A Simple Polypyrrole/Polyvinylidene Fluoride Membrane with Hydrophobic and Self-Floating Ability for Solar Water Evaporation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PPy
2.3. Fabrication of PVDF Membrane
2.4. Fabrication of PPy/PVDF Membrane
2.5. Fabrication of PPy/PVDF Membrane with Micro-Pyramids
2.6. Photothermal Performance Testing
2.7. Characterization
3. Results and Discussion
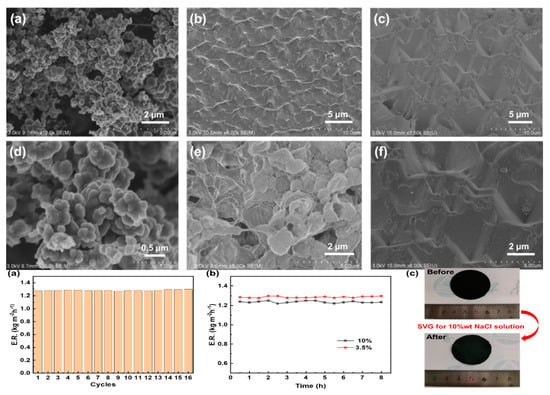
3.1. Material Fabrication and Morphology
3.2. Optical and Hydrophobic Property
3.3. Optimization of PPy/PVDF Membrane
3.4. Photothermal Property of PPy/PVDF(P)Membrane
3.5. Salt Tolerance and Stability of PPy/PVDF Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, F.; Guo, Y.; Zhou, X.; Shi, W.; Yu, G. Materials for solar-powered water evaporation. Nat. Rev. Mater. 2020, 5, 388–401. [Google Scholar] [CrossRef]
- Geng, H.; Xu, Q.; Wu, M.; Ma, H.; Zhang, P.; Gao, T.; Qu, L.; Ma, T.; Li, C. Plant leaves inspired sunlight-driven purifier for high-efficiency clean water production. Nat. Commun. 2019, 10, 1512. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, Y.; Guan, Y.; Liu, X.; Liu, H.; Xie, M.; Zhou, L.; Wei, C.; Yu, C.; Chen, Y. Cellulose nanofibril-stabilized pickeringemulsion and in situ polymerization lead to hybrid aerogel for high-efficiency solar steam generation. ACS Appl. Polym. Mater. 2020, 2, 4581–4591. [Google Scholar] [CrossRef]
- Qi, D.; Liu, Y.; Liu, Y.; Liu, Z.; Luo, Y.; Xu, H.; Zhou, X.; Zhang, J.; Yang, H.; Wang, W. Polymeric membranes with selective solution-diffusion for intercepting volatile organic compounds during solar-driven water remediation. Adv. Mater. 2020, 32, 2004401. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bai, Y.; Ge, C.; Ma, C.; Liang, W.; Zhang, X. Modified melamine foam-based flexible phase change composites: Enhanced photothermal conversion and shape memory properties. ACS Appl. Polym. Mater. 2021, 3, 3321–3333. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D.; Li, X.; Sun, H.; Zhao, H.; Li, Y.; Liu, X.; Shi, G. Photothermal membrane of CuS/polyacrylamide–carboxymethyl cellulose for solar evaporation. ACS Appl. Polym. Mater. 2021, 3, 2402–2410. [Google Scholar] [CrossRef]
- Xu, X.; Ozden, S.; Bizmark, N.; Arnold, C.; Datta, S.; Priestley, R. A bioinspired elastic hydrogel for solar-driven water purification. Adv. Mater. 2021, 33, 2007833. [Google Scholar] [CrossRef]
- Gao, M.; Peh, C.K.; Phan, H.T.; Zhu, L.; Ho, G.W. Solar absorber gel: Localized macro-nanoheat channeling for efficient plasmonic Au nanoflowers photothermic vaporization and triboelectric generation. Adv. Energy Mater. 2018, 8, 1800711. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Liang, W.; Yang, H.; Guan, T.; Zhao, B.; Sun, Y.; Chi, L.; Jiang, L. Rational design of plasmonic metal nanostructures for solar energy conversion. CCS Chem. 2021, 2127–2142. [Google Scholar] [CrossRef]
- Sun, H.; Li, X.; Chen, J.; Zhu, H.; Miao, H.; Li, Y.; Liu, X.; Shi, G. A novel photothermal, self-healing and anti-reflection water evaporation membrane. Soft Matter 2021, 17, 4730–4737. [Google Scholar] [CrossRef]
- Bae, K.; Kang, G.; Cho, S.; Park, W.; Kim, K.; Padilla, W. Flexible thin-film black gold membranes with ultrabroadband plasmonic nanofocusing for efficient solar vapourgeneration. Nat. Commun. 2015, 6, 10103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Tang, C.; Ma, J.; Liu, D.; Qi, D.; You, S.; Cui, F.; Wei, Y.; Wang, W. Low-tortuosity water microchannels boosting energy utilization for high water flux solar distillation. Environ. Sci. Technol. 2020, 54, 5150–5158. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Liu, S.; Ding, Y.; Wen, X.; Wang, K.; Shao, C.; Hong, X.; Liu, Y. A self-floating electrospunnanofiber mat for continuously high-efficiency solar desalination. Chemosphere 2021, 280, 130719. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Wang, J.; Song, W.; Fang, J.; Zhang, X. Self-floating hybrid hydrogels assembled with conducting polymer hollow spheres and silica aerogel microparticles for solar steam generation. J. Mater. Chem. A 2019, 7, 1244–1251. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Qu, D.; Jiang, W.; Chen, G.; An, L.; Zhuang, C.; Sun, Z. Self-floating nanostructured Ni–NiOx/Ni foam for solar thermal water evaporation. J. Mater. Chem. A 2019, 7, 8485–8490. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wang, P. Self-floating carbon nanotube membrane on macroporoussilica substrate for highly efficient solar-driven interfacial water evaporation. ACS Sustain. Chem. Eng. 2016, 4, 1223–1230. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Gao, M.; Peha, C.; Ho, G. Recent progress in solar-driven interfacial water evaporation: Advanced designs and applications. Nano Energy 2019, 57, 507–518. [Google Scholar] [CrossRef]
- Fan, Y.; Tian, Z.; Wang, F.; He, J.; Ye, X.; Zhu, Z.; Sun, H.; Liang, W.; Li, A. Enhanced solar-to-heat efficiency of photothermal materials containing an additional light-reflection layer for solar-driven interfacial water evaporation. ACS Appl. Energy Mater. 2021, 4, 2932–2943. [Google Scholar] [CrossRef]
- Chala, T.; Wu, C.; Chou, M.; Guo, Z. Melt electrospun reduced tungsten oxide/polylactic acid fiber membranes as a photothermal material for light-driven interfacial water evaporation. ACS Appl. Mater. Inter. 2018, 10, 28955–28962. [Google Scholar] [CrossRef]
- Ghasemi, H.; Ni, G.; Marconnet, A.M.; Loomis, J.; Yerci, S.; Miljkovic, N.; Chen, G. Solar steam generation by heat localization. Nat. Commun. 2014, 5, 4449. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Hu, X.; Zhuang, S.; Wang, Y.; Li, X.; Zhou, L.; Zhu, S.; Zhu, J. Flexible and salt resistant Janus absorbers by electrospinning for stable and efficient solar desalination. Adv. Energy Mater. 2018, 8, 1702884. [Google Scholar] [CrossRef]
- Zhou, X.; Fei, Z.; Guo, Y.; Zhang, Y.; Yu, G. A hydrogel-based antifouling solar evaporator for highly efficient water desalination. Energ. Environ. Sci. 2018, 11, 1985–1992. [Google Scholar] [CrossRef]
- Li, Z.; Xu, R.; Wei, N.; Song, X.; Yang, E.; Liu, Q.; Sui, Y.; Cui, H. 3D Network structure and hydrophobic Ni-G-WO3-x solar-driven interfacial evaporator for highly efficient steam generation. Sol. Energ. Mater. Sol. C. 2020, 217, 110593. [Google Scholar] [CrossRef]
- Ye, M.; Jia, J.; Wu, Z.; Qian, C.; Chen, R.; O'Brien, P.; Sun, W.; Dong, Y.; Ozin, G. Synthesis of black TiOx nanoparticles by Mg reduction of TiO2nanocrystals and their application for solar water evaporation. Adv. Energy Mater. 2017, 7, 1601811. [Google Scholar] [CrossRef]
- Wan, R.; Shi, G. Accelerated evaporation of water on graphene oxide. Phys. Chem. Chem. Phys. 2017, 19, 8843–8847. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Guo, D.; Cao, M.; Jiang, L. Floatable, self-cleaning, and carbon-black-based superhydrophobic gauze for the solar evaporation enhancement at the air–water interface. ACS Appl. Mater. Inter. 2015, 7, 13645–13652. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Duan, H.; Liu, Y.; Quan, X.; Tao, P.; Shang, W.; Wu, J.; Song, C.; Deng, T. The impact of surface chemistry on the performance of localized solar-driven evaporation system. Sci. Rep. 2015, 5, 13600. [Google Scholar] [CrossRef]
- Li, E.; Pan, Y.; Wang, C.; Liu, C.; Shen, C.; Pan, C.; Liu, X. Asymmetric superhydrophobic textiles for electromagnetic interference shielding, photothermal conversion, and solar water evaporation. ACS Appl. Mater. Inter. 2021, 13, 28996–29007. [Google Scholar] [CrossRef]
- Ito, Y.; Tanabe, Y.; Han, J.; Fujita, T.; Tanigaki, K.; Chen, M. Multifunctional porous graphene for high-efficiency steam generation by heat localization. Adv. Mater. 2015, 27, 4302–4307. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, B.; Wu, J.; Li, R.; Wang, P. Hydrophobic light-to-heat conversion membranes with self-healing ability for interfacial solar heating. Adv. Mater. 2015, 27, 4889–4894. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Li, X.; Li, Y.; Sun, J. Intumescent flame-retardant and self-healing superhydrophobic coatings on cotton fabric. ACS Nano 2015, 9, 4070–4076. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Wu, M.; Sun, J. All spraying processes for the fabrication of robust, self-healing, superhydrophobic coatings. Adv. Mater. 2014, 26, 3344–3348. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, H.; Gestos, A.; Fang, J.; Niu, H.; Ding, J.; Lin, T. Robust, electro-conductive, self-healing superamphiphobicfabric prepared by one-step vapour-phase polymerisation of poly(3,4-ethylenedioxythiophene) in the presence of fluorinated decyl polyhedral oligomeric silsesquioxane and fluorinated alkyl silane. Soft Matter 2013, 9, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, F.; Jin, Y.; Zhai, D.; Li, Y.; Ni, C.; Shi, G. Efficient gatherer of sunlight based on two-sided bio-inspired antireflective micro-pyramids with PPy/TiO2. Inorg. Chem. Commun. 2010, 110, 107604. [Google Scholar] [CrossRef]
- Shi, G.; Zhang, X.; Li, J.; Zhu, H.; Li, Y.; Zhang, L.; Ni, C.; Chi, L. Fabrication of 3D biomimetic composite coating with broadband antireflection, superhydrophilicity, and double p-n heterojunctions. Nano Res. 2017, 7, 2377–2385. [Google Scholar] [CrossRef]
