Serum Analysis of Women with Early-Stage Breast Cancer Using a Mini-Array of Tumor-Associated Antigens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. Expression and Purification of Recombinant Tumor-Associated Antigens (TAA)
2.3. Western-Blot Assays
2.4. Dot-Blot Assays
3. Results
3.1. Breast Cancer: TNM Staging
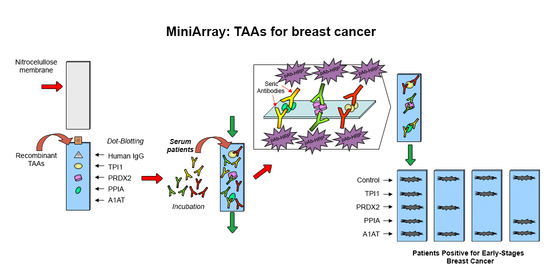
3.2. Manufacture of the Mini-Array
3.3. Presence of Antibodies Interacting with TAA Blotted on the Mini-Array
3.4. Evaluation of Diagnostic Values of the Mini-Array with Four TAA for the Diagnosis of Breast Cancer
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global Cancer Statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef]
- Ahmedin-Jemal, D.V.M.; Bray, F.; Melissa, M.; Jacques-Ferlay, M.E.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, M.P. Trends in breast cancer incidence, survival, and mortality. Lancet 2000, 356, 590–591. [Google Scholar] [CrossRef]
- Curado, M.P.; Edwards, B.; Shin, H.R.; Storm, H.; Ferlay, J.; Heanue, M.; Boyle, P. Cancer Incidence in Five Continents; IARC Scientific Publications: Lyon, France, 2007; Volume IX, p. 160. [Google Scholar]
- De Santis, C.E.; Ma, J.; Bryan, L.; Jemal, A. Breast cancer statistics, 2013. CA Cancer J. Clin. 2014, 64, 52–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Me, J.F.; Soerjomataram, I.; Siegel, R.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, A.T.; Brock, A. Twentieth Century Mortality Trends in England and Wales. Office for National Statistics. Health Stat. Q. 2003, 18, 5–17. [Google Scholar]
- Tabár, L.; Gad, A.; Holmberg, L.; Ljungquist, U.; Kopparberg County Project Group; Fagerberg, C.; Baldetorp, L.; Gröntoft, O.; Lundström, B.; Månson, J.; et al. Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet 1985, 1, 829–832. [Google Scholar] [CrossRef]
- Jesneck, J.L.; Mukherjee, S.; Yurkovetsky, Z.; Clyde, M.; Marks, J.R.; Lokshin, A.E.; Lo, J.Y. Do serum biomarkers really measure breast cancer? BMC Cancer 2009, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Chapman, C.; Murray, A.; Chakrabarti, J.; Thorpe, A.; Woolston, C.; Sahin, U.; Barnes, A.; Robertson, J. Autoantibodies in breast cancer: Their use as an aid to early diagnosis. Ann. Oncol. 2007, 18, 868–873. [Google Scholar] [CrossRef]
- Tan, E.M. Autoantibodies as reporters identifying aberrant cellular mechanisms in tumorigenesis. J. Clin. Investig. 2001, 108, 1411–1415. [Google Scholar] [CrossRef]
- Fernández-Grijalva, A.; Aguilar-Lemarroy, A.; Jave-Suárez, L.F.; Gutiérrez-Ortega, A.; Godinez-Melgoza, P.A.; Herrera-Rodríguez, S.; Mariscal-Ramirez, I.; Martínez-Velázquez, M.; Gawinowicz, M.A.; Martínez-Silva, M.G.; et al. Alpha 2HS-glycoprotein, a tumor-associated antigen (TAA) detected in Mexican patients with early-stage breast cancer. J. Proteom. 2015, 112, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Conley, B.A.; Taube, S.E. Prognostic and Predictive Markers in Cancer. Dis. Markers 2004, 20, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaenker, P.; Ziman, M. Serologic Autoantibodies as Diagnostic Cancer Biomarkers—A Review. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 2161–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downes, M.R.; Byrne, J.C.; Dunn, M.J.; Fitzpatrick, J.M.; Watson, R.W.G.; Pennington, S.R. Application of proteomic strategies to the identification of urinary biomarkers for prostate cancer: A review. Biomarkers 2006, 11, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Kang, U.-B.; Kim, D.H.; Yi, J.K.; Lee, J.W.; Noh, D.Y.; Lee, C.; Yu, M.-H. Identification of circulating endorepellin LG3 fragment: Potential use as a serological biomarker for breast cancer. Proteom. Clin. Appl. 2008, 2, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.; Choquet-Kastylevsky, G.; Joubert-Caron, R. Cancer Immunomics Using Autoantibody Signatures for Biomarker Discovery. Mol. Cell. Proteom. 2007, 6, 1115–1122. [Google Scholar] [CrossRef] [Green Version]
