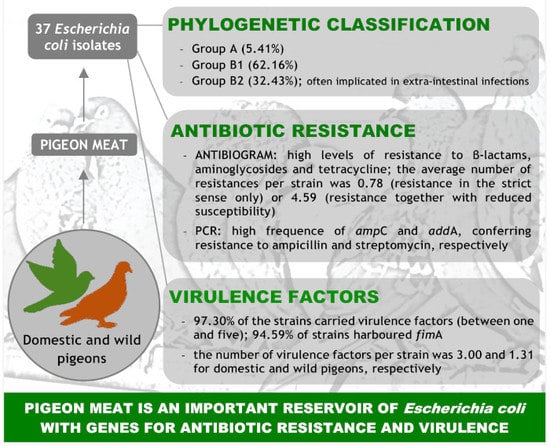
Phylogenetic Diversity, Antimicrobial Susceptibility and Virulence Characteristics of Escherichia coli Isolates from Pigeon Meat
Abstract
:1. Introduction
2. Results
2.1. Antibiotic Susceptibility
2.2. Genotypic Characterization
3. Discussion
3.1. Antibiotic Susceptibility
3.2. Genotypic Characterization
4. Materials and Methods
4.1. Strains
4.2. Antibiotic Susceptibility Testing
4.3. Phenotypic Determination of Extended Spectrum β-Lactamases (ESBLs)
4.4. Genotypic Characterization
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Brzuszkiewicz, E.; Thurmer, A.; Schuldes, J.; Leimbach, A.; Liesegang, H.; Meyer, F.D.; Boelter, J.; Petersen, H.; Gottschalk, G.; Daniel, R. Genome sequence analyses of two isolates from the recent Escherichia coli outbreak in Germany reveal the emergence of a new pathotype: Entero-Aggregative-Haemorrhagic Escherichia coli (EAHEC). Arch. Microbiol. 2011, 193, 883–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA and ECDC. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar]
- Hamadi, F.; Latrache, H.; Zahir, H.; Elghmari, A.; Timinouni, M.; Ellouali, M. The relation between Escherichia coli surface functional groups’ composition and their physicochemical properties. Braz. J. Microbiol. 2008, 39, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD. Antimicrobial Resistance. Tackling the Burden in the European Union. 2019. Available online: https://www.oecd.org/health/health-systems/AMR-Tackling-the-Burden-in-the-EU-OECD-ECDC-Briefing-Note-2019.pdf (accessed on 15 September 2019).
- Capita, R.; Alonso-Calleja, C. Antibiotic-resistant bacteria: A challenge for the food industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, Y.; Rao, D.; Zhang, Y.; Yang, K. Evidence for environmental dissemination of antibiotic resistance mediated by wild birds. Front. Microbiol. 2018, 9, 745. [Google Scholar] [CrossRef]
- Paulsen, P. Hygiene and microbiology of meat from wild game: An Australian view. In Game Meat Hygiene in Focus: Microbiology, Epidemiology, Risk Analysis and Quality Assurance; Paulsen, P., Bauer, A., Vodnansly, M., Winkelmayer, R., Smulders, F.J.M., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011; pp. 19–37. [Google Scholar]
- Radimersky, T.; Frolkova, P.; Janoszowska, D.; Dolejska, M.; Svec, P.; Roubalova, E.; Cikova, P.; Cizek, A.; Literak, I. Antibiotic resistance in faecal bacteria (Escherichia coli, Enterococcus spp.) in feral pigeons. J. Appl. Microbiol. 2010, 109, 1687–1695. [Google Scholar] [CrossRef]
- Allen, S.E.; Boerlin, P.; Janecko, N.; Lumsden, J.S.; Barker, I.K.; Pearl, D.L.; Reid-Smith, R.J.; Jardine, C. Antimicrobial resistance in generic Escherichia coli isolates from wild small mammals living in swine farm, residential, landfill, and natural environments in southern Ontario, Canada. Appl. Environ. Microbiol. 2011, 77, 882–888. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, A.; Igrejas, G.; Radhouani, H.; Correia, S.; