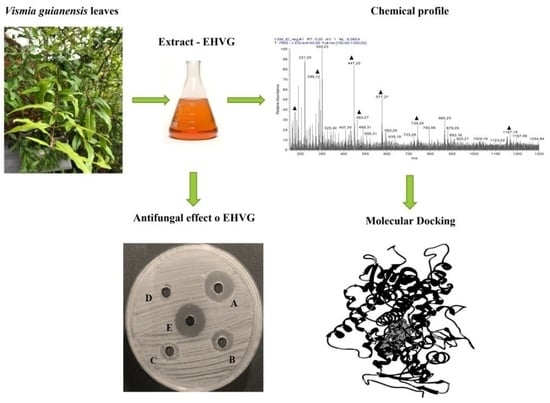
The Anti-Virulence Effect of Vismia guianensis against Candida albicans and Candida glabrata
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Plant Material and Extract
2.2. Chemical Profile of EHVG
2.3. EHVG Showed Low Toxicity Using Different Assays
2.4. Antifungal Activity of EHVG against C. albicans and C. glabrata
2.4.1. Treatment with EHVG Inhibits the Growth of C. albicans and C. glabrata
2.4.2. EHVG Inhibits C. albicans and C. glabrata Adhesion
2.4.3. EHVG Disrupts Early and Mature Biofilm Formation
2.5. In Silico Biological Activity and Toxicity for the Compounds Identified in EHVG
2.6. Compounds Present in EHVG, Especially Vismione D, Interact with CaCYP51
3. Discussion
4. Materials and Methods
4.1. Collection and Identification of Vismia guianensis
4.2. Preparation of the Extract
4.3. Characterization of the Plant Material
4.3.1. Particle Size Analysis of the Leaves
4.3.2. Ash Content and Extract Yield
4.4. Chemical Characterization
4.4.1. Analysis of EHVG by HPLC-PDA
4.4.2. Analysis of EHVG by FIA-ESI-IT-MSn
4.5. In Vitro Tests for Anti-Candida Activity
4.5.1. Isolation of Microorganisms
4.5.2. Inoculum Preparation
4.5.3. Determination of Minimum Inhibitory Concentration (MIC)
4.5.4. Determination of Minimum Fungicidal Concentration (MFC)
4.5.5. Time-Kill Assay
4.5.6. Adhesion Assay
4.5.7. Effect of EHVG on Biofilm Formation
4.6. Determination of EHVG Cytotoxicity by the MTT Assay
4.7. The Hemolytic Assay
4.8. Evaluation of Cell Viability by the Neutral Red Assay
4.9. In Silico Analysis
4.9.1. Ligands and Target Preparations
4.9.2. Molecular Docking
4.9.3. Pharmacokinetics and Toxicity Measurement
4.9.4. Pass Prediction
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Santos, G.C.O.; Vasconcelos, C.C.; Lopes, A.J.O.; Cartagenes, M.S.S.; Dualibe Filho, A.K.; Nascimento, F.R.F.; Ramos, R.M.; Pires, E.R.R.B.; Andrade, M.S.; Rocha, F.M.G.; et al. Candida infections and therapeutic strategies: Mechanisms of action for traditional and alternative agents. Front Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef] [PubMed]
- Seagle, E.E.; Willinam, S.L.; Chiller, T.M. Recent Trends in the Epidemiology of Fungal Infections. Infect. Dis. Clin. N. Am. 2021, 35, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Santana, D.P.; Ribeiro, E.L.; Menezes, A.C.S.; Naves, P.L.F. Novas abordagens sobre os fatores de virulência de Candida albicans. J. Medl. Biol. Sci. 2013, 12, 229–233. [Google Scholar]
- Lockhart, S. Current epidemiology of Candida infection. Clin. Microbiol. News 2014, 36, 131–136. [Google Scholar] [CrossRef]
- Lohse, M.B.; Gulati, M.; Johnson, A.D.; Nobile, C.J. Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018, 16, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, A.M.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Roscetto, E.; Contursi, P.; Vollaro, A.; Fusco, S.; Notomista, E.; Catania, M.R. Antifungical and anti-biofilm activity of the first cryptic antimicrobial peptide from an archaeal protein against Candida spp. clinical isolates. Sci. Rep. 2018, 8, 17570. [Google Scholar] [CrossRef] [Green Version]