- Shi, G.; Li, H.; Sang, X.; Wang, L.; Ni, C.; Li, Y. Micro-nano fabrication of hierarchical PPy/TiO2/Si by continuous self-assembly technology. Mater. Manuf. Process. 2018, 33, 378–382. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.; Liu, Y.; Abed, M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membrane Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Li, Y.; Feng, L.; Li, J.; Li, X.; Chen, J.; Wang, L.; Qi, D.; Liu, X.; Shi, G. Fabrication of an insect-like compound-eye SERS substrate with 3D Ag nano-bowls and its application in optical sensor. Sensor. Actuat. B-Chem. 2021, 330, 129357. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Wang, H.; Miao, H.; Zhu, H.; Liu, X.; Lin, H.; Shi, G. Fabrication of a three-dimensional bionic Si/TiO2/MoS2photoelectrode for efficient solar water splitting. ACS Appl. Energy Mater. 2021, 4, 730–736. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, L.; Zhu, H.; Miao, H.; Li, Y.; Liu, X.; Shi, G. Non contact metal-spiropyran-metal nanostructured substrates with Ag and Au@SiO2 nanoparticles deposited in nanohole arrays for surface-enhanced fluorescence and trace detection of metal ions. ACS Appl. Nano Mater. 2021, 4, 3780–3789. [Google Scholar] [CrossRef]
- Shi, G.; Chen, J.; Wang, L.; Wang, D.; Yang, J.; Li, Y.; Zhang, L.; Ni, C.; Chi, L. Titanium oxide/silicon moth-eye structures with antireflection, p-n heterojunctions, and superhydrophilicity. Langmuir 2016, 32, 10719–10724. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhu, Q.; Feng, L.; Li, X.; Zhu, H.; Miao, H.; Zeng, Z.; Wang, Y.; Li, Y.; Wang, L.; et al. Light-trapping SERS substrate with regular bioinspired arrays for detecting trace dyes. ACS Appl. Mater. Inter. 2021, 13, 11535–11542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, H.; Li, X.; Li, Y.; Jin, Y.; Liu, X.; Shi, G.; Wong, P.K. A hierarchical SiPN/CN/MoSx photocathode with low internal resistance and strong light-absorption for solar hydrogen production. Appl. Catal. B-Environ. 2022, 300, 120758. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, L.; Peh, C.; Ho, G. Solar absorber material and system designs for photothermal water vaporization towards clean water and energy production. Energy Environ. Sci. 2019, 12, 841–864. [Google Scholar] [CrossRef]
- Wu, X.; Chen, G.Y.; Owens, G. Photothermal materials: A key platform enabling highly efficient water evaporation driven by solar energy. Mater. Today Energy 2019, 12, 277–296. [Google Scholar] [CrossRef]
- Petrescu, F.I.T.; Calautit, J.K. About the Light Dimensions. Am. J. Appl. Sci. 2016, 13, 321–325. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Chen, J.; Zheng, J.; Chen, X.; Xu, H.; Petrescu, F.I.T.; Ungureanu, L.M.; Li, Y.; Shi, G. A Simple Polypyrrole/Polyvinylidene Fluoride Membrane with Hydrophobic and Self-Floating Ability for Solar Water Evaporation. Nanomaterials 2022, 12, 859. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12050859
Zhang S, Chen J, Zheng J, Chen X, Xu H, Petrescu FIT, Ungureanu LM, Li Y, Shi G. A Simple Polypyrrole/Polyvinylidene Fluoride Membrane with Hydrophobic and Self-Floating Ability for Solar Water Evaporation. Nanomaterials. 2022; 12(5):859. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12050859
Chicago/Turabian StyleZhang, Shenfeng, Jun Chen, Jixin Zheng, Xin Chen, Hongbo Xu, Florian Ion Tiberiu Petrescu, Liviu Marian Ungureanu, Ying Li, and Gang Shi. 2022. "A Simple Polypyrrole/Polyvinylidene Fluoride Membrane with Hydrophobic and Self-Floating Ability for Solar Water Evaporation" Nanomaterials 12, no. 5: 859. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12050859