen, F. Simple and Low-Cost Oil/Water Separation Based on the Underwater Superoleophobicity of the Existing Materials in Our Life or Nature. Front. Chem. 2020, 8, 507. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Z. pH-Responsive Bidirectional Oil-Water Separation Material. Chem. Commun. 2013, 49, 9416. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, Y.S.; Xiong, W.; Wang, M.; Fan, L.; Rabiee-Golgir, H.; Jiang, L.; Hou, W.; Huang, X.; Jiang, L.; et al. Highly Efficient and Recyclable Carbon Soot Sponge for Oil Cleanup. ACS Appl. Mater. Interfaces 2016, 4, 5924–5929. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Liu, K.; Jiang, L. Bioinspired Multifunctional Foam with Self-Cleaning and Oil/Water Separation. Adv. Funct. Mater. 2013, 23, 2881–2886. [Google Scholar] [CrossRef]
- Zhai, P.; Jia, H.; Zheng, Z.; Lee, C.; Su, H.; Wei, T.; Feng, S. Tuning Surface Wettability and Adhesivity of a Nitrogen-Doped Graphene Foam after Water Vapor Treatment for Efficient Oil Removal. Adv. Mater. Interfaces 2015, 2, 1500243. [Google Scholar] [CrossRef]
- Wen, G.; Guo, Z.; Liu, W. Biomimetic Polymeric Superhydrophobic Surfaces and Nanostructures: From Fabrication to Applications. Nanoscale 2017, 9, 3338–3366. [Google Scholar] [CrossRef]
- Genzer, J.; Efimenko, K. Recent Developments in Superhydrophobic Surfaces and Their Relevance to Marine Fouling: A Review. Biofouling 2006, 22, 339–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, L. Definition of Superhydrophobic States. Adv. Mater. 2007, 19, 3423–3424. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, Z. Superhydrophobic Nanocoatings: From Materials to Fabrications and to Applications. Nanoscale 2015, 7, 5922–5946. [Google Scholar]
- Wenzel, R.N. Resistance of Solid Surfaces to Wetting by Water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1994, 40, 546–551. [Google Scholar] [CrossRef]
- Li, J.; Jing, Z.; Zha, F.; Yang, Y.; Wang, Q.; Lei, Z. Facile Spray-Coating Process for the Fabrication of Tunable Adhesive Superhydrophobic Surfaces with Heterogeneous Chemical Compositions Used for Selective Transportation of Microdroplets with Different Volumes. ACS Appl. Mater. Interfaces 2014, 6, 8868–8877. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jiang, L. Switchable Adhesion on Liquid/Solid Interfaces. Adv. Funct. Mater. 2010, 20, 3753–3764. [Google Scholar] [CrossRef]
- Li, K.; Zeng, X.; Li, H.; Lai, X.; Ye, C.; Xie, H. Study on the Wetting Behavior and Theoretical Models of Polydimethylsiloxane/Silica Coating. Appl. Surf. Sci. 2013, 279, 458–463. [Google Scholar] [CrossRef]
- Cheng, Z.; Du, M.; Lai, H.; Zhang, N.; Sun, K. From Petal Effect to Lotus Effect: A Facile Solution Immersion Process for the Fabrication of Super-Hydrophobic Surfaces with Controlled Adhesion. Nanoscale 2013, 5, 2776–2783. [Google Scholar] [CrossRef]
- Xia, F.; Jiang, L. Bio-Inspired, Smart, Multiscale Interfacial Materials. Adv. Mater. 2008, 20, 2842–2858. [Google Scholar] [CrossRef]
- Erbil, H.Y.; Cansoy, C.E. Range of Applicability of the Wenzel and Cassie- Baxter Equations for Superhydrophobic Surfaces. Langmuir 2009, 25, 14135–14145. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.-Y.; Liu, Q.; Zhu, S.-P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of Robust Superhydrophobic Surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Gao, N.; Barthlott, W. Mimicking Natural Superhydrophobic Surfaces and Grasping the Wetting Process: A Review on Recent Progress in Preparing Superhydrophobic Surfaces. Adv. Colloid Interfaces 2011, 169, 80–105. [Google Scholar] [CrossRef]
- Verho, T.; Bower, C.; Andrew, P.; Franssila, S.; Ikkala, O.; Ras, R.H.A. Mechanically Durable Superhydrophobic Surfaces. Adv. Mater. 2011, 23, 673–678. [Google Scholar] [CrossRef]
- Bai, X.; Yang, Q.; Fang, Y.; Yong, J.; Bai, Y.; Zhang, J.; Hou, X.; Chen, F. Anisotropic, Adhesion-Switchable, and Thermal-Responsive Superhydrophobicity on the Femtosecond Laser-Structured Shape-Memory Polymer for Droplet Manipulation. Chem. Eng. J. 2020, 400, 125930. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Recent Advances in the Potential Applications of Bioinspired Superhydrophobic Materials. J. Mater. Chem. A 2014, 2, 16319–16359. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Yu, J.; Wang, M. Engineering Biomimetic Superhydrophobic Surfaces of Electrospun Nanomaterials. Nano Today 2011, 6, 510–530. [Google Scholar] [CrossRef]
- Simpson, J.T.; Hunter, S.R.; Aytug, T. Superhydrophobic Materials and Coatings: A Review. Rep. Prog. Phys. 2015, 78, 086501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lv, F.Y. A Review of the Recent Advances in Superhydrophobic Surfaces and the Emerging Energy-Related Applications. Energy 2015, 82, 1068–1087. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Y.; Ding, J.; Feng, L.; Wang, X.; Lin, T. Durable, Self-Healing Superhydrophobic and Superoleophobic Surfaces from Fluorinated-Decyl Polyhedral Oligomeric Silsesquioxane and Hydrolyzed Fluorinated Alkyl Silane. Angew. Chem. Int. Ed. 2011, 50, 11433–11436. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, Y.; Zhou, F.; Liu, W. Extreme Wettability and Tunable Adhesion: Biomimicking Beyond Nature? Soft Matter 2012, 8, 2070–2086. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Chen, F.; Zhang, D.; Du, G.; Si, J.; Yun, F.; Hou, X. Superhydrophobic PDMS Surfaces with Three-Dimensional (3D) Pattern-Dependent Controllable Adhesion. Appl. Surf. Sci. 2014, 288, 579–583. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Shi, L.; Li, J.; Guo, Z. Recent Progress of Double-Structural and Functional Materials with Special Wettability. J. Mater. Chem. 2012, 22, 799–815. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Yang, Q.; Farooq, U.; Hou, X. Photoinduced Switchable Underwater Superoleophobicity-Superoleophilicity on Laser Modified Titanium Surfaces. J. Mater. Chem. A 2015, 3, 10703–10709. [Google Scholar] [CrossRef]
- Xue, C.-H.; Ma, J.-Z. Long-Lived Superhydrophobic Surfaces. J. Mater. Chem. A 2013, 1, 4146–4161. [Google Scholar] [CrossRef]
- Liu, K.; Tian, Y.; Jiang, L. Bio-Inspired Superoleophobic and Smart Materials: Design, Fabrication, and Application. Prog. Mater. Sci. 2013, 58, 503–564. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Chen, F.; Zhang, D.S.; Du, G.Q.; Bian, H.; Si, J.H.; Hou, X. Bioinspired Superhydrophobic Surfaces with Directional Adhesion. RSC Adv. 2014, 4, 8138–8143. [Google Scholar] [CrossRef]
- Han, J.; Cai, M.; Lin, Y.; Liu, W.; Luo, X.; Zhang, H.; Zhong, M. 3D Re-Entrant Nanograss on Microcones for Durable Superamphiphobic Surfaces via Laser-Chemical Hybrid Method. Appl. Surf. Sci. 2018, 456, 726–736. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, Q.; Fang, Y.; Yong, J.L.; Chen, F.; Hou, X. Underwater Anisotropic 3D Superoleophobic Tracks Applied for the Directional Movement of Oil Droplets and the Microdroplets Reaction. Adv. Mater. Interfaces 2019, 6, 1900067. [Google Scholar] [CrossRef]
- Bhushan, B. Biomimetics: Lessons from Nature-An Overview. Phil. Trans. R. Scc. A 2009, 367, 1445–1486. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.J.; Zheng, Y.M.; Zhai, J.; Jiang, L. Bioinspired Super-Antiwetting Interfaces with Special Liquid-Solid Adhesion. Acc. Chem. Res. 2010, 43, 368–377. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Superhydrophobic and Superoleophobic Properties in Nature. Mater. Today 2015, 18, 273–285. [Google Scholar] [CrossRef]
- Stratakis, E.; Bonse, J.; Heitz, J.; Siegel, J.; Tsibidis, G.D.; Skoulas, E.; Papadopoulos, A.; Mimidis, A.; Joel, A.-C.; Comanns, P.; et al. Laser Engineering of Biomimetic Surfaces. Mater. Sci. Eng. R Rep. 2020, 141, 100562. [Google Scholar] [CrossRef]
- Parker, A.R.; Lawrence, C.R. Water Capture by a Desert Beetle. Nature 2001, 414, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jiang, L. Water-Repellent Legs of Water Striders. Nature 2004, 432, 36. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, X.; Jiang, L. Directional Adhesion of Superhydrophobic Butterfly Wings. Soft Matter 2007, 3, 178–182. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal Effect: A Superhydrophobic State with High Adhesive Force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yan, X.; Yao, X.; Xu, L.; Zhang, K.; Zhang, J.; Yang, B.; Jiang, L. The Dry-Style Antifogging Properties of Mosquito Compound Eyes and Artificial Analogues Prepared by Soft Lithography. Adv. Mater. 2007, 19, 2213–2217. [Google Scholar] [CrossRef]