- Colombo, A.L.; Garnica, M.; Camargo, L.F.A.; Cunha, C.A.; Bandeira, A.C.; Borghi, D.; Campos, T.; Senna, A.L.; Didier, M.E.V.; Dias, V.C.; et al. Candida glabrata: An emerging pathogen in Brazilian tertiary care hospitals. Med. Mycol. 2013, 51, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431. [Google Scholar] [CrossRef]
- Brandão, H.N.; David, J.P.; Couto, R.D.; Nascimento, J.A.P.; David, J.M. Química e farmacologia de quimioterápicos antineoplásicos derivados de plantas. Quim. Nova 2010, 33, 1359–1369. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.R.; Andrade Neto, J.B.; Silva, C.R.; Campos, R.D.S.; Costa Silva, R.A.; Freitas, D.D.; Nascimento, F.B.; Andrade, L.N.D.; Sampaio, L.S.; Grangeiro, T.B.; et al. Berberine antifungal activity in Fluconazole-resistant pathogenic yeasts: Action mechanism evaluated by flow cytometry and biofilm growth inhibition in Candida spp. Antimicrob. Agents Chemother. 2016, 60, 3551–3557. [Google Scholar] [CrossRef] [PubMed]
- Camelo, S.R.P.; Costa, R.S.; Ribeiro-Costa, R.M.; Barbosa, W.L.R.; Vasconcelos, F.; Vieira, J.M.S.; Silva Junior, J.O.C. Phytochemical evaluation and antimicrobial activity of ethanolic extract of Vismia guianensis (Aubl.) Choisy. Int. J. Pharm. Sci. Res. 2011, 2, 3224–3229. [Google Scholar]
- Gonzales, J.G.; delle Monache, F.; delle Monache, G.; Marini-Bettolo, G.B. Chemistry of the genus Vismia. Part VII.Vismione A from the leaves of Vismia guianensis. Planta Medica 1980, 40, 347–350. [Google Scholar]
- Guerra, R.N.M. Immunosuppressive Activity of Vismia reichardtiana Fruits. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 1997. [Google Scholar]
- Politi, M.; Sanogo, R.; Ndjoko, K.; Guilet, D.; Wolfender, J.L.; Hostettmann, K.; Morelli, I. HPLC-UV/PAD; HPLC-MS Analyses of leaf and root extracts of Vismia guianensis and isolation and identification of two new bianthrones. Phytochem. Anal. 2004, 15, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Tala, M.F.; Jeanne, E.; Lantovololona, R.; Talontsi, F.M.; Wabo, H.K.; Tane, P.; Laatsch, H. Anthraquinones and triterpenoids from seeds of Vismia guianensis. Biochem. Syst. Ecol. 2013, 50, 310–312. [Google Scholar] [CrossRef]
- Álvarez, E.R.; Gil, J.H.G.; Jímenez, O.J.G.; Posada, C.M.A.; Rojano, B.A.; García, C.M.P.; Durango, D.L.R. Actividad antioxidante y contenido fenólico de los extractos provenientes de las bayas de especies del género Vismia (Guttiferae). Vitae 2008, 15, 165–172. [Google Scholar]
- Lins, A.; Agra, M.; Conceição, D.; Pinto, F.; Camara, C.; Silva, T. Chemical Constituents and Antioxidant Activity from Aerial Parts of Clusia paralicola and Vismia guianensis. Rev. Virtual Quím. 2016, 8, 157–168. [Google Scholar] [CrossRef]
- Zaiter, A.; Becker, L.; Karam, M.C.; Dicko, A. Effect of particle size on antioxidant activity and catechin content of green tea powders. J. Food Sci. Technol. 2016, 53, 2025–2032. [Google Scholar] [CrossRef] [Green Version]
