Increase and Change in the Pattern of Antibiotic Use in Serbia (2010–2019)
Abstract
:1. Introduction
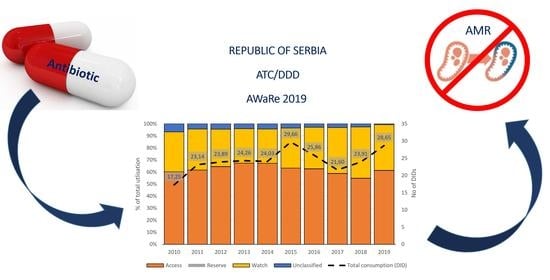
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tacconelli, E.; Pezzani, M.D. Public health burden of antimicrobial resistance in Europe. Lancet Infect. Dis. 2019, 19, 4–6. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization. Turning Plans into Action for Antimicrobial Resistance (AMR): Working Paper 2.0: Implementation and Coordination; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Horvat, O.; Mijatović, V.; Milijasevic, B.; Tomás, A.; Kusturica, M.P.; Tomic, Z.; Sabo, A. Are There Striking Differences in Outpatient Use of Antibiotics Between South Backa District, Serbia, and Some Scandinavian Countries? Front. Public Health 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Popović, R.; Tomić, Z.; Tomas, A.; Anđelić, N.; Vicković, S.; Jovanović, G.; Bukumirić, D.; Horvat, O.; Sabo, A. Five-year surveillance and correlation of antibiotic consumption and resistance of Gram-negative bacteria at an intensive care unit in Serbia. J. Chemother. 2020, 32, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Kusturica, M.P.; Tomić, Z.; Bukumiric, Z.; Ninkovic, L.; Tomás, A.; Stilinović, N.; Sabo, A. Home pharmacies in Serbia: An insight into self-medication practice. Int. J. Clin. Pharm. 2015, 37, 373–378. [Google Scholar] [CrossRef]
- Ministry of health of Republic of Serbia. Working Group for the Development of National Good Clinical Practice Guideline for Rational Antibiotic Use; Ministry of health of Republic of Serbia: Belgrade, Serbia, 2018. [Google Scholar]
- World Health Organization. Republic of Serbia: National Antibiotic Resistance Control Programme for the Period 2019–2021; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Radonjić, V.; Lj, Đ. Trade and Consumption of the Medicinal Products—Annual Reports 2006; Medicines and Medical Devices Agency of Serbia: Belgrade, Serbia, 2007. [Google Scholar]
- World Health Organization. WHO Collaborating Centre for Drug Statistics Methodology. Anatomical Therapeutic Chemical (ATC) Classification Index-Including Defined Daily Doses (DDDs) for Plain Substances; WHO-Oslo: Oslo, Norway, 2008. [Google Scholar]
- Vlahovic-Palcevski, V.; Gantumur, M.; Radošević, N.; Palčevski, G.; Stichele, R.V. Coping with changes in the Defined Daily Dose in a longitudinal drug consumption database. Pharm. World Sci. 2010, 32, 125–129. [Google Scholar] [CrossRef]
- Kusama, Y.; Ishikane, M.; Tanaka, C.; Tsuzuki, S.; Muraki, Y.; Ohmagari, N. What is the impact of the change in DDD of amoxicillin and amoxicillin combined with β-lactamase inhibitors on nationwide surveillance of antimicrobial use? J. Antimicrob. Chemother. 2019, 74, 3119–3121. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Batut, M.J. Annual Report on Communicable Diseases. 2019. Available online: http://www.batut.org.rs/download/izvestaji/GodisnjiIzvestajOZaraznimBolestima2018.pdf (accessed on 27 March 2021).
- Institute for Health Metrics and Evaluation (IHME). Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results. 2020. Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 27 March 2021).
- European Centre for Disease Prevention and Control. Antimicrobial Consumption: Annual Epidemiological Report for 2019; ECDC: Stockholm, Sweden, 2020.
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [Green Version]
- Sulis, G.; Adam, P.; Nafade, V.; Gore, G.; Daniels, B.; Daftary, A.; Das, J.; Gandra, S.; Pai, M. Antibiotic prescription practices in primary care in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003139. [Google Scholar] [CrossRef]
- Sharland, M.; Pulcini, C.; Harbarth, S.; Zeng, M.; Gandra, S.; Mathur, S.; Magrini, N. Classifying antibiotics in the WHO Essential Medicines List for optimal use—Be AWaRe. Lancet Infect. Dis. 2018, 18, 18–20. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Releases the 2019 AWaRe Classification Antibiotics; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization. Adopt AWaRe: Handle Antibiotics with Care. Available online: https://adoptaware.org (accessed on 10 March 2021).
- Martínez-González, N.A.; Di Gangi, S.; Pichierri, G.; Neuner-Jehle, S.; Senn, O.; Plate, A. Time Trends and Factors Associated with Antibiotic Prescribing in Swiss Primary Care (2008 to 2020). Antibiotics 2020, 9, 837. [Google Scholar] [CrossRef]
- Nguyen, N.V.; Do, N.T.T.; Nguyen, C.T.K.; Tran, T.K.; Ho, P.D.; Nguyen, H.H.; Vu, H.T.L.; Wertheim, H.F.L.; Van Doorn, H.R.; Lewycka, S. Community-level consumption of antibiotics according to the AWaRe (Access, Watch, Reserve) classification in rural Vietnam. JAC-Antimicrob. Resist. 2020, 2, 48. [Google Scholar] [CrossRef]
- Machado-Alba, J.; Valladales-Restrepo, L.; Gaviria-Mendoza, A.; Machado-Duque, M.; Figueras, A. Patterns of Antibiotic Prescription in Colombia: Are There Differences between Capital Cities and Municipalities? Antibiotics 2020, 9, 389. [Google Scholar] [CrossRef]
- AlJadeeah, S.; Wirtz, V.J.; Nagel, E. Outpatient Antibiotic Dispensing for the Population with Government Health Insurance in Syria in 2018–2019. Antibiotics 2020, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Zhussupova, G.; Utepova, D.; Orazova, G.; Zhaldybayeva, S.; Skvirskaya, G.; Tossekbayev, K. Evaluation of Antibiotic Use in Kazakhstan for the Period 2017–2019 Based on WHO Access, Watch and Reserve Classification (AWaRe 2019). Antibiotics 2021, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Gandra, S.; Kotwani, A. Need to improve availability of “access” group antibiotics and reduce the use of “watch” group antibiotics in India for optimum use of antibiotics to contain antimicrobial resistance. J. Pharm. Policy Pract. 2019, 12, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.S.; Antonić, R.A.; Bagi, B.I.; Ilić, I.M.; Kočović, A.G.; Milosavljević, M.N.; Nedović, N.M.; Pejčić, A.V.; Vapljanin, M.Z.; Šabanović, A.M. Inappropriate prescribing of antibiotics to the patients with acute bronchitis. Vojnosanit. Pregl. 2019, 76, 684–689. [Google Scholar] [CrossRef] [Green Version]
- Elseviers, M.M.; Ferech, M.; Stichele, R.H.V.; Goossens, H. The ESAC project group Antibiotic use in ambulatory care in Europe (ESAC data 1997–2002): Trends, regional differences and seasonal fluctuations. Pharmacoepidemiol. Drug Saf. 2006, 16, 115–123. [Google Scholar] [CrossRef]
- Goossens, H.; Ferech, M.; Coenen, S.; Stephens, P. European Surveillance of Antimicrobial Consumption Project Group Comparison of Outpatient Systemic Antibacterial Use in 2004 in the United States and 27 European Countries. Clin. Infect. Dis. 2007, 44, 1091–1095. [Google Scholar] [CrossRef]
- Quality Indicators for Antibiotic Consumption in the Community. Available online: https://www.ecdc.europa.eu/en/antimicrobial-consumption/database/quality-indicators (accessed on 10 March 2021).
- Wojkowska-Mach, J.; Godman, B.; Glassman, A.; Kurdi, A.; Pilc, A.; Rozanska, A.; Skoczyński, S.; Wałaszek, M.; Bochenek, T. Antibiotic consumption and antimicrobial resistance in Poland; findings and implications. Antimicrob. Resist. Infect. Control. 2018, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Central Asian and Eastern European Surveillance of Antimicrobial Resistance: Annual Report 2019; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Central Asian and Eastern European Surveillance of Antimicrobial Resistance: Annual Report 2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Jinks, T.; Lee, N.; Sharland, M.; Rex, J.; Gertler, N.; Diver, M.; Jones, I.; Jones, K.; Mathewson, S.; Chiara, F.; et al. A time for action: Antimicrobial resistance needs global response. Bull. World Health Organ. 2016, 94, 558–558A. [Google Scholar] [CrossRef] [PubMed]
- Lum, E.P.; Page, K.; Whitty, J.A.; Doust, J.; Graves, N. Antibiotic prescribing in primary healthcare: Dominant factors and trade-offs in decision-making. Infect. Dis. Health 2018, 23, 74–86. [Google Scholar] [CrossRef] [Green Version]
- Hürlimann, D.; Limacher, A.; Schabel, M.; Zanetti, G.; Berger, C.; Mühlemann, K.; Kronenberg, A.; Group, S.S.W. Improve-ment of antibiotic prescription in outpatient care: A cluster-randomized intervention study using a sentinel surveillance network of physicians. J. Antimicrob. Chemother. 2015, 70, 602–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donà, D.; Barbieri, E.; Daverio, M.; Lundin, R.; Giaquinto, C.; Zaoutis, T.; Sharland, M. Implementation and impact of pedi-atric antimicrobial stewardship programs: A systematic scoping review. Antimicrob. Resist. Infect. Control. 2020, 9, 1–12. [Google Scholar]
- Chan, W.V.; Pearson, T.A.; Bennett, G.C.; Cushman, W.C.; Gaziano, T.A.; Gorman, P.N.; Handler, J.; Krumholz, H.M.; Kush-ner, R.F.; MacKenzie, T.D. ACC/AHA special report: Clinical practice guideline implementation strategies: A summary of systematic reviews by the NHLBI Implementation Science Work Group: A report of the American College of Cardiolo-gy/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e122–e137. [Google Scholar]
- Pinto, D.; Heleno, B.; Rodrigues, D.S.; Papoila, A.L.; Santos, I.; Caetano, P.A. Effectiveness of educational outreach visits compared with usual guideline dissemination to improve family physician prescribing—an 18-month open clus-ter-randomized trial. Implement. Sci. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Coxeter, P.; Del Mar, C.B.; McGregor, L.; Beller, E.M.; Hoffmann, T.C. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database Syst. Rev. 2015, 2015, CD010907. [Google Scholar] [CrossRef] [Green Version]
- Dyar, O.J.; Beović, B.; Vlahović-Palčevski, V.; Verheij, T.; Pulcini, C. How can we improve antibiotic prescribing in primary care? Expert Rev. Anti. Infect. Ther. 2016, 14, 403–413. [Google Scholar] [CrossRef]
- Budd, E.; Cramp, E.; Sharland, M.; Hand, K.; Howard, P.; Wilson, P.; Wilcox, M.; Muller-Pebody, B.; Hopkins, S. Adaptation of the WHO Essential Medicines List for national antibiotic stewardship policy in England: Being AWaRe. J. Antimicrob. Chemother. 2019, 74, 3384–3389. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.C.; Turnidge, J.; Collignon, P.; Looke, D.; Barton, M.; Gottlieb, T. Control of Fluoroquinolone Resistance through Successful Regulation, Australia. Emerg. Infect. Dis. 2012, 18, 1453–1460. [Google Scholar] [CrossRef]
- Balinskaite, V.; Johnson, A.P.; Holmes, A.; Aylin, P. The Impact of a National Antimicrobial Stewardship Program on Antibiotic Prescribing in Primary Care: An Interrupted Time Series Analysis. Clin. Infect. Dis. 2018, 69, 227–232. [Google Scholar] [CrossRef]
- Mölstad, S.; Löfmark, S.; Carlin, K.; Erntell, M.; Aspevall, O.; Blad, L.; Hanberger, H.; Hedin, K.; Hellman, J.; Norman, C.; et al. Lessons learnt during 20 years of the Swedish strategic programme against antibiotic resistance. Bull. World Health Organ. 2017, 95, 764–773. [Google Scholar] [CrossRef]
- Avent, M.L.; Cosgrove, S.E.; Price-Haywood, E.G.; Van Driel, M.L. Antimicrobial stewardship in the primary care setting: From dream to reality? BMC Fam. Pract. 2020, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- WHO Collaborating Centre for Drug Statistics Methodology. Available online: https://www.whocc.no/atc_ddd_alterations__cumulative/ddd_alterations/ (accessed on 14 March 2021).
- Bergman, U.; Popa, C.; Tomson, Y.; Wettermark, B.; Einarson, T.; Åberg, H.; Sjöqvist, F. Drug utilization 90%—A simple method for assessing the quality of drug prescribing. Eur. J. Clin. Pharmacol. 1998, 54, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Coenen, S.; Ferech, M.; Haaijer-Ruskamp, F.M.; Butler, C.C.; Stichele, R.H.V.; Verheij, T.J.M.; Monnet, D.L.; Little, P.; Goossens, P.L.H. The ESAC project group European Surveillance of Antimicrobial Consumption (ESAC): Quality indicators for outpatient antibiotic use in Europe. Qual. Saf. Health Care 2007, 16, 440–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Antibiotic Class | Antibiotic Utilization (DID) per Year | Regression | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | β | p | |
| J01C | 5.68 | 7.70 | 7.97 | 7.58 | 9.41 | 11.47 | 8.63 | 6.70 | 8.29 | 11.26 | 0.532 | 0.113 |
| J01D | 3.43 | 3.31 | 4.26 | 3.61 | 3.84 | 4.60 | 4.81 | 3.66 | 2.95 | 4.38 | 0.651 | 0.534 |
| J01F | 3.23 | 5.12 | 4.01 | 4.06 | 3.93 | 5.82 | 5.04 | 3.67 | 5.16 | 5.54 | 0.505 | 0.137 |
| J01M | 2.10 | 2.66 | 3.68 | 3.04 | 3.29 | 3.82 | 3.43 | 3.70 | 3.86 | 3.23 | 0.667 | 0.035 |
| Quality Indicator | Year | Regression | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | β | p | |
| J01CE_% * | 1.08% | 0.00% | 0.72% | 0.47% | 0.94% | 0.41% | 0.39% | 0.39% | 0.33% | 0.26% | −0.57 | 0.086 |
| J01CR_% * | 6.57% | 11.40% | 7.41% | 7.63% | 7.83% | 10.57% | 10.15% | 9.56% | 18.87% | 21.41% | 0.772 | 0.009 |
| J01DD + J01DE_% * | 4.01% | 2.55% | 2.06% | 2.09% | 1.03% | 2.83% | 3.71% | 5.12% | 6.05% | 5.53% | 0.664 | 0.036 |
| J01MA_% * | 6.64% | 7.49% | 11.35% | 8.94% | 10.17% | 10.11% | 11.05% | 14.79% | 14.30% | 11.16% | 0.786 | 0.007 |
| J01_B/N | 1.42 | 2.34 | 1.37 | 1.92 | 1.15 | 2.07 | 1.95 | 2.39 | 6.00 | 4.16 | 0.692 | 0.027 |
| ATC/DDD | 2010 | 2019 | % Change | β | Significance. |
|---|---|---|---|---|---|
| J01AA07-tetracycline | 0.12 | 0.02 | −82.42% | −0.85 | 0.002 |
| J01AA12-tigecycline | 0.001 | 0.01 | 510.00% | 0.722 | 0.018 |
| J01CR02-amoxicillin, clavulanic acid | 1.09 | 5.93 | 442.00% | 0.812 | 0.014 |
| J01DB04-cefazolin | 0.02 | 0.09 | 331.50% | 0.643 | 0.045 |
| J01DB05-cefadroxil | 0.04 | 0.10 | 161.63% | 0.728 | 0.064 |
| J01DC04-cefaclor | 0.13 | 0.02 | −83.66% | −0.776 | 0.008 |
| J01DD02-ceftazidime | 0.02 | 0.04 | 92.65% | 0.639 | 0.047 |
| J01DD08-cefixime | 0.65 | 1.40 | 114.55% | 0.772 | 0.009 |
| J01DD13-cefpodoxime | 0.04 * | 0.14 | 3296.25% | 0.847 | 0.008 |
| J01FA06-roxithromycin | 0.5 | 0.12 | −76.71% | −0.662 | 0.037 |
| J01FA09-clarithromycin | 0.94 | 1.64 | 74.79% | 0.814 | 0.004 |
| J01FF01-clindamycin | 0.04 | 0.608 | 1421.00% | 0.888 | 0.001 |
| J01GB03-gentamicin | 0.5 | 0.39 | −21.58% | −0.695 | 0.026 |
| J01MA02-ciprofloxacin | 0.62 | 1.62 | 160.56% | 0.689 | 0.028 |
| J01MA12-levofloxacin | 0.09 * | 1.33 | 1375.56% * | 0.919 | p < 0.001 |
| J01MB04-pipemidic acid | 0.99 | 0.13 | −86.26% | −0.884 | 0.001 |
| J01XA01-vancomycin | 0.03 | 0.051 | 70.00% | 0.717 | 0.020 |
| J01XX01-fosfomycin | 0.02 | 0.095 | 373.20% | 0.931 | p < 0.001 |
| J01XX08-linezolid | 0.0004 * | 0.006 | 1318.65% | 0.98 | p < 0.001 |
| No. | 2010 | 2015 | 2019 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ATC INN | DID | % | ATC INN | DID | % | ATC INN | DID | % | |
| 1 | J01CA04-amoxicillin (A) | 3.09 | 17.93 | J01CA04-amoxicillin (A) | 7.68 | 25.89 | J01CR02-amoxicillin; clavulanic acid (A) | 5.93 | 20.70 |
| 2 | J01DB01-cefalexin (A) | 2.40 | 13.91 | J01DB01-cefalexin (A) | 3.60 | 12.14 | J01CA04-amoxicillin (A) | 4.96 | 17.30 |
| 3 | J01AA02-doxycycline (A) | 1.38 | 8.00 | J01FA10-azithromycin (Wa) | 3.19 | 10.76 | J01FA10-azithromycin (Wa) | 2.83 | 9.87 |
| 4 | J01CA01-ampicillin (A) | 1.31 | 7.59 | J01CR02-amoxicillin; clavulanic acid (A) | 3.06 | 10.31 | J01DB01-cefalexin (A) | 2.29 | 7.99 |
| 5 | J01FA10-azithromycin (Wa) | 1.27 | 7.36 | J01AA02-doxycycline (A) | 2.20 | 7.42 | J01FA09-clarithromycin (Wa) | 1.64 | 5.74 |
| 6 | J01CR02- amoxicillin, clavulanic acid (A) | 1.09 | 6.34 | J01MA02-ciprofloxacin (Wa) | 1.90 | 6.42 | J01MA02-ciprofloxacin (Wa) | 1.62 | 5.64 |
| 7 | J01MB04-pipemidic acid (NC) | 0.99 | 5.74 | J01FA09-clarithromycin (Wa) | 1.46 | 4.92 | J01AA02-doxycycline (A) | 1.47 | 5.12 |
| 8 | J01FA09-clarithromycin (Wa) | 0.94 | 5.45 | J01MB04-pipemidic acid (NC) | 0.90 | 3.03 | J01DD08-cefixime (Wa) | 1.39 | 4.87 |
| 9 | J01DD08-cefixime (Wa) | 0.65 | 3.77 | J01DD08-cefixime (Wa) | 0.77 | 2.60 | J01MA12-levofloxacin (Wa) | 1.33 | 4.64 |
| 10 | J01MA02-ciprofloxacin (Wa) | 0.62 | 3.59 | J01MA12-levofloxacin (Wa) | 0.74 | 2.49 | J01EE01-sulfamethoxazole; trimethoprim (A) | 0.89 | 3.10 |
| 11 | J01GB03-gentamicin (A) | 0.50 | 2.90 | J01CA01-ampicillin (A) | 0.61 | 2.06 | J01FF01-clindamycin (A) | 0.61 | 2.12 |
| 12 | J01FA06-roxithromycin (Wa) | 0.50 | 2.90 | J01GB03-gentamicin (A) | 0.61 | 2.06 | J01DD04-ceftriaxone (Wa) | 0.47 | 1.65 |
| 13 | J01DD04-ceftriaxone (Wa) | 0.40 | 2.32 | J01DC02-cefuroxime (Wa) | 0.41 | 1.45 | |||
| 14 | J01FA01-erythromycin (Wa) | 0.35 | 2.03 | ||||||
| 15 | J01MA06-norfloxacin (Wa) | 0.25 | 1.45 | ||||||
| DU90% | 1–15 | 15.75 | 91.29 | 1–12 | 26.72 | 90.09 | 1–13 | 25.83 | 90.18 |
| 16–43 | 1.50 | 8.71 | 13–48 | 2.94 | 9.91 | 14–49 | 2.81 | 9.82 | |
| Total | 43 | 17.25 | 100.00 | 48 | 29.66 | 100.00 | 49 | 28.65 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomas, A.; Pavlović, N.; Stilinović, N.; Horvat, O.; Paut-Kusturica, M.; Dugandžija, T.; Tomić, Z.; Sabo, A. Increase and Change in the Pattern of Antibiotic Use in Serbia (2010–2019). Antibiotics 2021, 10, 397. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10040397
Tomas A, Pavlović N, Stilinović N, Horvat O, Paut-Kusturica M, Dugandžija T, Tomić Z, Sabo A. Increase and Change in the Pattern of Antibiotic Use in Serbia (2010–2019). Antibiotics. 2021; 10(4):397. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10040397
Chicago/Turabian StyleTomas, Ana, Nebojša Pavlović, Nebojša Stilinović, Olga Horvat, Milica Paut-Kusturica, Tihomir Dugandžija, Zdenko Tomić, and Ana Sabo. 2021. "Increase and Change in the Pattern of Antibiotic Use in Serbia (2010–2019)" Antibiotics 10, no. 4: 397. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10040397