- Madrid, F.F. Autoantibodies in breast cancer sera: Candidate biomarkers and reporters of tumorigenesis. Cancer Lett. 2007, 230, 187–198. [Google Scholar] [CrossRef]
- Ren, P.; Ye, H.; Dai, L.; Liu, M.; Liu, X.; Chai, Y.; Shao, Q.; Li, Y.; Lei, N.; Peng, B.; et al. Peroxiredoxin 1 is a tumor-associated antigen in esophageal squamous cell carcinoma. Oncol. Rep. 2013, 30, 2297–2303. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.Y.; Megliorino, R.; Peng, X.X.; Tan, E.M.; Chen, Y.; Chan, E.K. Antibody detection using tumor-associated antigen mini-array in immunodiagnosing human hepatocellular carcinoma. J. Hepatol. 2006, 46, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.; Keyser, B.; Lin, Z.T.; Wu, T. Autoantibodies as Potential Biomarkers in Breast Cancer. Biosens. Basel 2018, 8, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaenker, P.; Gray, E.; Ziman, M. Autoantibody Production in Cancer—The Humoral Immune Response toward Autologous Antigens in Cancer Patients. Autoimmun. Rev. 2016, 15, 477–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.; Shi, J.; Wang, P.; Song, C.; Wang, K.; Zhang, J.Y.; Ye, H. Tumour-Associated Autoantibodies as Diagnostic Biomarkers for Breast Cancer: A Systematic Review and Meta-Analysis. Scand. J. Immunol. 2016, 83, 393–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preiss, S.; Kammertoens, T.; Lampert, C.; Willimsky, G.; Blankenstein, T. Tumor-induced antibodies resemble the response to tissue damage. Int. J. Cancer 2005, 115, 456–462. [Google Scholar] [CrossRef]
- Liu, S.; Tan, Q.; Song, Y.; Shi, Y.; Shi, Y.K. Anti-p53 autoantibody in blood as a diagnostic biomarker for colorectal cancer: A meta-analysis. Scand. J. Immunol. 2019, 91, e12829. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Shi, J.X.; Xing, M.T.; Dai, L.P.; Li, J.T.; Zhang, J.Y. Evaluation of serum autoantibodies against tumor-associated antigens as biomarkers in lung cancer. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Shi, J.X.; Zhang, H.F.; Xing, M.T.; Li, P.; Dai, L.P.; Luo, C.L.; Wang, X.; Wang, P.; Ye, H.; et al. Serum autoantibodies against a panel of 15 tumor-associated antigens in the detection of ovarian cancer. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [Green Version]
- Pardoll, D.M. Does the immune system see tumors as foreign or self? Annu. Rev. Immunol. 2003, 21, 807–839. [Google Scholar] [CrossRef]
- Anderson, K.S.; Cramer, D.W.; Sibani, S.; Wallstrom, G.; Wong, J.; Park, J.; Qiu, J.; Vitonis, A.; LaBaer, J. Autoantibody signature for the serologic detection of ovarian cancer. J. Proteome Res. 2015, 14, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Linnebacher, M.; Maletzki, C. Tumor-infiltrating B cells. Oncoimmunology 2012, 1, 1186–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Árias, E.; Aguilar-Lemarroy, A.; Jave-Suárez, L.F.; Morgan-Villela, G.; Mariscal-Ramírez, I.; Martinez-Velazquez, M.; Alvarez, A.H.; Gutiérrez-Ortega, A.; Hernández-Gutiérrez, R. Alpha 1-antitrypsin: A novel tumor-associated antigen identified in patients with early-stage breast cancer. Electrophoresis 2012, 33, 2130–2137. [Google Scholar] [CrossRef]