- Seo, E.K.; Mukherjee, R.; Wani, M.C.; Wall, M.E.; Navarro, H.; Farnsworth, N.R.; Kinghorn, A.D. New bioactive aromatic compounds from Vismia guianensis. Phytochemistry 2000, 55, 35–42. [Google Scholar] [CrossRef]
- Hussain, H.; Hussain, J.; Al-Harrasi, A.; Saleem, M.; Green, I.R.; van Ree, T.; Ghulam, A. Chemistry and biology of genus Vismia. Pharm. Biol. 2012, 11, 1448–1462. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.D.C.; Silva, A.G.D.; Silva, A.P.S.D.; Lima, V.L.M.; Bezerra Silva, P.C.; Rocha, S.K.L.D.; Navarro, D.M.D.; Correia, M.T.D.S.; Napoleão, T.H.; Silva, M.V.D.; et al. Essential oils from leaves of medicinal plants of Brazilian flora: Chemical composition and activity against Candida Species. Medicines 2017, 4, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachikonye, M.; Mukanganyama, S. Antifungal and drug efflux inhibitory activity of selected flavonoids against Candida albicans and Candida krusei. J. Biol. Act. Prod. Nat. 2016, 6, 223–236. [Google Scholar]
- Oliveira, A.H.; Oliveira, G.G.; Carnevale Neto, D.F.; Portuondo, A.; Batista-Duharte, A.; Carlos, I.Z. Anti-inflammatory activity of Vismia guianensis (Aubl.) Pers. extracts and antifungal activity against Sporothrix schenckii. J. Ethnopharmacol. 2017, 195, 266–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, A.J.; Mata-Santos, T.; Carrion, L.L.; Rodrigues, K.; Fenalti, J.M.; Mesquita, D.W.O.; Scaini, C.J.; Martins, D.; Mesquita, A.S.S.; Silva, P.E.A.; et al. Evaluation of antifungal, antimycobacterial and larvicide activity of the Duroia macrophylla and D. saccifera. J. Epidemiol. Infect. Control 2016, 6, 108–124. [Google Scholar] [CrossRef] [Green Version]
- Seleem, D.; Benso, B.; Noguti, J.; Pardi, V.; Murata, R.M. In vitro and in vivo antifungical activity of Lichochalcone-A against Candida albicans biofilms. PLoS ONE 2016, 11, e0157188. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, T.Y.; Friggeri, L.; Wawrzak, Z.; QI, A.; Hoekstra, W.J.; Schotzinger, R.J.; York, J.D.; Guengerich, F.; Lepesheva, G.I. Structural analyses of Candida albicans sterol 14α-demethylase complexed with azole drugs address the molecular basis of azole-mediated inhibition of fungal sterol biosynthesis. J. Biol. Chem. 2017, 292, 6728–6743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, A.G.; Scotti, L.; Scotti, M.T.; Mendonça, F.J.B.; Melo, N.S.R.; Alves, R.S.; De Gomez-García, O.; Andrade-Pavón, D.; Campos-Aldrete, E.; Ballinas-Indilí, R.; et al. Synthesis, molecular docking, and antimycotic evaluation of some 3-acyl imidazo[1,2-a] pyrimidines. Molecules 2018, 23, 599. [Google Scholar]
- Oliveira-Santos, G.C. Efeito de Terminalia catappa L. em leveduras de Candida: Avaliação In Sílico, In Vitro e In Vivo. Ph.D. Thesis, Rede Nordeste de Biotecnologia (Renorbio) Universidade Federal do Maranhão, Sao Luís, Brazil, 2018. [Google Scholar]
- Ahmad, A.; Khan, A.; Manzoor, N.; Khan, L.A. Evolution of ergosterol biosynthesis inhibitors as fungicidal against Candida. Microb. Pathoge 2010, 48, 35–41. [Google Scholar] [CrossRef]