- Barthlott, W.; Schimmel, T.; Wiersch, S.; Koch, K.; Brede, M.; Barczewski, M.; Walheim, S.; Weis, A.; Kaltenmaier, A.; Leder, A.; et al. The Salvinia Paradox: Superhydrophobic Surfaces with Hydrophilic Pins for Air Retention Under Water. Adv. Mater. 2010, 22, 2325–2328. [Google Scholar] [CrossRef]
- Zheng, Y.; Bai, H.; Huang, Z.; Tian, X.; Nie, F.Q.; Zhao, Y.; Zhai, J.; Jiang, L. Directional Water Collection on Wetted Spider Silk. Nature 2010, 463, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, J.-N.; Wu, S.-Z.; Chen, Q.-D.; Zhao, S.; Zhang, H.; Sun, H.-B.; Jiang, L. Three-Level Biomimetic Rice-Leaf Surfaces with Controllable Anisotropic Sliding. Adv. Funct. Mater. 2011, 21, 2927–2932. [Google Scholar] [CrossRef]
- Ju, J.; Bai, H.; Zheng, Y.; Zhao, T.; Fang, R.; Jiang, L. A Multi-Structural and Multi-Functional Integrated Fog Collection System in Cactus. Nat. Commun. 2012, 3, 1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milionis, A.; Loth, E.; Bayer, I.S. Recent Advances in the Mechanical Durability of Superhydrophobic Materials. Adv. Colloid Interfaces 2016, 229, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Choi, C.-H.; Kim, C.-J. Superhydrophobic Drag Reduction in Laminar Flows: A Critical Review. Exp. Fluids 2016, 57, 176. [Google Scholar] [CrossRef] [Green Version]
- Yong, J.; Yang, Q.; Hou, X.; Chen, F. Relationship and Interconversion between Superhydrophilicity, Superhydrophobicity, Underwater Superoleophilicity, Underwater Superoleophobicity, Underwater Superaerophilicity, and Underwater Superaerophobicity: A Mini-Review. Front. Chem. 2020, 8, 828. [Google Scholar] [CrossRef]
- Das, S.; Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. A Review on Superhydrophobic Polymer Nanocoatings: Recent Development and Applications. Ind. Eng. Chem. Res. 2018, 57, 2727–2745. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M.; Joseph, G.B.; Durairaj, R.B.; Mageshwaran, G. Superhydrophobic Surfaces: A Review on Fundamentals, Applications, and Challenges. J. Coat. Technol. Res. 2018, 15, 231–250. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of Superhydrophobic Coatings as a Corrosion Barrier: A Review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Shi, Y.; Dong, Z.; Jiang, L. Bioinspired Designs of Superhydrophobic and Superhydrophilic Materials. ACS Central Sci. 2018, 4, 1102–1112. [Google Scholar]
- Phuong, N.-T.; Tran, H.N.; Plamondon, C.O.; Tuduri, L.; Vo, D.-V.N.; Nanda, S.; Mishra, A.; Chao, H.-P.; Bajpai, A.K. Recent Progress in the Preparation, Properties and Applications of Superhydrophobic Nano-Based Coatings and Surfaces: A Review. Prog. Org. Coat. 2019, 132, 235–256. [Google Scholar]
- Sam, E.K.; Sam, D.K.; Lv, X.; Liu, B.; Xiao, X.; Gong, S.; Yu, W.; Chen, J.; Liu, J. Recent Development in the Fabrication of Self-Healing Superhydrophobic Surfaces. Chem. Eng. J. 2019, 373, 531–546. [Google Scholar]
- Darband, G.B.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and Engineering of Superhydrophobic Surfaces: Review of Corrosion Resistance, Chemical and Mechanical Stability. Arab. J. Chem. 2020, 13, 1763–1802. [Google Scholar] [CrossRef]
- Dalawai, S.P.; Mohamed, M.A.S.; Latthe, S.S.; Xing, R.; Sutar, R.S.; Nagappan, S.; Ha, C.-S.; Sadasivuni, K.K.; Liu, S. Recent Advances in Durability of Superhydrophobic Self-Cleaning Technology: A Critical Review. Prog. Org. Coat. 2020, 138, 105381. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, D.S.; Yang, Q.; Yong, J.; Du, G.Q.; Si, J.H.; Yun, F.; Hou, X. Bioinspired Wetting Surface via Laser Microfabrication. ACS Appl. Mater. Interfaces 2013, 5, 6777–6792. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Yang, C.; Yu, N.; Shang, Y.; Sun, Y.; Wang, L.; Liu, C. Superhydrophobic TiO2/Polyvinylidene Fluoride Composite Surface with Reversible Wettability Switching and Corrosion Resistance. Chem. Eng. J. 2016, 290, 37–44. [Google Scholar] [CrossRef]
- Wang, L.; Gong, Q.; Zhan, S.; Jiang, L.; Zheng, Y. Robust Anti-Icing Performance of a Flexible Superhydrophobic Surface. Adv. Mater. 2016, 28, 7729–7735. [Google Scholar] [CrossRef]
- Cho, E.-C.; Chang-Jian, C.-W.; Chen, H.-C.; Chuang, K.-S.; Zheng, J.-H.; Hsiao, Y.-S.; Lee, K.-C.; Huang, J.-H. Robust Multifunctional Superhydrophobic Coatings with Enhanced Water/Oil Separation, Self-Cleaning, Anti-Corrosion, and Anti-Biological Adhesion. Chem. Eng. J. 2017, 314, 347–357. [Google Scholar] [CrossRef]
- Li, L.; Bai, Y.; Li, L.; Wang, S.; Zhang, T. A Superhydrophobic Smart Coating for Flexible and Wearable Sensing Electronics. Adv. Mater. 2017, 29, 1702517. [Google Scholar] [CrossRef]
- Feng, K.; Hung, H.-Y.; Liu, J.; Li, M.; Zhou, C.; Liu, M. Fabrication of High Performance Superhydrophobic Coatings by Spray-Coating of Polysiloxane Modified Halloysite Nanotubes. Chem. Eng. J. 2018, 331, 744–754. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Yang, Q.; Zhang, D.S.; Bian, H.; Du, G.Q.; Si, J.H.; Meng, X.W.; Xou, X. Controllable Adhesive Superhydrophobic Surfaces Based on PDMS Microwell Arrays. Langmuir 2013, 29, 3274–3279. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.-Q.; Liu, Y.; Han, B.; Wang, H.; Han, D.-D.; Wang, J.-N.; Zhang, Y.-L.; Sun, H.-B. Direct Laser Writing of Superhydrophobic PDMS Elastomers for Controllable Manipulation via Marangoni Effect. Adv. Funct. Mater. 2017, 24, 1702946. [Google Scholar] [CrossRef]
- Fang, Y.; Yong, J.L.; Chen, F.; Huo, J.; Yang, Q.; Zhang, J.; Hou, X. Bioinspired Fabrication of Bi/Tridirectionally Anisotropic Sliding Superhydrophobic PDMS Surfaces by Femtosecond Laser. Adv. Mater. Interfaces 2018, 5, 1701245. [Google Scholar] [CrossRef]
- Gong, D.; Long, J.; Jiang, D.; Fan, P.; Zhang, H.; Li, L.; Zhong, M. Robust and Stable Transparent Superhydrophobic Polydimethylsiloxane Films by Duplicating via a Femtosecond Laser-Ablated Template. ACS Appl. Mater. Interfaces 2016, 8, 17511–17518. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, H.; Zhai, J.; Song, Y.; Jiang, L.; Zhu, D. Super-Hydrophobic Surface of Aligned Polyacrylonitrile Nanofibers. Angew. Chem. 2002, 114, 1269–1271. [Google Scholar] [CrossRef]
- Fang, Y.; Yong, J.; Chen, F.; Huo, J.; Yang, Q.; Bian, H.; Du, G.; Hou, X. Durability of the Tunable Adhesive Superhydrophobic PTFE Surfaces for Harsh Environment Applications. Appl. Phys. A 2016, 122, 827. [Google Scholar] [CrossRef]
- Pernites, R.B.; Ponnapati, R.R.; Advincula, R.C. Superhydrophobic-Superoleophilic Polythiophene Films with Tunable Wetting and Electrochromism. Adv. Mater. 2011, 23, 3207–3213. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S.; Darmanin, T.; Guittard, F. Elaboration of Voltage and Ion Exchange Stimuli-Responsive Conducting Polymers with Selective Switchable Liquid-Repellency. ACS Appl. Mater. Interfaces 2014, 6, 7953–7960. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, L.; Zhang, Z.; Wu, S.; Li, G.; Wu, P.; Hu, Y.; Li, J.; Chu, J.; Wu, D. Biomimetic Surfaces with Anisotropic Sliding Wetting by Energy-Modulation Femtosecond Laser Irradiation for Enhanced Water Collection. RSC Adv. 2017, 7, 11170–11179. [Google Scholar] [CrossRef] [Green Version]
- Yin, K.; Yang, S.; Dong, X.; Chu, D.; Duan, J.-A.; He, J. Robust Laser-Structured Asymmetrical PTFE Mesh for Underwater Directional Transportation and Continuous Collection of Gas Bubbles. Appl. Phys. Lett. 2018, 112, 243701. [Google Scholar] [CrossRef]
- Yong, J.; Zhang, C.; Bai, X.; Zhang, J.; Yang, Q.; Hou, X.; Chen, F. Designing “Supermetalphobic” Surfaces that Greatly Repel Liquid Metal by Femtosecond Laser Processing: Does the Surface Chemistry or Microstructure Play a Crucial Role? Adv. Mater. Interfaces 2020, 7, 1901931. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Niu, H.; Gestos, A.; Wang, X.; Li, T. Fluoroalkyl Silane Modified Silicone Rubber/Nanoparticle Composite: A Super Durable, Robust Superhydrophobic Fabric Coating. Adv. Mater. 2012, 24, 2409–2412. [Google Scholar] [CrossRef]
- Bai, X.; Yong, J.; Shan, C.; Fang, Y.; Hou, X.; Chen, F. Remote, Selective, and in Situ Manipulation of Liquid Droplets on a Femtosecond Laser-Structured Superhydrophobic Shape-Memory Polymer by Near-Infrared Light. Sci. China Chem. 2021, 64, 861–872. [Google Scholar] [CrossRef]
- Li, Y.; Han, J.; Cai, M.; Liu, W.; Luo, X.; Zhang, H.; Zhong, M. Durable and Robust Transparent Superhydrophobic Glass Surfaces Fabricated by a Femtosecond Laser with Exceptional Water Repellency and Thermostability. J. Mater. Chem. A 2018, 6, 9049–9056. [Google Scholar]
