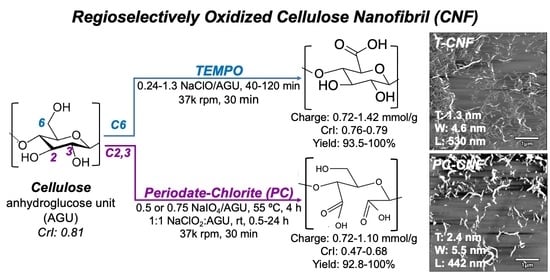
3.1. T-CNF from TEMPO-Mediated Oxidation and Blending
The TEMPO-mediated C6 regioselective oxidation of the dissolving pulp was conducted at 1.5, 3, 5, and 8 mmol of NaClO per gram of cellulose to produce TEMPO-oxidized cellulose (T-Cell) series that were designated as T-Cell1.5, T-Cell3, T-Cell5, and T-Cell8, respectively (
Figure 1a). These TEMPO oxidation reactions correspond to their respective 0.24, 0.48, 0.81, and 1.30 NaClO/anhydroglucose (AGU) ratios. The conversion of cellulose C6 hydroxyls to carboxyl groups was quantified via conductivity titration (Equation (1)) to show the surface charge of the T-Cell increased from 0.72 to 1.42 mmol/g with an increasing NaClO/AGU ratio from 0.241 to 0.81, then slightly increased to 1.48 mmol/g at 1.30 NaClO/AGU (
Figure 1b). The length of time taken to complete the TEMPO oxidation reaction also exhibited the same trend, i.e., it increased from 40 to 100 min with an increasing NaClO/AGU ratio from 0.24 to 0.81, then a much smaller incline in time to 110 min with a considerable increase to 1.3 NaClO/AGU (
Figure 1b). Thus, the TEMPO oxidation of pulp cellulose at 5 mmol of NaClO per gram of cellulose, corresponding to a 0.81 NaClO/AGU molar ratio, was deemed optimal to convert the most surface C6 hydroxyls (23% conversion) to carboxyl functional groups.
The four TEMPO-oxidized celluloses (T-Cell) with varied surface charges from 0.72 to 1.42 mmol/g of surface carboxyls were high-speed blended at a 0.1
w/
v% concentration for varying lengths of time from 1 to 60 min, then centrifuged (5k rpm, 15 min) to separate the CNF in the supernatants (
Figure 2a,
Figure S1). At all oxidation levels, substantial precipitates were observed from the shortest 1 min blending, indicative of an insufficient shearing to disintegrate any of the TEMPO-oxidized cellulose significantly. With a higher oxidation and longer blending, less precipitates were present, showing more TEMPO-oxidized cellulose being disintegrated to be dispersed in the supernatants. With an increasing oxidation, the supernatants of the blended suspensions at any given blending time also appeared to be increasingly opaque, indicating increasing CNF quantities in the supernatants.
These observations are consistent with the expectation that more TEMPO-oxidized cellulose requires less shearing to be disintegrated into nanocelluloses due to a greater extent of reduced hydrogen bonding and an increased electrostatic repulsion force in the non-crystalline domains. However, the least oxidized T-Cell1.5 produced modestly increasing yields with longer blending, producing only 43.6% T-CNF1.5 in large bundles and partly fibrillated structures even from the longest 60 min blending (
Figure 2b). The substantial bundles and merely 43.6% yield observed for T-Cell1.5 blended for a respective 30 and 60 min gave a clear indication that the 0.72 mmol/g charge produced from 0.24 NaClO/AGU was too low to be effectively fibrillated into T-CNF. T-Cell3 with a 1.10 mmol/g charge produced by TEMPO oxidation at 0.49 NaClO/AGU was deemed the threshold level for T-CNF production. From the more oxidized T-Cell3, T-Cell5, and T-Cell8, T-CNF production was much more strongly dependent upon the length of blending, producing 50.8% T-CNF3, 84.2% T-CNF5, and 95.7% T-CNF8 after only 10 min, then further increasing to a respective 82.1, 93.5, and 100% from a moderate blending time of 30 min, illustrating the synergistic effects of TEMPO oxidation and mechanical blending in facilitating the disintegration of TEMPO-oxidized cellulose into T-CNFs.