- Cheng, F.X.; Li, W.H.; Liu, G.X.; Tang, Y. In silico ADMET prediction: Recent advances, current challenges and future trends. Curr. Top. Med. Chem. 2013, 13, 1273–1289. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, H.L.; Zhou, Z.W.; Long, H.Z.; Luo, H.Y.; Wen, D.D.; Cheng, L.; Gao, L.C. Isoliensinine: A Natural Compound with “Drug-Like” Potential. Front. Pharmacol. 2021, 12, 630385. [Google Scholar] [CrossRef]
- Poroikov, V.; Filimonov, D. PASS: Prediction of biological activity spectra for substances predictive toxicology. In Predictive Toxicology, 1st ed.; Helma, C., Ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- ANVISA—Farmacopeia Brasileira, Vol 2—Agência Nacional de Vigilância Sanitária. Brasília: 1. Substâncias Farmacêuticas Químicas, Vegetais e Biológicas. 2. Medicamentos e Correlatos. 3. Especificações e Métodos de Análise. 2010; 546p, 1v/il. Available online: https://www.gov.br/anvisa/pt-br/assuntos/farmacopeia/farmacopeia-brasileira/6a-edicao-volume-2 (accessed on 8 August 2022).
- Medeiros, A.C.; Almeida, E.; Quintino-da-Rocha, C.; Tangerina, M.; Lima-Neto, J.S.; Silva, A.; Rocha, C.; Martins, L. Antiparasitic activities of hydroethanolic extracts of Ipomoea imperati (Vahl) Griseb. (Convolvulaceae). PLoS ONE 2019, 14, e0211372. [Google Scholar]
- Silva, A.F.; Rocha, C.Q.; Silva, L.C.N.; Carvalho-Júnior, A.R.; Mendes, I.N.F.V.; Araruna, A.B.; Motta, E.P.; Silva, R.S.; Campos, C.D.L.; Farias, J.R.; et al. Antifungal and anti-virulence activities of hydroalcoholic extract and fractions of Platonia insignis leaves against vaginal isolates of Candida species. Pathogens 2020, 9, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CLSI—Clinical and Laboratory Standards Institute—Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved M27-A3; CLSI: Wayne, PA, USA, 2012.
- Ostrosky, E.A.; Mizumoto, M.K.; Lima, M.E.L.; Kaneko, T.M.; Nishikawa, S.O.; Freitas, B.R. Methods for evaluation of the antimicrobial activity and determination of Minimum Inhibitory Concentration (MIC) of plant extracts. Braz. J. Pharmacogn. 2008, 18, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Sidiqui, Z.N.; Farooq, F.; Musthafa, T.N.M.; Ahmad, A.; Khan, A.U. Synthesis, characterization and antimicrobial evaluation of novel halopyrazole derivatives. J. Saudi. Chem. Soc. 2013, 17, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Zago, C.E.; Silva, S.; Sanitá, P.V.; Barbugli, P.A.; Dias, C.M.I.; Lordello, V.B.; Vergani, C.E. Dynamics of biofilm formation and the interaction between Candida albicans and methicillin-susceptible (MSSA) and -resistant Staphylococcus aureus (MRSA). PLoS ONE 2015, 13, e0123206. [Google Scholar] [CrossRef] [Green Version]
- Gulati, M.; Lohse, M.B.; Ennis, C.L.; Gonzalez, R.E.; Perry, A.M.; Bapat, P.; Valle Arevalo, A.; Rodriguez, D.L.; Nobile, C.J. In vitro culturing and screening of Candida albicans biofilms. Curr. Protoc. Microbiol. 2018, 50, e60. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Rajan, S.; Wong, S.W.; Tsang, D.N.C.; Lai, C.K.C.; Samaranayake, L.P.; Jin, L. Antinfungal susceptibility in serum and virulence determinants of Candida isolates from Hong Kong. Front. Microbiol. 2016, 7, 216. [Google Scholar] [CrossRef] [Green Version]
- Seneviratne, C.J.; Silva, W.J.; Jin, L.J.; Samaranayake, Y.H.; Samaranayake, L.P. Architectural analysis, viability assessment and growth kinetics of Candida albicans and Candida glabrata biofilms. Arch. Oral Biol. 2009, 54, 1052–1060. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lomabardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Sliwoski, G.; Kothiwale, S.; Meiler, J.; Lowe, E.W. Computational methods in drug discovery. Pharmocol. Rev. 2014, 66, 334–395. [Google Scholar] [CrossRef] [Green Version]
- Mojumdar, M.; Paul, A.; Kabir, M.S.H.; Rahman, G.; Zohora, F.T.; Hasan, M.S.; Ahmed, T.; Rahman, M.R.; Akter, Y.; Rahman, M. Molecular docking and PASS prediction for analgesic activity of some isolated compounds from acalypha indica l and ADME/T property analysis of the compounds. World J. Pharm. 2016, 5, 1761–1770. [Google Scholar]
- Goel, R.K.; Singh, D.; Lagunin, A.; Poroikov, V. PASS-assisted exploration of new therapeutic potential of natural products. Med. Chem. Res. 2011, 20, 1509–1514. [Google Scholar] [CrossRef]
- Khurana, N.; Ishar, M.P.S.; Gajbhiye, A.; Goel, R.K. PASS assisted prediction and pharmacological evaluation of novel nicotinic analogs for nootropic activity in mice. Eur. J. Pharmacol. 2011, 662, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Rudik, A.V.; Dmitriev, A.V.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.V. PASS-based prediction of metabolites detection in biological systems. SAR QSAR Environ. Res. 2019, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]












| Hydromodule | Dry Residue (X ± SD) a | Total Residue | Extract Yield (%) |
|---|---|---|---|
| 1:5 | 21 ± 0.0018 | 210 | 11 |
| 1:10 | 13 ± 0.0004 | 258 | 13 |
| 1:15 | 8 ± 0.0006 | 119 | 12 |
| 1:20 | 7 ± 0.0017 | 15 | 13 |
| Number | [M−H]− | MSn Ions | Proposed Compound |
|---|---|---|---|
| 1 | 191 | 173, 111, 85 | Quinic acid |
| 2 | 431 | 269 | Isovitexin |
| 3 | 447 | 429, 357 | Isoorientin |
| 4 | 289 | Catechin | |
| 5 | 447 | 429, 301, 269, 229 | Orientin |
| 6 | 431 | 285, 163 | Kaempferol-O-rhamnoside |
| 7 | 731 | 285, 255 | Kaempferol galactoside-rhamnoside |
| 8 | 1153 | 1001, 983, 789 | Catechin tetramer |
| 9 | 463 | 301, 283, 273, 229, 179, 121 | Quercetin glycoside |
| 10 | 577 | 425, 407, 285, 257, 213 | Catechin dimer |
| 11 | 1167 | 1015, 863, 711 | A-type procyanidin trimer |
| 12 | 409 | 273, 255 | Vismione D |
| 13 | 283 | 269, 239 | Anthraquinone F |
| 14 | 285 | Kaempferol |
| Candida Strain | Vismia guianensis (EHVG) | Antifungal | |||
|---|---|---|---|---|---|
| MIC a | MFC a | MFC/MIC Ratio | Ampho B b | Flu c | |
| C. glabrata (ATCC 2001) d | 3.125 | 6.25 | 2 | 0.25 | 16 |
| C. albicans (ATCC 90028) | 3.125 | 3.125 | 1 | 1 | 8 |
| C. albicans (ATCC 14053) | 6.25 | 6.25 | 1 | 0.5 | 8 |