- Zhang, D.; Chen, F.; Yang, Q.; Yong, J.; Bian, H.; Ou, Y.; Si, J.H.; Meng, X.W.; Hou, X. A Simple Way to Achieve Pattern-Dependent Tunable Adhesion in Superhydrophobic Surfaces by a Femtosecond Laser. ACS Appl. Mater. Interfaces 2012, 4, 4905–4912. [Google Scholar] [CrossRef] [PubMed]
- Larmour, I.A.; Bell, S.E.J.; Saunders, G.C. Remarkably Simple Fabrication of Superhydrophobic Surafces Using Electroless Galvanic Deposition. Angew. Chem. 2007, 119, 1740–1742. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Huo, J.; Fang, Y.; Yang, Q.; Zhang, J.; Hou, X. Femtosecond Laser Induced Hierarchical ZnO Superhydrophobic Surfaces with Switchable Wettability. Chem. Commun. 2015, 51, 9813–9816. [Google Scholar] [CrossRef]
- Cheng, Z.; Feng, L.; Jiang, L. Tunable Adhesive Superhydrophobic Surafces for Superaramagnetic Microdroplets. Adv. Funct. Mater. 2008, 18, 3219–3225. [Google Scholar] [CrossRef]
- Bai, X.; Yang, Q.; Fang, Y.; Zhang, J.; Yong, J.L.; Hou, X.; Chen, F. Superhydrophobicity-Memory Surfaces Prepared by a Femtosecond Laser. Chem. Eng. J. 2020, 383, 123143. [Google Scholar] [CrossRef]
- Nakajima, A.; Fujishima, A.; Hashimoto, K.; Watannabe, T. Preparation of Transparent Superhydrophobic Boehmite and Silica Films by Sublimation of Aluminum Acetylacetonate. Adv. Mater. 1999, 11, 1365–1368. [Google Scholar] [CrossRef]
- Chiou, N.-R.; Lu, C.; Guan, J.; Lee, L.J.; Epstein, A.J. Growth and Alignment of Polyaniline Nanofibres with Superhydrophobic, Superhydrophilic and Other Properties. Nat. Nanotechnol. 2007, 2, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Liu, M.; Jiang, L. Recent Development in Polymeric Superoleophobic Surfaces. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1209–1224. [Google Scholar] [CrossRef]
- Yun, G.-T.; Jung, W.-B.; Oh, M.S.; Jang, G.M.; Baek, J.; Kim, N.; Im, S.G.; Jung, H.-T. Springtail-Inspired Superomniphobic Surface with Extreme Pressure Resistance. Sci. Adv. 2018, 4, eaat4978. [Google Scholar] [CrossRef] [Green Version]
- Hensel, R.; Finn, A.; Helbig, R.; Braun, H.-G.; Neinhuis, C.; Fischer, W.-J.; Werner, C. Biologically Inspired Omniphobic Surfaces by Reverse Imprint Lithography. Adv. Mater. 2014, 26, 2029–2033. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhang, X.; Li, Q.; Liu, C.; Ye, T.; Liu, J.; Xu, H.; Zhang, X.; Jiang, J.L.C.; Xue, L.; et al. Springtail-Inspired Superamphiphobic Ordered Nanohoodoo Arrays with Quasi-Doubly Reentrant Structures. Small 2020, 16, 2000779. [Google Scholar] [CrossRef]
- Chu, Z.; Seeger, S. Superamphiphobic Surfaces. Chem. Soc. Rev. 2014, 43, 2784–2798. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Y.; Huang, J.; Chen, Z.; Chen, G.; Lai, Y. Bioinspired Surfaces with Superamphiphobic Properties: Concepts, Synthesis, and Applications. Adv. Funct. Mater. 2018, 28, 1707415. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing Superoleophobic Surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef] [Green Version]
- Tuteja, A.; Choi, W.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Robust Omniphobic Surfaces. Proc. Natl. Acad. Sci. USA 2008, 105, 18200–18205. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, A.; Taylor, J.A.; Lifton, V.; Sidorenko, A.A.; Salamon, T.R.; Lobaton, E.J.; Kolodner, A.P.; Krupenkin, T.N. Nanonails: A Simple Geometrical Approach to Electrically Tunable Superlyophobic Surfaces. Langmuir 2008, 24, 9–14. [Google Scholar] [CrossRef]
- Im, M.; Im, H.; Lee, J.-H.; Yoon, J.-B.; Choi, Y.-K. A Robust Superhydrophobic and Superoleophobic Surface with Inverse-Trapezoidal Microstructures on a Large Transparent Flexible Substrate. Soft Matter 2010, 6, 1401–1404. [Google Scholar] [CrossRef]
- Zhao, H.; Law, K.-Y.; Sambhy, V. Fabrication, Surface Properties, and Origin of Superoleophobicity for a Model Textured Surface. Langmuir 2011, 27, 5927–5935. [Google Scholar] [CrossRef]
- Kota, A.-K.; Li, Y.; Mabry, J.-M.; Tuteja, A. Hierarchically Structured Superoleophobic Surfaces with Ultralow Contact Angle Hysteresis. Adv. Mater. 2012, 24, 5838–5843. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Law, K.-Y. Directional Self-Cleaning Superoleophobic Surface. Langmuir 2012, 28, 11812–11818. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wu, T.; Zhang, W.; Ling, S.; Xiang, R.; Gui, X.; Zhu, Y.; Tang, Z. Engineering Superlyophobic Surfaces on Curable Materials based on Facile and Inexpensive Microfabrication. J. Mater. Chem. A 2014, 2, 6952–6959. [Google Scholar] [CrossRef]
- Emarati, S.-M.; Mozammel, M. Theoretical, Fundamental and Experimental Study of Liquid-Repellency and Corrosion Resistance of Fabricated Superamphiphobic Surface on Al Alloy 2024. Chem. Eng. J. 2020, 387, 124046. [Google Scholar] [CrossRef]
- Wong, W.-S.-Y.; Corrales, T.-P.; Naga, A.; Baumli, P.; Kaltbeitzel, A.; Kappl, M.; Papadopoulos, P.; Vollmer, D.; Butt, H.-J. Microdroplet Contaminants: When and Why Superamphiphobic Surfaces are Not Self-Cleaning. ACS Nano 2020, 14, 3836–3846. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wang, D.; Guo, Z. Highly Fluorinated F-APP-TiO2 Particle with Hierarchical Core-Shell Structure and Its Application in Multifunctional Superamphiphobic Surface: Mechanical Robustness, Self-Recovery and Flame Retardancy. J. Colloid Interfaces Sci. 2020, 560, 777–786. [Google Scholar] [CrossRef]
- Han, X.; Peng, J.; Jiang, S.; Xiong, J.; Song, Y.; Gong, X. Robust Superamphiphobic Coatings Based on Raspberry-like Hollow SnO2 Composites. Langmuir 2020, 36, 11044–11053. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, Y.; Wang, J.; Sun, Y.; Zhang, J.; Li, Y. Superamphiphobic Aluminum Alloy with Low Sliding Angles and Acid-Alkali Liquids Repellency. Mater. Des. 2020, 188, 108479. [Google Scholar] [CrossRef]
- Kota, A.-K.; Kwon, G.; Tuteja, A. The Design and Applications of Superomniphobic Surfaces. NPG Asia Mater. 2014, 6, e109. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.-L.; Kim, C.-J. Turning a Surface Superrepellent even to Completely Wetting Liquids. Science 2014, 346, 1096–1100. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Wu, S.; Chen, Q.; Zhao, S.; Zhang, H.; Jiao, J.; Piersol, J.-A.; Wang, J.; Sun, H.; Jiang, L. Facile Creation of Hierarchical PDMS Microstructures with Extreme Underwater Superoleophobicity for Anti-Oil Application in Microfluidic Channels. Lab Chip 2011, 11, 3873–3879. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lin, L.; Liu, Z.X.M.; Wang, S.; Jiang, L. Filefish-Inspired Surface Design for Anisotropic Underwater Oleophobicity. Adv. Funct. Mater. 2014, 24, 809–816. [Google Scholar] [CrossRef]
- Li, M.; Wang, B.; Heng, L.; Jiang, L. Surface-Independent Reversible Transition of Oil Adhesion under Water Induced by Lewis Acid-Base Interactions. Adv. Mater. Interfaces 2014, 1, 1400298. [Google Scholar] [CrossRef]
- Xu, L.-P.; Zhao, J.; Su, B.; Liu, X.; Peng, J.; Liu, Y.; Liu, H.; Yang, G.; Jiang, L.; Wen, Y.; et al. An Ion-Induced Low-Oil-Adhesion Organic/Inorganic Hybrid Film for Stable Superoleophobicity in Seawater. Adv. Mater. 2013, 25, 606–611. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Xue, Z.; Gao, J.; Meng, J.; Wang, S.; Jiang, L. Clam’s Shell Inspired High-Energy Inorganic Coatings with Underwater Low Adhesive Superoleophobicity. Adv. Mater. 2012, 24, 3401–3405. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, H.; Lai, H.; Du, Y.; Fu, K.; Li, C.; Yu, J.; Zhang, N.; Sun, K. Regulating Underwater Oil Adhesion on Superoleophobic Copper Films through Assembling n-Alkanoic Acids. ACS Appl. Mater. Interfaces 2015, 7, 20410–20417. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Li, W.; Liu, H. Bioinspired in Situ Growth of Conversion Films with Underwater Superoleophobicity and Excellent Self-Cleaning Performance. ACS Appl. Mater. Interfaces 2013, 5, 10904–10911. [Google Scholar] [CrossRef]
- Zhang, E.; Cheng, Z.; Lv, T.; Li, L.; Liu, Y. The Design of Underwater Superoleophobic Ni/NiO Microstructures with Tunable Oil Adhesion. Nanoscale 2015, 7, 19293–19299. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.; Wei, C.D.Z.; Zhu, Y.; Jiang, L. Reversible Underwater Switching between Superoleophobicity and Superoleophilicity on Conducting Polymer Nanotube Arrays. Soft Matter 2011, 7, 4163–4165. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Zhang, Q.; Meng, C.; Zhang, T.; Zhai, J. Underwater Superoleophobic Porous Membrane Based on Hierarchical TiO2 Nanotubes: Multifunctional Integration of Oil-Water Separation, Flow-through Photocatalysis and Self-Cleaning. J. Mater. Chem. A 2015, 3, 1279–1286. [Google Scholar] [CrossRef]