For T-Cell3, T-Cell5, and T-Cell8, the short 10 min of blending produced T-CNFs in a reduced thickness from 2.4 to 1.5 nm but in similar lengths between 462 to 486 nm (
Figure 2c). Lengthening the blending to 30 min produced T-CNFs in an only slightly reduced thickness from 1.3 to 1.8 nm and lengths from 551 to 486 nm. In addition, widths of the T-CNFs also decreased from 6.6 nm to 4.6–4.9 nm when the surface charge increased from 1.10 mmol/g to 1.42–1.48 mmol/g. Overall, neither increasing the TEMPO oxidation level, i.e., the surface charges, nor the lengths of blending exhibited a clear trend in affecting T-CNF thickness, but T-CNF lengths seemed slightly shortened when the surface charge increased from 1.42 to 1.48 mmol/g, possibly by breaking the β-1,4-glycosidic link. Most significantly, all T-CNFs from the 30 min blending of T-Cell with 1.1 to 1.48 mmol/g charges were very homogeneous and in good to excellent yields of 82.1, 93.5, to 100%. Even 10 min of blending could produce 84.2 and 95.7% T-CNFs from the more charged 1.42 and 1.48 mmol/g T-Cell (
Figure 2d).
Most significantly, the synergistic effects of TEMPO oxidation and mechanical blending are clearly evident and versatile to produce T-CNFs with surface charges ranging from 1.10 to 1.48 mmol/g but in similar dimensional attributes of a 1.3–2.4 nm thickness, 4.6–6.6 nm width, and 254–481 nm length (
Figure 2e). Overall, T-Cell5 TEMPO oxidized at 0.81 NaClO/AGU molar ratios requires only 10 min of blending to produce an 84.2% T-CNF5 that could be maximized to 93.5% T-CNF5 with 30 min of blending. The even more oxidized T-Cell8 from a TEMPO oxidation at a 1.30 NaClO/AGU molar ratio could produce a 95.7% T-CNF8 with only 10 min blending or an impressive 100% could be converted with 30 min of blending. All T-CNFs are long-ribbon-like, i.e., highly dimensionally anisotropic with W/T ratios ranging from 2.7 to 4.1 and L/T ratios ranging from ca. 200 to 408 (
Figure S1).
To further investigate the degree of substitution for T-CNFs, an
1H NMR was performed after the solvent changed from the aqueous dispersion to the D
2O (
Figure 3a). Under the assumption that all anomeric protons of amorphous and crystalline surface AGU of T-CNF are detectable by the
1H NMR, the degree of substitution (DS) of the T-CNF surface hydroxyls to carboxyls could be quantified. The cellulose anomeric proton was the sum of the integration of areas for all anomeric H1 to H5 proton peaks then averaged by five. Unmodified hydroxyls could be estimated by integrating the areas of H6 and H6′ then dividing by two protons. The DS of hydroxyls to carboxyls could be estimated by one minus the unmodified hydroxyl to anomeric proton ratio:
The DS for T-CNF3 to T-CNF8 varied from 0.83, to 0.75, to 0.86, showing no clear association with surface charges, which may be due to the small portion of existing hydroxyls on the C6 (
Figure 3b). The downfield peak at δ 4.4 was assigned to the cellulosic anomeric proton (H1) similar to the chemical shift at δ 4.5 reported in dissolved cellulose [
6]. The H6 and H6′ peaks for T-CNF3-8 appeared at δ 3.97 and δ 3.85, comparable to the δ 3.65–3.88 range for dissolved MCC in NaOD/D
2O [
7]. Multiple overlapping peaks between δ 3.45–3.80 were assigned to H3, H4 and H5, matching those at δ 3.34–3.66 of the TEMPO-CNF [
8]. The furthest up-field cellulosic peak at δ 3.2–3.3 coincided with the chemical shift of H2 at δ 3.2 for T-CNF in D
2O [
8].
The 0.81 CrI of dissolving pulp cellulose was reduced slightly to the 0.76 to 0.79 range for all T-CNFs via the coupled TEMPO oxidation and shear force from blending (
Figure 3c). The indifferent CrI values indicate that the crystallinities of these T-CNFs are independent from the levels of TEMPO oxidation and blending time. The high yields as well as high and indifferent CrI are consistent with the relatively mild nature of TEMPO oxidation and the fact that all T-CNFs isolated in the supernatants were dialyzed and were thus free of lower molecular mass fragments.