| C. albicans (SC 5314) | 6.25 | 6.25 | 1 | 0.5 | 16 |
| A1 e C. albicans | 6.25 | 12.5 | 2 | 1 | 8 |
| A2 C. albicans | 3.125 | 3.125 | 1 | 0.5 | 8 |
| A3 C. albicans | 6.25 | 6.25 | 1 | 0.25 | 4 |
| A4 C. albicans | 3.125 | 6.25 | 2 | 0.5 | 16 |
| A5 C. albicans | 3.125 | 3.125 | 1 | 0.5 | 16 |
| A6 C. albicans | 3.125 | 3.125 | 1 | 0.5 | 16 |
| A7 C. albicans | 6.25 | 6.25 | 1 | 1 | 16 |
| Activity/ Compounds | Antifungal | Anti-Inflammatory | Antioxidant | |||
|---|---|---|---|---|---|---|
| Pa a | Pi b | Pa | Pi | Pa | Pi | |
| Vismione D | 0.684 | 0.011 | 0.606 | 0.030 | 0.478 | 0.008 |
| Catechin | 0.552 | 0.023 | 0.548 | 0.044 | 0.810 | 0.003 |
| Kaempferol | 0.495 | 0.031 | 0.676 | 0.019 | 0.856 | 0.003 |
| Quercetin | 0.490 | 0.032 | 0.689 | 0.017 | 0.872 | 0.003 |
| Anthraquinone | 0.351 | 0.063 | 0.410 | 0.090 | - | - |
| Fluconazole | 0.726 | 0.008 | - | - | - | - |
| Amphotericin | 0.977 | 0.000 | 0.330 | 0.136 | - | - |
| Ligand | ΔGbind (kcal/mol) * | Ki (μM) ** |
|---|---|---|
| Vismione D | −10.96 | 0.009 |
| Anthraquinone F | −7.92 | 1.56 |
| Catechin | −6.97 | 7.79 |
| Kaempferol | −6.70 | 12.35 |
| Quercetin | −5.60 | 20.98 |
| Posaconazole | −8.43 | 0.57 |
| Fluconazole | −6.89 | 11.61 |
| Identification | Type of Strain |
|---|---|
| ATCC 2001 a—C. glabrata | Reference |
| ATCC 90,028—C. albicans | Reference |
| ATCC 14,053—C. albicans | Reference |
| ATCC MYA 2876 (SC 5314)—C. albicans | Reference–Wild type |
| A1 C. albicans | Clinical isolate–vagina |
| A2 C. albicans | Clinical isolate–vagina |
| A3 C. albicans | Clinical isolate–vagina |
| A4 C. albicans | Clinical isolate–vagina |
| A5 C. albicans | Clinical isolate–vagina |
| A6 C. albicans | Clinical isolate–vagina |
| A7 C. albicans | Clinical isolate–oral |
| A8 C. albicans | Clinical isolate–oral |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motta, E.P.; Farias, J.R.; Costa, A.A.C.d.; Silva, A.F.d.; Oliveira Lopes, A.J.; Cartágenes, M.d.S.S.; Nicolete, R.; Abreu, A.G.; Fernandes, E.S.; Nascimento, F.R.F.; et al. The Anti-Virulence Effect of Vismia guianensis against Candida albicans and Candida glabrata. Antibiotics 2022, 11, 1834. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics11121834
Motta EP, Farias JR, Costa AACd, Silva AFd, Oliveira Lopes AJ, Cartágenes MdSS, Nicolete R, Abreu AG, Fernandes ES, Nascimento FRF, et al. The Anti-Virulence Effect of Vismia guianensis against Candida albicans and Candida glabrata. Antibiotics. 2022; 11(12):1834. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics11121834
Chicago/Turabian StyleMotta, Elizangela Pestana, Josivan Regis Farias, Arthur André Castro da Costa, Anderson França da Silva, Alberto Jorge Oliveira Lopes, Maria do Socorro Sousa Cartágenes, Roberto Nicolete, Afonso Gomes Abreu, Elizabeth Soares Fernandes, Flavia Raquel Fernandes Nascimento, and et al. 2022. "The Anti-Virulence Effect of Vismia guianensis against Candida albicans and Candida glabrata" Antibiotics 11, no. 12: 1834. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics11121834