- Ge, D.; Yang, L.; Wang, C.; Lee, E.; Zhang, Y.; Yang, S. A Multi-Functional Oil-Water Separator from A Selectively Pre-Wetted Superamphiphobic Paper. Chem. Commun. 2015, 51, 6149–6152. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, D.; Yang, Y.; Li, J.; Zha, F.; Lei, Z. A Prewetting Induced Underwater Superoleophobic or Underoil (Super) hydrophobic Waste Potato Residue-Coated Mesh for Selective Efficient Oil/Water Separation. Green Chem. 2016, 18, 541–549. [Google Scholar] [CrossRef]
- Huang, X.; Mutlu, H.; Theato, P. A Bioinspired Hierarchical Underwater Superoleophobic Surface with Reversible PH Response. Adv. Mater. Interfaces 2020, 7, 2000101. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-Q.; Zhang, Y.-L.; Fu, X.-Y.; Sun, H.-B. Bioinspired Underwater Superoleophobic Membrane Based on a Graphene Oxide Coated Wire Mesh for Efficient Oil/Water Separation. ACS Appl. Mater. Interfaces 2015, 7, 20930–20936. [Google Scholar] [CrossRef]
- Liu, W.; Xiang, S.; Liu, X.; Yang, B. Underwater Superoleophobic Surface Based on Silica Hierarchical Cylinder Arrays with a Low Aspect Ratio. ACS Nano 2020, 14, 9166–9175. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Chen, F.; Yang, Q.; Yong, J.; Fang, Y.; Zhang, J.; Hou, X. Underwater Transparent Miniature “Mechanical Hand” Based on Femtosecond Laser-Induced Controllable Oil-Adhesive Patterned Glass for Oil Droplet Manipulation. Langmuir 2017, 33, 3659–3665. [Google Scholar] [CrossRef]
- Zhou, D.-L.; Yang, D.; Han, D.; Zhang, Q.; Chen, F.; Fu, Q. Fabrication of Superhydrophilic and Underwater Superoleophobic Membranes for Fast and Effective Oil/Water Separation with Excellent Durability. J. Membr. Sci. 2021, 620, 118898. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Yang, Q.; Zhang, D.S.; Farooq, U.; Du, G.Q.; Hou, X. Bioinspired Underwater Superoleophobic Surface with Ultralow Oil-Adhesion Achieved by Femtosecond Laser Microfabrication. J. Mater. Chem. A 2014, 2, 8790–8795. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Yang, Q.; Du, G.; Shan, C.; Bian, H.; Farooq, U.; Hou, X. Bioinspired Transparent Underwater Superoleophobic and Anti-Oil Surfaces. J. Mater. Chem. A 2015, 3, 9379–9384. [Google Scholar] [CrossRef]
- Xi, M.; Yong, J.; Chen, F.; Yang, Q.; Hou, X. Femtosecond Laser-Induced Superhygrophobic Surface: Beyond Superhydrophobicity and Repelling Various Complex Liquids. RSC Adv. 2019, 9, 6650–6657. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.-Y.; Xue, C.-H.; An, Q.-F.; Jia, S.-T.; Xu, M.-M. Fabrication of Superhydrophobic Coatings with Edible Materials for Superrepelling Non-Newtonian Liquid Foods. Chem. Eng. J. 2019, 371, 833–841. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.-R.; Carmalt, C.-J.; Parkin, I.-P. Robust Self-Cleaning Surfaces that Function when Exposed to Either Air or Oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef]
- Pan, S.-J.; Kota, A.-K.; Mabry, J.-M.; Tuteja, A. Superomniphobic Surfaces for Effective Chemical Shielding. J. Am. Chem. Soc. 2013, 135, 578–581. [Google Scholar] [CrossRef]
- Ragesh, P.; Ganesh, V.-A.; Nair, S.-V.; Nair, A.-S. A Review on “Self-Cleaning and Multifunctional Materials”. J. Mater. Chem. A 2014, 2, 14773–14797. [Google Scholar] [CrossRef]
- Nishimoto, S.; Bhushan, B. Bioinspired Self-Cleaning Surfaces with Superhydrophobicity, Superoleophobicity, and Superhydrophilicity. RSC Adv. 2013, 3, 671–690. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Chen, F.; Zhang, D.; Bian, H.; Ou, Y.; Si, J.; Du, G.; Hou, X. Stable Superhydrophobic Surface with Hierarchical Mesh-Porous Structure Fabricated by a Femtosecond Laser. Appl. Phys. A 2013, 111, 243–249. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Chen, F.; Zhang, D.; Farooq, U.; Du, G.; Hou, X. A Simple Way to Achieve Superhydrophobicity, Controllable Water Adhesion, Anisotropic Sliding, and Anisotropic Wetting Based on Femtosecond-Laser-Induced Line-Patterned Surfaces. J. Mater. Chem. A 2014, 2, 5499–5507. [Google Scholar] [CrossRef]
- Kreder, M.-J.; Alvarenga, J.; Kim, P.; Aizenberg, J. Design of Anti-Icing Surfaces: Smooth, Textured or Slippery? Nat. Rev. Mater. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Chu, D.; Singh, S.-C.; Zhan, J.Y.Z.; Sun, X.; Duan, J.; Guo, C. Superamphiphobic Surfaces with Controllable Adhesion Fabricated by Femtosecond Laser Bessel Beam on PTFE. Adv. Mater. Interfaces 2019, 6, 1900550. [Google Scholar] [CrossRef]
- Lv, J.; Song, Y.; Jiang, L.; Wang, J. Bio-Inspired Strategies for Anti-Icing. ACS Nano 2014, 8, 3152–3169. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Li, Z.; Yu, Z.; Singh, S.; Guo, C. Superhydrophobic Al Surfaces with Properties of Anticorrosion and Reparability. ACS Omega 2018, 3, 17425–17429. [Google Scholar] [CrossRef]
- Shi, F.; Niu, J.; Liu, J.; Liu, F.; Wang, Z.; Feng, X.; Zhang, X. Towards Understanding Why a Superhydrophobic Coating Is Needed by Water Striders. Adv. Mater. 2007, 19, 2257–2261. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, J.; Chen, Z.; Lai, Y. Bioinspired Special Wettability Surfaces: From Fundamental Research to Water Harvesting Applications. Small 2017, 13, 1602992. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Yang, Q.; Zhang, D.; Du, G.; Si, J.; Yun, F.; Hou, X. Femtosecond Laser Weaving Superhydrophobic Patterned PDMS Surfaces with Tunable Adhesion. J. Phys. Chem. C 2013, 117, 24907–24912. [Google Scholar] [CrossRef]
- Wang, M.; Chen, C.; Ma, J.; Xu, J. Preparation of Superhydrophobic Cauliflower-Like Silica Nanospheres with Tunable Water Adhesion. J. Mater. Chem. 2011, 21, 6962–6967. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Chen, F.; Bian, H.; Du, G.; Farooq, U.; Hou, X. Reversible Underwater Lossless Oil Droplet Transportation. Adv. Mater. Interfaces 2015, 2, 1400388. [Google Scholar] [CrossRef]
- Jokinen, V.; Sainiemi, L.; Franssila, S. Complex Droplets on Chemically Modified Silicon Nanograss. Adv. Mater. 2008, 20, 3453–3456. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Chen, F.; Du, G.; Shan, C.; Farooq, U.; Wang, J.; Hou, X. Using “Underwater Superoleophobic Pattern” to Make Liquid Lens Array. RSC Adv. 2015, 5, 40907–40911. [Google Scholar] [CrossRef]
- Songok, J.; Tuominen, M.; Teisala, H.; Haapanen, J.; Mäkelä, J.; Kuusipalo, J.; Toivakka, M. Paper-Based Microfluidics: Fabrication Technique and Dynamics of Capillary-Driven Surface Flow. ACS Appl. Mater. Interfaces 2014, 6, 20060–20066. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, T.; Ge, P.; Xue, P.; Ye, S.; Chen, H.; Li, Z.; Zhang, J.; Yang, B. Controlling Flow Behavior of Water in Microfluidics with a Chemically Patterned Anisotropic Wetting Surface. Langmuir 2015, 31, 4032–4039. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.-W.; Choi, S.-S.; Lee, S.-H.; Kim, B.; Lee, S.-N.; Park, M.-C.; Kim, P.; Hwang, S.-Y.; Suh, K.-Y. Microfluidic Separation and Enrichment of Human Breast Cancer Cells by Adhesion Difference. Lab Chip 2007, 7, 1461–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, A.; Quaglio, M.; Marasso, S.-L.; Chiodoni, A.; Cocuzza, M.; Bongiovanni, R. Direct Photolithography of Perfluoropolyethers for Solvent-Resistant Microfluidics. Langmuir 2013, 29, 15711–15718. [Google Scholar] [CrossRef]
- Shen, L.; Wang, B.; Wang, J.; Fu, J.; Picart, C.; Ji, J. Asymmetric Free-Standing Film with Multifunctional Anti-Bacterial and Self-Cleaning Properties. ACS Appl. Mater. Interfaces 2012, 4, 4476–4483. [Google Scholar] [CrossRef] [Green Version]
- Stratakis, E.; Ranella, A.; Fotakis, C. Biomimetic Micro/Nanostructured Functional Surfaces for Microfluidic and Tissue Engineering Applications. Biomicrofluidics 2011, 5, 013411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, Z.; Kabbash, M.E.I.; Cheng, J.; Zhang, J.; Singh, S.; Guo, C. Highly Floatable Superhydrophobic Metallic Assembly for Aquatic Applications. ACS Appl. Mater. Interfaces 2019, 11, 48512–48517. [Google Scholar] [CrossRef] [Green Version]
- Wan, Z.; Chen, J. Human Errors are Behind Most Oil-Tanker Spills. Nature 2018, 560, 161–163. [Google Scholar] [CrossRef]
- Deepwater Horizon Oil Spill. Available online: https://en.jinzhao.wiki/wiki/Deepwater_Horizon_oil_spill (accessed on 31 December 2021).