3.2. PC-CNF from Periodate–Chlorite Oxidation and Blending
The sequential periodate–chlorite oxidation of cellulose was conducted at two primary sodium periodate oxidant levels, i.e., 0.5:1 and 0.75:1 NaIO
4:AGU molar ratios, at 55 °C for 4 h followed by secondary sodium chlorite oxidation at a 1:1 NaClO
2:AGU molar ratio at an ambient temperature over varying lengths of time from 0.5 h to up to 24 h. Generally, a higher primary oxidant level led to higher charges and yields of PC-CNF (
Figure 4a,b,
Figure S2). With an increasing secondary NaClO
2 oxidation time, the PC-CNF charges slightly increased from 0.72 to 0.89 mmol/g and from 1.06 to 1.16 mmol/g at 0.5:1 and 0.75:1 NaIO
4:AGU molar ratios, respectively (
Figure 4a). The PC-CNF yields, on the other hand, significantly increased from 49.3% to 99.6% with a longer secondary oxidation, with the most drastic increases occurring between 6 and 12 h at the lower primary oxidation level. In contrast, yields were impressively consistent at 100% and irrespective of the lengths of the reaction time at the higher primary oxidation level (
Figure 4b). PC-CNF yields were linked with a higher charge, showing an over 90% yield when surface charges exceeded 0.88 mmol/g (
Figure 4c). The PC-CNFs produced from the higher primary 0.75:1 NaIO
4:AGU oxidant level were generally thinner and shorter but similar in widths to those from the lower primary 0.5:1 NaIO
4:AGU oxidant level (
Figure 4d–f). With a longer secondary oxidation, both the PC-CNF thickness and length reduced slightly, i.e., the thickness lowered from 2.7 to 2.1 nm and from 2.1 to 1.8 nm whereas the length shortened from 533 to 464 nm and from 407 to 285 nm at 0.5:1 and 0.75:1 NaIO
4:AGU, respectively (
Figure 4d). The widths of all PC-CNFs were in between 5.4 and 5.9 nm, seemingly independent of either the primary NaIO
4 levels or the lengths of secondary oxidation (
Figure 4e). All PC-CNFs are dimensionally anisotropic, W/T and L/T ratios exceed 1 and 100, respectively.
The quality of the PC-CNFs was further elucidated by imaging the diluted supernatants. Substantial bundles observed from the lower 0.5:1 NaIO
4/AGU primary oxidation and shorter secondary oxidation up to 6 h gave clear evidence of the partial defibrillation of the 56.3% in the supernatant (
Figure 5). Even with the much improved 92.8% yield from the longer 12 h of secondary oxidation, some bundled structures were still observed by both AFM and TEM. The bundle thickness reduced from 7.0 nm to 4.2 nm as the secondary oxidation lengthened from 0.5 h to 12 h, then no fiber bundle was observed at 18 h. From the lower primary oxidation at 0.5:1 NaIO
4/AGU, the relative uniform PC-CNF (T: 2.1 ± 0.7 nm) in a high yield (95.5%) required longer secondary oxidation at 15 h (
Figure S2). On the contrary, the higher primary oxidation at 0.75:1 NaIO
4/AGU produced exclusively isolated PC-CNFs, irrespective of the lengths of the secondary oxidation and, impressively, from as brief as only 0.5 h. However, a longer secondary chlorite oxidation did reduce PC-CNF dimensions as noted earlier (
Figure 4), indicative of β-1,4-glycosidic link chain scission. All PC-CNFs are dimensionally anisotropic. The W/T ratio was 2.7 and L/T ratios ranged from 117 to 167 among the few with corresponding T, W, and L values.
The crystallinity of all the PC-CNFs were significantly lower than the 0.81 CrI of the original dissolving pulp cellulose (
Figure 6a,b). From the primary oxidation at the lower 0.5:1 NaIO
4/AGU ratio, the CrI was significantly reduced to 0.67 and remained there with increasing lengths of secondary oxidation time from 0.5 to 9 h, then lowered further to 0.55 at 12 h. From the primary oxidation at the higher 0.75:1 NaIO
4/AGU molar ratio, the CrI was also significantly reduced to 0.60 at 6 h then lowered even further to 0.46 at 9 h of secondary oxidation time. That most significant CrI reduction was observed with even a brief secondary oxidation supports the notion that breaking the C2-C3 bond in the primary periodate oxidation imposes the most significant effect on reducing the crystallinity, and periodate oxidation occurs not only on the amorphous chains but also across the amorphous-crystalline boundaries into the crystalline domains to reduce crystallinity. A longer secondary chlorite oxidation of C2,C3 dialdehydes into dicarboxls further reduces the crystallinity.
3.3. Regioselective C6 vs. C2,C3 Carboxylation Comparison
Both TEMPO-mediated and sequential periodate–chlorite oxidation reactions involve the heterogeneous oxidation of respective C6 and C2,C3 hydroxyls in the less-ordered and the amorphous-crystalline interfacial regions of the micro-scale cellulose. In the TEMPO-mediated oxidation involving NaBr and NaClO, both TEMPO and NaBr serve as catalysts and NaClO as the primary oxidant. NaClO oxidizes NaBr into the much stronger secondary hypobromite NaBrO oxidant that oxidizes TEMPO to the N-oxoammonium ion to convert cellulose C6 hydroxyls to aldehydes. The C6 aldehyde-to-carboxyl conversion requires another equivalent N-oxoammonium ion. The N-oxoammonium ions reduce to hydroxylamine then are oxidized back to TEMPO nitroxyl radicals to repeat the cycle. Therefore, each C6 hydroxyl-to-carboxyl oxidation requires two molar equivalents of TEMPO/NaBr/NaClO. With a 0.81 CrI of the dissolving pulp cellulose, the threshold 0.49 NaClO/AGU oxidant level that yielded 82.1% T-CNF3.30 is slightly higher than two times that of the 0.19 molar equivalent of the non-crystalline cellulose fraction and is stoichiometrically consistent when taking the amorphous-crystalline interfacial areas into account. Above the threshold, TEMPO oxidized at 0.81 NaClO/AGU carrying a 1.4 mmol/g charge produced 84.2% T-CNF with only 10 min of blending or 93.5% T-CNF with 30 min of blending. A higher TEMPO oxidation at 1.3 NaClO/AGU could produce 95.7% T-CNF with only 10 min of blending or an impressive 100% converted with 30 min of blending.