- Desmetz, C.; Bascoul-Mollevi, C.; Rochaix, P.; Lamy, P.-J.; Kramar, A.; Rouanet, P.; Maudelonde, T.; Mangé, A.; Solassol, J. Identification of a New Panel of Serum Autoantibodies Associated with the Presence of In situ Carcinoma of the Breast in Younger Women. Clin. Cancer Res. 2009, 15, 4733–4741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Yu, J.; Sreekumar, A.; Varambally, S.; Shen, R.; Giacherio, D.; Mehra, R.; Montie, J.E.; Pienta, K.J.; Sanda, M.G.; et al. Autoantibody Signatures in Prostate Cancer. N. Eng. J. Med. 2005, 353, 1224–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; La Torre, I.G.D.; Gutiérrez-Rivera, M.C.; Wang, B.; Liu, Y.; Dai, L.; Qian, W.; Zhang, J.Y. Detection of autoantibodies to multiple tumor-associated antigens (TAAs) in the immunodiagnosis of breast cancer. Tumor Biol. 2015, 36, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Sun, C.; Ren, P.; Dai, L.; Peng, B.; Wang, K.; Qian, W.; Zhang, J.Y. Mini-array of multiple tumor-associated antigens (TAAs) in the immunodiagnosis of breast cancer. Oncol. Lett. 2013, 5, 663–668. [Google Scholar] [CrossRef] [Green Version]



| Patient | Age (Years) | Stage | Pathology | Tumor Size cm | Ab vs. | |||
|---|---|---|---|---|---|---|---|---|
| TPI1 | PRDX2 | PPIA | A1AT | |||||
| 1 | 49 | T1 N0 M0 | ID | <2 | + | + | + | + |
| 2 | 50 | T2 N1 M0 | IL | >2, <5 | + | + | + | + |
| 3 | 46 | T1 N0 M0 | ID | <2 | − | + | − | + |
| 4 | 48 | T1 N0 M0 | ID | <2 | + | − | + | − |
| 5 | 45 | T1 N0 M0 | ID | <2 | + | − | − | + |
| 6 | 40 | T2 N0 M0 | ID | >2, <5 | − | + | − | + |
| 7 | 44 | T2 N1 M0 | ID | >2, <5 | − | + | − | + |
| 8 | 46 | T2 N0 M0 | IL | >2, <5 | + | − | + | − |
| 9 | 41 | T1 N0 M0 | IL | <2 | − | + | + | + |
| 10 | 40 | T1 N0 M0 | IL | <2 | + | + | + | + |
| 11 | 37 | T1 N0 M0 | ID | <2 | + | − | − | + |
| 12 | 44 | T1 N2 M0 | ID | <2 | + | − | + | + |
| Patient | Age (Years) | Stage | Pathology | Tumor Size cm | Ab vs. | |||
|---|---|---|---|---|---|---|---|---|
| TPI1 | PRDX2 | PPIA | A1AT | |||||
| 1 | 49 | T0 N0 M0 | N | n/d | − | − | − | + |
| 2 | 43 | T0 N0 M0 | N | n/d | − | − | − | − |
| 3 | 47 | T0 N0 M0 | N | n/d | − | − | − | + |
| 4 | 46 | T0 N0 M0 | N | n/d | − | − | − | − |
| 5 | 50 | T0 N0 M0 | N | n/d | − | + | − | − |
| 6 | 45 | T0 N0 M0 | N | n/d | − | − | − | − |
| 7 | 41 | T0 N0 M0 | N | n/d | − | − | − | − |
| 8 | 47 | T0 N0 M0 | N | n/d | − | − | + | − |
| 9 | 38 | T0 N0 M0 | N | n/d | − | − | − | − |
| 10 | 48 | T0 N0 M0 | N | n/d | − | − | − | − |
| 11 | 46 | T0 N0 M0 | N | n/d | − | − | − | − |
| 12 | 50 | T0 N0 M0 | N | n/d | − | − | − | − |
| TAA | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | p Value |
|---|---|---|---|---|---|
| TPI1 | 1 | 0.75 | 0.6667 | 1 | 0.0013 |
| (**) | |||||
| PRDX2 | 0.875 | 0.6875 | 0.5833 | 0.9167 | 0.0272 |
| (*) | |||||
| PPIA | 0.875 | 0.6875 | 0.5833 | 0.9167 | 0.0272 |
| (*) | |||||
| A1AT | 0.8333 | 0.8333 | 0.8333 | 0.8333 | 0.0033. |
| (**) | |||||
| All | 0.8889 | 0.7333 | 0.6667 | 0.9167 | <0.0001 |
| (****) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oaxaca-Camacho, A.R.; Ochoa-Mojica, O.R.; Aguilar-Lemarroy, A.; Jave-Suárez, L.F.; Muñoz-Valle, J.F.; Padilla-Camberos, E.; Núñez-Hernández, J.A.; Herrera-Rodríguez, S.E.; Martínez-Velázquez, M.; Carranza-Aranda, A.S.; et al. Serum Analysis of Women with Early-Stage Breast Cancer Using a Mini-Array of Tumor-Associated Antigens. Biosensors 2020, 10, 149. https://0-doi-org.brum.beds.ac.uk/10.3390/bios10100149
Oaxaca-Camacho AR, Ochoa-Mojica OR, Aguilar-Lemarroy A, Jave-Suárez LF, Muñoz-Valle JF, Padilla-Camberos E, Núñez-Hernández JA, Herrera-Rodríguez SE, Martínez-Velázquez M, Carranza-Aranda AS, et al. Serum Analysis of Women with Early-Stage Breast Cancer Using a Mini-Array of Tumor-Associated Antigens. Biosensors. 2020; 10(10):149. https://0-doi-org.brum.beds.ac.uk/10.3390/bios10100149
Chicago/Turabian StyleOaxaca-Camacho, Alma Rosa, Oscar René Ochoa-Mojica, Adriana Aguilar-Lemarroy, Luis F. Jave-Suárez, José Francisco Muñoz-Valle, Eduardo Padilla-Camberos, Juan Antonio Núñez-Hernández, Sara E. Herrera-Rodríguez, Moisés Martínez-Velázquez, Ahtziri Socorro Carranza-Aranda, and et al. 2020. "Serum Analysis of Women with Early-Stage Breast Cancer Using a Mini-Array of Tumor-Associated Antigens" Biosensors 10, no. 10: 149. https://0-doi-org.brum.beds.ac.uk/10.3390/bios10100149