- Gao, X.; Zhou, J.; Du, R.; Xie, Z.; Deng, S.; Liu, R.; Liu, Z.; Zhang, J. Robust Superhydrophobic Foam: A Graphdiyne-Based Hierarchical Architecture for Oil/Water Separation. Adv. Mater. 2016, 28, 168–173. [Google Scholar] [CrossRef]
- Cortese, B.; Caschera, D.; Federici, F.; Ingo, G.; Gigli, G. Superhydrophobic Fabrics for Oil-Water Separation through a Diamond like Carbon (DLC) Coating. J. Mater. Chem. A 2014, 2, 6781–6789. [Google Scholar] [CrossRef]
- Zhou, W.; Li, S.; Liu, Y.; Xu, Z.; Wei, S.; Wang, G.; Lian, J.; Jiang, Q. Dual Superlyophobic Copper Foam with Good Durability and Recyclability for High Flux, High Efficiency, and Continuous Oil-Water Separation. ACS Appl. Mater. Interfaces 2018, 10, 9841–9848. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Fan, H.; Wang, Y.; Zhou, C.; Ren, F.; Wu, S.; Li, G.; Hu, Y.; Li, J.; et al. A Janus Oil Barrel with Tapered Microhole Arrays for Spontaneous High-Flux Spilled Oil Absorption and Storage. Nanoscale 2017, 9, 15796–15803. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhao, J.; Oderinde, Q.; Han, J.; Liu, Z.; Gao, B.; Xiong, R.; Zhang, Q.; Jiang, S.; Huang, C. Durable Superhydrophobic and Superoleophilic Electrospun Nanofibrous Membrane for Oil-Water Emulsion Separation. J. Colloid Interf. Sci. 2018, 532, 12–23. [Google Scholar] [CrossRef]
- Ren, G.; Song, Y.; Li, X.; Zhou, Y.; Zhang, Z.; Zhu, X. A Superhydrophobic Copper Mesh as an Advanced Platform for Oil-Water Separation. Appl. Surf. Sci. 2018, 428, 520–525. [Google Scholar] [CrossRef]
- Latthe, S.-S.; Kodag, V.-S.; Sutar, R.-S.; Bhosale, A.-K.; Nagappan, S.; Ha, C.-S.; Sadasivuni, K.-K.; Kulal, S.-R.; Liu, S.; Xing, R. Sawdust-Based Superhydrophobic Pellets for Efficient Oil-Water Separation. Mater. Chem. Phys. 2020, 243, 122634. [Google Scholar] [CrossRef]
- Ma, W.; Ding, Y.; Zhang, M.; Gao, S.; Li, Y.; Huang, C.; Fu, G. Nature-Inspired Chemistry toward Hierarchical Superhydrophobic, Antibacterial and Biocompatible Nanofibrous Membranes for Effective UV Shielding, Self-Cleaning and Oil-Water Separation. J. Hazard. Mater. 2020, 384, 121476. [Google Scholar] [CrossRef]
- Ge, B.; Yang, X.; Li, H.; Zhao, L.; Ren, G.; Miao, X.; Pu, X.; Li, W. A Durable Superhydrophobic BiOBr/PFW Cotton Fabric for Visible Light Response Degradation and Oil/Water Separation Performance. Colloid Surf. A 2020, 585, 124027. [Google Scholar] [CrossRef]
- Yu, T.; Halouane, F.; Mathias, D.; Barras, A.; Wang, Z.; Lv, A.; Lu, S.; Xu, W.; Meziane, D.; Tiercelin, N.; et al. Preparation of Magnetic, Superhydrophobic/Superoleophilic Polyurethane Sponge: Separation of Oil/Water Mixture and Demulsification. Chem. Eng. J. 2020, 384, 123339. [Google Scholar] [CrossRef]
- Xue, C.-H.; Li, Y.-R.; Hou, J.-L.; Zheng, L.; Ma, J.-Z. Self-Roughened Superhydrophobic Coatings for Continuous Oil-Water Separation. J. Mater. Chem. A 2015, 3, 10248–10253. [Google Scholar] [CrossRef]
- Yong, J.; Fang, Y.; Chen, F.; Huo, J.; Yang, Q.; Bian, H.; Du, G.; Hou, X. Femtosecond Laser Ablated Durable Superhydrophobic PTFE Films with Micro-Through-Holes for Oil/Water Separation: Separating Oil from Water and Corrosive Solutions. Appl. Surf. Sci. 2016, 389, 1148–1155. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhang, L.; Song, J.; Wang, X.; Liu, H. Superhydrophobic Nickel-Electroplated Carbon Fibers for Versatile Oil/Water Separation with Excellent Reusability and High Environmental Stability. ACS Appl. Mater. Interfaces 2020, 12, 24390–24402. [Google Scholar] [CrossRef]
- Wang, S.; Song, Y.; Jiang, L. Microscale and Nanoscale Hierarchical Structured Mesh Films with Superhydrophobic and Superoleophilic Properties Induced by Long-Chain Fatty Acids. Nanotechnology 2007, 18, 015103. [Google Scholar] [CrossRef]
- Li, J.; Kang, R.; Tang, X.; She, H.; Yang, Y.; Zha, F. Superhydrophobic Meshes That Can Repel Hot Water and Strong Corrosive Liquids Used for Efficient Gravity-Driven Oil/Water Separation. Nanoscale 2016, 8, 7638–7648. [Google Scholar] [CrossRef]
- Baig, U.; Matin, A.; Gondal, M.A.; Zubar, S.M. Facile Fabrication of Superhydrophobic, Superoleophilic Photocatalytic Membrane for Efficient Oil-Water Separation and Removal of Hazardous Organic Pollutants. J. Clean. Prod. 2019, 208, 904–915. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Xu, X.; Guo, F.; Zhu, X.; Men, X.; Ge, B. Robust and Durable Superhydrophobic Cotton Fabrics for Oil/Water Separation. ACS Appl. Mater. Interfaces 2013, 5, 7208–7214. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Gao, S.; Huang, J.; Li, S.; Zhu, T.; Cheng, Y.; Zhao, Y.; Chen, Z.; Lai, Y. A Self-Roughened and Biodegradable Superhydrophobic Coating with UV Shielding, Solar-Induced Self-Healing and Versatile Oil-Water Separation Ability. J. Mater. Chem. A 2019, 7, 2122–2128. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, M.; Liu, Z.; Kang, M.; Huang, C.; Fu, G. Fabrication of Highly Durable and Robust Superhydrophobic-Superoleophilic Nanofibrous Membranes Based on a Fluorine-Free System for Efficient Oil/Water Separation. J. Membr. Sci. 2019, 570–571, 303–313. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Z.; Guo, J.; Shen, H.; Zhang, H.; Zhao, N.; Zhao, Y.; Chen, L.; Liang, S.; Jin, Y.; et al. Facile Fabrication of Robust Superhydrophobic Porous Materials and Their Application in Oil/Water Separation. J. Mater. Chem. A 2015, 3, 23252–23260. [Google Scholar] [CrossRef]
- Du, R.; Gao, X.; Feng, Q.-L.; Zhao, Q.-C.; Li, P.; Li, S.-B.; Shi, L.-R.; Zhang, J. Microscopic Dimensions Engineering: Stepwise Manipulation of the Surface Wettability on 3D Substrates for Oil/Water Separation. Adv. Mater. 2016, 28, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Chu, D.; Dong, X.; Wang, C.; Duan, J.-A.; He, J. Femtosecond Laser Induced Robust Periodic Nanoripple Structured Mesh for Highly Efficient Oil-Water Separation. Nanoscale 2017, 9, 14229–14235. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.K.; Bae, S.; Jeon, H.; Kim, M.; Cho, S.J.; Lim, G. An Underwater Superoleophobic Nanofibrous Cellulosic Membrane for Oil/Water Separation with High Separation Flux and High Chemical Stability. Nanoscale 2018, 10, 3037–3045. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Ji, S.; Zhang, J.; Liu, Q.; He, F.; Peng, S.; Li, Y. Tannic Acid Encountering Ovalbumin: A Green and Mild Strategy for Superhydrophilic and Underwater Superoleophobic Modification of Various Hydrophobic Membranes for Oil/Water Separation. J. Mater. Chem. A 2018, 6, 13959–13967. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, G.; Bai, R.; Shen, S.; Zhou, X.; Wyman, I. Fabrication of Superhydrophilic and Underwater Superoleophobic Membranes via an in situ Crosslinking Blend Strategy for Highly Efficient Oil/Water Emulsion Separation. J. Membr. Sci. 2019, 569, 60–70. [Google Scholar] [CrossRef]
- Dong, Y.; Huang, C.; Yang, X.-Y. Underwater Superoleophobic and Underoil Superhydrophobic Surface Made by Liquid-Exfoliated MoS2 for On-Demand Oil-Water Separation. Chem. Eng. J. 2019, 361, 322–328. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Zhou, C.; Wen, X.; Xu, S.; Cheng, J.; Pi, P. A Durable Underwater Superoleophobic and Underoil Superhydrophobic Fabric for Versatile Oil/Water Separation. Chem. Eng. J. 2019, 370, 1218–1227. [Google Scholar] [CrossRef]
- Bo, Z.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Superamphiphilic and Underwater Superoleophobic Membrane for Oil/ Water Emulsion Separation and Organic Dye Degradation. J. Membr. Sci. 2020, 598, 117804. [Google Scholar] [CrossRef]
- Chen, C.; Chen, L.; Chen, S.; Yu, Y.; Weng, D.; Mahmood, A.; Wang, G.; Wang, J. Preparation of Underwater Superoleophobic Membranes via TiO2 Electrostatic Self-Assembly for Separation of Stratified Oil/Water Mixtures and Emulsions. J. Membr. Sci. 2020, 602, 117976. [Google Scholar] [CrossRef]
- Helali, N.; Rastgar, M.; Ismail, M.F.; Sadrzadeh, M. Development of Underwater Superoleophobic Polyamide-Imide (PAI) Microfiltration Membranes for Oil/Water Emulsion Separation. Sep. Purif. Technol. 2020, 238, 116451. [Google Scholar] [CrossRef]