In a sequential periodate–chlorite oxidation, sodium periodate breaks the C2-C3 bonds in the anhydroglucose ring in the primary oxidation to form C2,C3 dialdehydes that are subsequently oxidized by sodium chlorite into dicarboxylates in the secondary oxidation. The 0.5 primary oxidation NaIO4/AGU ratio, i.e., over two times that of the 0.19 molar equivalent of the non-crystalline cellulose fraction, was deemed necessary as the primary at 0.25 NaIO4/AGU was insufficient for the secondary oxidation to proceed. Such a significant excess of the primary NaIO4 oxidant suggests its lower accessibility to the C2-C3 bonds than the N-oxoammonium ion to the primary C6 hydroxyls in the case of the TEMPO.
The TEMPO oxidation is a milder oxidation reaction at an ambient temperature and more time efficient due to the built-in sequential oxidation mechanism in its one-pot reaction, whereas the periodate–chlorite oxidation involves a two-step oxidation which is required to be conducted in the dark and at a moderately elevated 55 °C temperature. The T-CNFs produced also possess a wider range of 0.72–1.48 mmol/g charges on C6 compared to the narrower charge range of 0.72–1.16 mmol/g on the C2 and C3 of PC-CNF.
The optimally synthesized T-CNFs were averaged to be 1.3 nm thick, 4.6 nm wide, and 254 nm long in a 93.5% yield from a TEMPO oxidation at a 0.81:1 NaClO:AGU molar ratio and 30 min of blending. The optimally produced PC-CNFs were 2.4 nm thick, 5.5 nm wide, and 442 nm long on average in the 92.8% yield from the primary sodium periodate oxidation at a 0.5:1 NaIO4:AGU molar ratio and 12 h of secondary sodium chlorite oxidation at a 1:1 NaIO4:AGU molar ratio and 30 min of blending. Both T-CNFs and PC-CNFs are long-ribbon-like, i.e., highly dimensionally anisotropic with W/T ratios ranging from 2.7 to 4.1 for T-CNFs and 2.7 for one PC-CNF and with L/T ratios ranging from ca. 200 to 408 for T-CNFs and 117–167 for two PC-CNFs. Both regioselective oxidizations could be easily tuned by optimizing the respective oxidant to the accessible AGU in the dissolving pulp to convert over 90% of the cellulose into T-CNFs and PC-CNFs. The minimal charge levels for efficient shear force blending into CNF were slightly higher for the T-Cell at 1.1 mmol/g than the PC-Cell’s 0.88 mmol/g. The dimensional attributes of both T-CNFs and PC-CNFs fall within similar ranges of thicknesses (1.3–2.4 nm T-CNF, 1.8–2.7 nm PC-CNF), widths (4.6–6.6 nm T-CNF, 5.5–5.9 nm PC-CNF), and lengths (254–481 nm T-CNF, 247–442 nm PC-CNF).
TEMPO-mediated oxidation is an energy- and time-efficient way to prepare slightly thinner and shorter C6 carboxylated CNFs; sequential periodate–chlorite oxidation is versatile to produce a slightly longer C2,C3 dicarboxylated CNF in which the dialdehyde intermediates are also amenable to further chemical reactions to generate multiple types of functionalized nanocelluloses as well as other reactive nanomaterials.
Though both TEMPO and PC oxidation reactions have been reported individually on various cellulose sources, these regioselectively oxidized and well-characterized C6 and C2,C3 carboxylated CNFs from unmodified cellulose represent the first comparable series of nanocellulose. Generated from mass-produced, commercially available, and relatively pure dissolving pulp, these CNFs represent the first comparable series of regioselectively carboxylated nanocellulose references and standards. Specifically, these regioselectively carboxylated CNF series are presented as a range of well-characterized C6 and C2,C3 carboxylated CNFs along with C6 sulfated CNFs [
19] for the development of a toolbox of safer-by-design substance evaluation tools linking the biological behavior of functionalized CNFs to their surface chemistries, charges, and dimensions [
20]. These approaches may be further expanded to apply to less-purified or other sources of cellulose as well as to advance future scalable and industrially relevant product and application development.