- Li, G.; Fan, H.; Ren, F.; Zhou, C.; Zhang, Z.; Xu, B.; Wu, S.; Hu, Y.; Zhu, W.; Li, J.; et al. Multifunctional Ultrathin Aluminum Foil: Oil/Water Separation and Particle Filtration. J. Mater. Chem. A 2016, 4, 18832–18840. [Google Scholar] [CrossRef]
- Zhang, E.; Cheng, Z.; Lv, T.; Qian, Y.; Liu, Y. Anti-Corrosive Hierarchical Structured Copper Mesh Film with Superhydrophilicity and Underwater Low Adhesive Superoleophobicity for Highly Efficient Oil-Water Separation. J. Mater. Chem. A 2015, 3, 13411–13417. [Google Scholar] [CrossRef]
- Gao, X.; Xu, L.-P.; Xue, Z.; Feng, L.; Peng, J.; Wen, Y.; Wang, S.; Zhang, X. Dual-Scaled Porous Nitrocellulose Membranes with Underwater Superoleophobicity for Highly Efficient Oil/Water Separation. Adv. Mater. 2014, 26, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Chen, F.; Yang, Q.; Bian, H.; Du, G.; Shan, C.; Huo, J.; Fang, Y.; Hou, X. Oil-Water Separation: A Gift from the Desert. Adv. Mater. Interfaces 2016, 3, 1500650. [Google Scholar] [CrossRef] [Green Version]
- Wen, Q.; Di, J.; Jiang, L.; Yu, J.; Xu, R. Zeolite-Coated Mesh Film for Efficient Oil-Water Separation. Chem. Sci. 2013, 4, 591–595. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, W.B.; Shi, Z.; Wang, D.; Jin, J.; Jiang, L. Nanowire-Haired Inorganic Membranes with Superhydrophilicity and Underwater Ultralow Adhesive Superoleophobicity for High-Efficiency Oil/Water Separation. Adv. Mater. 2013, 25, 4192–4198. [Google Scholar] [CrossRef]
- Song, J.; Li, S.; Zhao, C.; Lu, Y.; Zhao, D.; Sun, J.; Carmalt, T.C.J.; Deng, X.; Parkin, I.P. A Superhydrophilic Cement-Coated Mesh: An Acid, Alkali, and Organic Reagent-Free Material for Oil/Water Separation. Nanoscale 2018, 10, 1920–1929. [Google Scholar] [CrossRef]
- Dai, J.; Wang, L.; Wang, Y.; Tian, S.; Tian, X.; Xie, A.; Zhang, R.; Yan, Y.; Pan, J. Robust Nacrelike Graphene Oxide-Calcium Carbonate Hybrid Mesh with Underwater Superoleophobic Property for Highly Efficient Oil/Water Separation. ACS Appl. Mater. Interfaces 2020, 12, 4482–4493. [Google Scholar] [CrossRef]
- Chen, P.; Xu, Z. Mineral-Coated Polymer Membranes with Superhydrophilicity and Underwater Superoleophobicity for Effective Oil/Water Separation. Sci. Rep. 2013, 3, 2776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, M.; Xue, L.; Liu, F.; Jiang, L. An Intelligent Superwetting PVDF Membrane Showing Switchable Transport Performance for Oil/Water Separation. Adv. Mater. 2014, 26, 2943–2948. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Hou, X.; Chen, F. Endowing Metal Surfaces with Underwater Superoleophobicity by Femtosecond Laser Processing for Oil-Water Separation Application. Front. Phys. 2020, 8, 305. [Google Scholar] [CrossRef]
- Li, X.; Shan, H.; Zhang, W.; Li, B. 3D Printed Robust Superhydrophilic and Underwater Superoleophobic Composite Membrane for High Efficient Oil/Water Separation. Sep. Purif. Technol. 2020, 237, 116324. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Shi, R.; Chen, L.; Fan, M. A Robust Salt-Tolerant Superoleophobic Chitosan/Nanofibrillated Cellulose Aerogel for Highly Efficient Oil/Water Separation. Carbohydr. Polym. 2018, 200, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Yin, Z.; Cao, X.; Xie, X.; Tan, C.; Huang, X.; Chen, B.; Chen, F.; Yang, Q.; Bu, X.; et al. Carbon Fiber Aerogel Made from Raw Cotton: A Novel, Efficient and Recyclable Sorbent for Oils and Organic Solvents. Adv. Mater. 2013, 25, 5916–5921. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Hie, H.; Huang, H.-X.; Turng, L.-S. Magnetically Driven Superhydrophobic Silica Sponge Decorated with Hierarchical Cobalt Nanoparticles for Selective Oil Absorption and Oil/Water Separation. Chem. Eng. J. 2018, 337, 541–551. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Huang, H.-X.; Peng, X.-F.; Turng, L.-S. Superhydrophobic Graphene/Cellulose/Silica Aerogel with Hierarchical Structure as Superabsorbers for High Efficiency Selective Oil Absorption and Recovery. Ind. Eng. Chem. Res. 2018, 57, 1745–1755. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Ge, M.; Cao, C.; Deng, S.; Zhang, S.; Chen, G.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. Robust Flower-Like TiO2 @Cotton Fabrics with Special Wettability for Effective Self-Cleaning and Versatile Oil/Water Separation. Adv. Mater. Interfaces 2015, 2, 1500220. [Google Scholar] [CrossRef]
- Wu, F.; Pickett, K.; Panchal, A.; Liu, M.; Lvov, Y. Superhydrophobic Polyurethane Foam Coated with Polysiloxane-Modified Clay Nanotubes for Efficient and Recyclable Oil Absorption. ACS Appl. Mater. Interfaces 2019, 11, 25445–25456. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; Zhang, T.; Qiu, F.; Yue, X.; Yang, D. Sustainable, Flexible, and Superhydrophobic Functionalized Cellulose Aerogel for Selective and Versatile Oil/Water Separation. ACS Sustain. Chem. Eng. 2019, 7, 9984–9994. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kwon, T.-H.; Im, H.; Moon, D.-I.; Baek, D.J.; Seol, M.-L.; Duarte, J.P.; Choi, Y.-K. A Polydimethylsiloxane (PDMS) Sponge for the Selective Absorption of Oil from Water. ACS Appl. Mater. Interfaces 2011, 3, 4552–4556. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Zheng, Z.; Mao, N.; Zhang, N.; Hu, W.; Zhang, J. Fluorosurfactants-Directed Preparation of Homogeneous and Hierarchical-Porosity CMP Aerogels for Gas Sorption and Oil Cleanupl. Adv. Sci. 2015, 2, 1400006. [Google Scholar] [CrossRef]
- Du, R.; Feng, Q.; Ren, H.; Zhao, Q.; Gao, X.; Zhang, J. Hybrid-Dimensional Magnetic Microstructure Based 3D Substrates for Remote Controllable and Ultrafast Water Remediation. J. Mater. Chem. A 2016, 4, 938–945. [Google Scholar] [CrossRef]
- Yu, S.; Tan, H.; Wang, J.; Liu, X.; Zhou, K. High Porosity Supermacroporous Polystyrene Materials with Excellent Oil-Water Separation and Gas Permeability Properties. ACS Appl. Mater. Interfaces 2015, 7, 6745–6753. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Pan, Q.; Liu, F. Facile Removal and Collection of Oils from Water Surfaces through Superhydrophobic and Superoleophilic Sponges. J. Phys. Chem. C 2011, 115, 17464–17470. [Google Scholar] [CrossRef]
- Yang, S.; Shen, C.; Chen, L.; Wang, C.; Rana, M.; Lv, P. Vapor-Liquid Deposition Strategy to Prepare Superhydrophobic and Superoleophilic Graphene Aerogel for Oil-Water Separation. ACS Appl. Nano Mater. 2018, 1, 531–540. [Google Scholar] [CrossRef]
- Zhu, Q.; Pan, Q. Mussel-Inspired Direct Immobilization of Nanoparticles and Application for Oil-Water Separation. ACS Nano 2014, 8, 1402–1409. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Liu, Y.; Li, L.; Li, H.; Peng, X.-F.; Zhou, H. Highly Durable Superhydrophobic Polymer Foams Fabricated by Extrusion and Supercritical CO2 Foaming for Selective Oil Absorption. ACS Appl. Mater. Interfaces 2019, 11, 7479–7487. [Google Scholar] [CrossRef]
- Huang, Z.-S.; Quan, Y.-Y.; Mao, J.-J.; Wang, Y.-L.; Lai, Y.; Zheng, J.; Chen, Z.; Wen, K.; Li, H. Multifunctional Superhydrophobic Composite Materials with Remarkable Mechanochemical Robustness, Stain Repellency, Oil-water Separation and Sound-Absorption Properties. Chem. Eng. J. 2019, 358, 1610–1619. [Google Scholar] [CrossRef]
- Qiu, S.; Li, Y.; Li, G.; Zhang, Z.; Li, Y.; Wu, T. Robust Superhydrophobic Sepiolite-Coated Polyurethane Sponge for Highly Efficient and Recyclable Oil Absorption. ACS Sustain. Chem. Eng. 2019, 7, 5560–5567. [Google Scholar] [CrossRef]
- He, J.; Zhao, H.; Li, X.; Su, D.; Zhang, F.; Ji, H.; Liu, R. Superelastic and Superhydrophobic Bacterial Cellulose/silica Aerogels with Hierarchical Cellular Structure for Oil Absorption and Recovery. J. Hazard. Mater. 2018, 346, 199–207. [Google Scholar] [CrossRef]
- Ge, J.; Shi, L.A.; Wang, Y.C.; Zhao, H.Y.; Yao, H.B.; Zhu, Y.B.; Zhang, Y.; Zhu, H.W.; Wu, H.A.; Yu, S.H. Joule-Heated Graphene-Wrapped Sponge Enables Fast Clean-up of Viscous Crude-Oil Spill. Nat. Nanotechnol. 2017, 12, 434. [Google Scholar] [CrossRef]
- Wu, X.; Lei, Y.; Li, S.; Huang, J.; Teng, L.; Chen, Z.; Yue, Y. Photothermal and Joule Heating-Assisted Thermal Management Sponge for Efficient Cleanup of Highly Viscous Crude Oil. J. Hazard. Mater. 2021, 403, 124090. [Google Scholar] [CrossRef]
- Li, J.; Yan, L.; Tang, X.; Feng, H.; Hu, D.; Zha, F. Robust Superhydrophobic Fabric Bag Filled with Polyurethane Sponges Used for Vacuum-Assisted Continuous and Ultrafast Absorption and Collection of Oils from Water. Adv. Mater. Interfaces 2016, 3, 1500770. [Google Scholar] [CrossRef]
- Song, J.; Huang, S.; Lu, Y.; Bu, X.; Mates, J.E.; Ghosh, A.; Ganguly, R.; Carmalt, C.J.; Parkin, I.P.; Xu, W.; et al. Self-Driven One-Step Oil Removal from Oil Spill on Water via Selective-Wettability Steel Mesh. ACS Appl. Mater. Interfaces 2014, 6, 19858–19865. [Google Scholar] [CrossRef] [PubMed]
- Matafonova, G.; Batoev, V. Recent Advances in Application of UV Light-emitting Diodes for Degrading Organic Pollutants in Water Through Advanced Oxidation Processes: A Review. Water Res. 2018, 132, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Idaira, P.-F.; Ali, N.; Verónica, P.; Jared, L.A.; Juan, H.A.; Ana, M.A. Utilization of Highly Robust and Selective Crosslinked Polymeric Ionic Liquid-based Sorbent Coatings in Direct-Immersion Solid-Phase Microextraction and High-performance Liquid Chromatography for Determining Polar Organic Pollutants in Waters. Talanta 2016, 158, 125–133. [Google Scholar]
- Tran, V.S.; Ngo, H.H.; Guo, W.; Zhang, J.; Liang, S.; Ton-That, C.; Zhang, X. Typical Low Cost Biosorbents for Adsorptive Removal of Specific Organic Pollutants from Water. Bioresour. Technol. 2015, 182, 353–363. [Google Scholar] [CrossRef]
- Sajida, M.; Ihsanullaha, M.K.N.; Baigb, N.; Osmanb, A.M. Removal of Heavy Metals and Organic Pollutants from Water Using Dendritic Polymers Based Adsorbents: A Critical Review. Sep. Purif. Technol. 2018, 191, 400–423. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Hou, X.; Chen, F. Underwater Superpolymphobicity: Concept, Achievement, and Applications. Nano Sel. 2021, 2, 1011–1022. [Google Scholar] [CrossRef]
- Yong, J.; Singh, S.C.; Zhan, Z.; Mohamed, E.; Chen, F.; Guo, C. Femtosecond Laser-Produced Underwater “Superpolymphobic” Nanorippled Surfaces: Repelling Liquid Polymers in Water for Application of Controlling Polymer Shape and Adhesion. ACS Appl. Nano Mater. 2019, 2, 7362–7371. [Google Scholar] [CrossRef]
- Yong, J.; Zhan, Z.; Singh, S.C.; Chen, F.; Guo, C. Femtosecond Laser-Structured Underwater “Superpolymphobic” Surfaces. Langmuir 2019, 35, 9318–9322. [Google Scholar] [CrossRef] [Green Version]
- Yong, J.; Zhan, Z.; Singh, S.C.; Chen, F.; Guo, C. Microfludic Channels Fabrication Based on Underwater Superpolymphobic Microgrooves Produced by Femtosecond Laser Direct Writing. ACS Appl. Polym. Mater. 2019, 1, 2819–2825. [Google Scholar] [CrossRef] [Green Version]
- Yong, J.; Zhang, C.; Bai, X.; Zhang, J.; Yang, Q.; Hou, X.; Chen, F. Filtration and Removal of Liquid Polymers from Water (Polymer/Water Separation) by Use of the Underwater Superpolymphobic Mesh Produced with a Femtosecond Laser. J. Colloid Interf. Sci. 2020, 585, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Tian, Y.; Tian, X.; Li, W.; Han, X.; Kong, T.; Wang, L. Design of Multi-Scale Textured Surfaces for Unconventional Liquid Harnessing. Mater. Today 2021, 43, 62–83. [Google Scholar] [CrossRef]
- Liu, X.; Yang, F.; Guo, J.; Fu, J.; Guo, Z. New Insights into Unusual Droplets: From Mediating the Wettability to Manipulating the Locomotion Modes. Chem. Commun. 2020, 56, 14757–14788. [Google Scholar] [CrossRef]
- George, J.E.; Chidangil, S.; George, S.D. Recent Progress in Fabricating Superaerophobic and Superaerophilic Surfaces. Adv. Mater. Interfaces 2017, 4, 1601088. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, P.; Wang, J.; Jiang, L. Superwettability of Gas Bubbles and Its Application: From Bioinspiration to Advanced Materials. Adv. Mater. 2017, 29, 1703053. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-C.; Hou, J.; Wan, L.-S.; Chen, V.; Xu, Z.-K. Janus Membranes with Asymmetric Wettability for fine Bubble Aeration. Adv. Mater. Interfaces 2016, 3, 1500774. [Google Scholar] [CrossRef]
- Waldman, R.Z.; Yang, H.-C.; Mandia, D.J.; Nealey, P.F.; Elam, J.W.; Darling, S.B. Janus Membranes via Diffusion-Controlled Atomic Layer Deposition. Adv. Mater. Interfaces 2018, 5, 1800658. [Google Scholar] [CrossRef]
- Xu, W.; Lu, Z.; Sun, X.; Jiang, L.; Duan, X. Superwetting Electrodes for Gas-Involving Electrocatalysis. Acc. Chem. Res. 2018, 51, 1590–1598. [Google Scholar] [CrossRef]
- Lu, Z.; Sun, M.; Xu, T.; Li, Y.; Xu, W.; Chang, Z.; Ding, Y.; Sun, X.; Jiang, L. Superaerophobic Electrodes for Direct Hydrazune Fuel Cells. Adv. Mater. 2015, 27, 2361–2366. [Google Scholar] [CrossRef]
- Lu, Z.; Li, Y.; Lei, X.; Liu, J.; Sun, X. Nanoarray Based “Superaerophobic” Surfaces for Gas Evolution Reaction Electrodes. Mater. Horiz. 2015, 2, 294–298. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Xu, T.; Lu, Z.; Wu, X.; Wan, P.; Sun, X.; Jiang, L. Under-Water Superaerophobic Pine-Shaped Pt Nano-Array Electrode for Ultrahigh-Performance Hydrogen Evolution. Adv. Funct. Mater. 2015, 25, 1737–1744. [Google Scholar] [CrossRef]
- Li, Z.; Hu, R.; Song, J.; Liu, L.; Qu, J.; Song, W.; Cao, C. Gas-Liquid-Solid Triphase Interfacial Chemical Reactions Associated with Gas Wettability. Adv. Mater. Interfaces 2021, 8, 2001636. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Li, M.; Yang, Q.; Fang, Y.; Huo, J.; Hou, X. Remarkably Simple Achievement of Superhydrophobicity, Superhydrophilicity, Underwater Superoleophobicity, Underwater Superoleophilicity, Underwater Superaerophobicity, and Underwater Superaerophilicity on Femtosecond Laser Ablated PDMS Surfaces. J. Mater. Chem. A 2017, 5, 25249–25257. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Gao, J.; Xue, Z.; Chen, L.; Lin, L.; Jiang, L.; Wang, S. Bioinspired Oil Strider Floating at the Oil/Water Interface Supported by Huge Superoleophobic Force. ACS Nano 2012, 6, 5614–5620. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Fan, H.; Zhang, G.; Zhao, S.; Cui, J.; Yan, Y. Facile Construction of Gas Diode Membrane towards in situ Gas Consumption via Coupling Two Chemical Reactions. J. Colloid Interfaces Sci. 2019, 557, 282–290. [Google Scholar] [CrossRef]
- Huo, J.; Yang, Q.; Yong, J.; Fan, P.; Lu, Y.; Hou, X.; Chen, F. Underwater Superaerophobicity/Superaerophilicity and Unidirectional Bubble Passage Based on the Femtosecond Laser-Structured Stainless Steel Mesh. Adv. Mater. Interfaces 2020, 7, 1902128. [Google Scholar] [CrossRef]
- Hu, Y.; Qiu, W.; Zhang, Y.; Zhang, Y.; Li, C.; Li, J.; Wu, S.; Zhu, W.; Wu, D.; Chu, J. Channel-Controlled Janus Membrane Fabricated by Simultaneous Laser Ablation and Nanoparticles Deposition for Underwater Bubbles Manipulation. Appl. Phys. Lett. 2019, 114, 173701. [Google Scholar] [CrossRef]
- Song, J.; Liu, Z.; Wang, X.; Liu, H.; Lu, Y.; Deng, X.; Carmalt, C.J.; Parkin, I.P. High-Efficiency Bubble Transportation in an Aqueous Environment on a Serial Wedge-Shaped Wettability Pattern. J. Mater. Chem. A 2019, 7, 13567–13576. [Google Scholar] [CrossRef]
- Yong, J.; Singh, S.C.; Zhan, Z.; Huo, J.; Chen, F.; Guo, C. Reducing Adhesion for Dispensing Tiny Water/Oil Droplets and Gas Bubbles by Femtosecond Laser-Treated Needle Nozzles: Superhydrophobicity, Superoleophobicity, and Superaerophobicity. ChemNanoMat 2019, 5, 241–249. [Google Scholar] [CrossRef]
- Pei, C.; Peng, Y.; Zhang, Y.; Tian, D.; Liu, K.; Jiang, L. An Integrated Janus Mesh: Underwater Bubble Antibuoyancy Unidirectional Penetration. ACS Nano 2018, 12, 5489–5494. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, H.; Li, Y.; Zhang, H.; Zhang, G.; Lu, Z.; Sun, X.; Jiang, L. Superaerophobic RuO2-Based Nanostructured Electrode for High-Performance Chlorine Evolution Reaction. Small 2017, 13, 1602240. [Google Scholar] [CrossRef]
- Yu, X.; Yu, Z.-Y.; Zhang, X.-L.; Zheng, Y.-R.; Duan, Y.; Gao, Q.; Wu, R.; Sun, B.; Gao, M.-R.; Wang, G.; et al. “Superaerophobic” Nickel Phosphide Nanoarray Catalyst for Efficient Hydrogen Evolution at Ultrahigh Current Densities. J. Am. Chem. Soc. 2019, 141, 7537–7543. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Cheng, X.; Wang, H.; Lei, C.; Pardiwal, S.; Yang, B.; Li, Z.; Zhang, Q.; Lei, L.; Wang, S.; et al. A Superaerophobic Bimetallic Selenides Heterostructure for Efficient Industrial-Level Oxygen Evolution at Ultra-High Current Densities. Nano Micro Lett. 2020, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Dorrer, C.; Rühe, J. Superaerophobicity: Repellence of Air Bubbles from Submerged, Surface-Engineered Silicon Substrates. Langmuir 2012, 28, 14968–14973. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Xu, X.; He, M.; Su, B. Controllable Manipulation of Bubbles in Water by Using Underwater Superaerophobic Grapheneoxide/Gold-Nanoparticle Composite Surface. Nanoscale 2018, 10, 231–238. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Li, W.; Huo, J.; Fang, Y.; Yang, Q.; Bian, H.; Hou, X. Underwater Superaerophobic and Superaerophilic Nanoneedles-Structured Meshes for Water/Bubbles Separation: Removing or Collecting Gas Bubbles in Water. Glob. Chall. 2018, 2, 1700133. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, W.; Yu, X.; Zhang, H.; Li, Y.; Sun, X.; Wang, X.; Wang, H.; Wang, J.; Luo, J.; et al. Ultrahigh Hydrogen Evolution Perfoemance of Under-Water “Superaerophobic” MoS2 Nanostructured Electrodes. Adv. Mater. 2014, 26, 2683–2687. [Google Scholar] [CrossRef]
- Fu, X.; Hou, J.; Chen, C.; Li, J.; Yue, L.; Chen, X.; Zhao, L.; Ran, G.; Xia, X.; Gong, Y.; et al. Superhydrophobic and Superaerophilic Hierarchical Pt@MIL-101/PVDF Composite for Hydrogen Water Isotope Exchange Reactions. J. Hazard. Mater. 2019, 380, 120904. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, H.; Han, Y.; Huang, L.; Chen, Y.; Liu, J.; Wang, X.; Liu, X.; Ling, S. Superaerophilic Wedge-Shaped Channels with Precovered Air Film for Efficient Subaqueous Bubble/Jet Transportation and Continuous Oxygen Supplementation. ACS Appl. Mater. Interfaces 2019, 11, 23808–23814. [Google Scholar] [CrossRef]
- Li, Z.; Cao, C.; Zhu, Z.; Liu, J.; Song, W.; Jiang, L. Superaerophilic Materials are Surprising Catalysts: Wettability-Induced Excellent Hydrogenation Activity under Ambient H2 Pressure. Adv. Mater. Interfaces 2018, 5, 1801259. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, Z.; Zhao, Y.; Wen, X.; Xu, W.; Zhong, Y.; Bai, L.; Liu, W.; Zhang, Y.; Zhang, Y.; et al. Superaerophilic Copper Nanowires for Efficient and Switchable CO2 Electroreduction. Nanoscale Horiz. 2019, 4, 490–494. [Google Scholar] [CrossRef]
- Tu, C.; Yang, Q.; Chen, Y.; Ye, Y.; Wang, Y.; Du, P.; Yang, S.; Bao, F.; Yin, Z.; Jiang, R.; et al. Anisotropic Spreading of Bubbles on Superaerophilic Straight Trajectories Beneath a Slide in Water. Water 2020, 12, 798. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, Y.; Zhang, D.; Li, L.; Wang, T.; Chen, J. Preparation of Superaerophilic Copper Mesh for Underwater Gas Collection by Combination of Spraying Technology and Fame Treatment. Appl. Phys. A 2020, 126, 24. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; He, Z.; Gong, H.; Xu, X.; Zhao, M.; Qin, H. Efficient Gas Transportation Using Bioinspired Superhydrophobic Yarn as the Gas-Siphon Underwater. ACS Appl. Mater. Interfaces 2020, 12, 18174–18181. [Google Scholar] [CrossRef]
- Huo, J.; Yong, J.; Chen, F.; Yang, Q.; Fang, Y.; Hou, X. Trapped Air-Induced Reversible Transition between Underwater Superaerophilicity and Superaerophobicity on the Femtosecond Laser-Ablated Superhydrophobic PTFE Surfaces. Adv. Mater. Interfaces 2019, 6, 1900262. [Google Scholar] [CrossRef]
- Xiao, X.; Li, S.; Zhu, X.; Xiao, X.; Zhang, C.; Jiang, F.; Yu, C.; Jiang, L. Bioinspired Two-Dimensional Structure with Asymmetric Wettability Barriers for Unidirectional and Long-Distance Gas Bubble Delivery Underwater. Nano Lett. 2021, 21, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Shi, L.-A.; Huang, Z.; Hu, Y.; Wu, S.; Li, J.; Wu, D.; Chu, J. Microhole-Arrayed PDMS with Controllable Wettability Gradient by One-Step Femtosecond Laser Drilling for Ultrafast Underwater Bubble Unidirectional Self-Transport. Adv. Mater. Interfaces 2019, 6, 1900297. [Google Scholar] [CrossRef]
- Xue, X.; Wang, R.; Lan, L.; Wang, J.; Xue, Z.; Jiang, L. Reliable Manipulation of Gas Bubble Size on Superaerophilic Cones. ACS Appl. Mater. Interfaces 2018, 10, 5099–5106. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Cao, M.; Zhang, C.; Bei, Z.; Li, K.; Yu, C.; Jiang, L. Directional and Continuous Transport of Gas Bubbles on Superaerophilic Geometry-Gradient Surfaces in Aqueous Environments. Adv. Funct. Mater. 2018, 28, 1705091. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, W.; Ma, J.; Li, Y.; Sun, X.; Jiang, L. Superaerophilic Carbon-Nanotube-Array Electrode for High-Performance Oxygen Reduction Reaction. Adv. Mater. 2016, 28, 7155–7161. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Chen, F.; Huo, J.; Fang, Y.; Yang, Q.; Zhang, J.; Hou, X. Femtosecond Laser Induced Underwater Superaerophilic and Superaerophobic PDMS Sheet with Through-Microholes for Air Bubbles Selectively Passing Through and Further Collecting Underwater Gas. Nanoscale 2018, 10, 3688–3696. [Google Scholar] [CrossRef]
- Yong, J.; Singh, S.C.; Zhan, Z.; Chen, F.; Guo, C. Substrate-Independent, Fast, and Reversible Switching between Underwater Superaerophobicity and Aerophilicity on the Femtosecond Laser-Induced Superhydrophobic Surfaces for Selectively Repelling or Capturing Bubbles in Water. ACS Appl. Mater. Interfaces 2019, 11, 8667–8675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, C.; Yong, J.; Yang, Q.; Chen, F.; Huo, J.; Zhuang, J.; Jiang, Z.; Hou, X. Reversible Switch between Underwater Superaerophilicity and Superaerophobicity on the Superhydrophobic Nanowire-Haired Mesh for Controlling Underwater Bubble Wettability. AIP Adv. 2018, 8, 045001. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wu, Y.; Su, B.; Wang, J.; Song, Y.; Jiang, L. Terminating Marine Methane Bubbles by Superhydrophobic Sponges. Adv. Mater. 2012, 24, 5884–5889. [Google Scholar] [CrossRef]
- Li, N.; Chen, W.; Chen, G.; Wan, X.; Tian, J. Low-Cost, Sustainable, and Environmentally Sound Cellulose Absorbent with High Efficiency for Collecting Methane Bubbles from Seawater. ACS Sustain. Chem. Eng. 2018, 6, 6370–6377. [Google Scholar] [CrossRef]
- Zhu, S.; Li, J.; Cai, S.; Bian, Y.; Chen, C.; Xu, B.; Su, Y.; Hu, Y.; Wu, D.; Chu, J. Unidirectional Transport and Effective Collection of Underwater CO2 Bubbles Utilizing Ultrafast-Laser-Ablated Janus Foam. ACS Appl. Mater. Interfaces 2020, 12, 18110–18115. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Zhuang, J.; Bai, X.; Huo, J.; Yang, Q.; Hou, X.; Chen, F. Water/Gas Separation Based on the Selective Bubble-Passage Effect of Underwater Superaerophobic and Superaerophilic Meshes Processed by a Femtosecond Laser. Nanoscale 2021, 13, 10414–10424. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Huo, J.; Hou, X.; Chen, F. Underwater Gas Self-Transportation along Femtosecond Laser-Written Open Superhydrophobic Surface Microchannels (<100 µm) for Bubble/Gas Manipulation. Int. J. Extrem. Manuf. 2022, 4, 015002. [Google Scholar] [CrossRef]
































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, J.; Yang, Q.; Hou, X.; Chen, F. Emerging Separation Applications of Surface Superwettability. Nanomaterials 2022, 12, 688. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12040688
Yong J, Yang Q, Hou X, Chen F. Emerging Separation Applications of Surface Superwettability. Nanomaterials. 2022; 12(4):688. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12040688
Chicago/Turabian StyleYong, Jiale, Qing Yang, Xun Hou, and Feng Chen. 2022. "Emerging Separation Applications of Surface Superwettability" Nanomaterials 12, no. 4: 688. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12040688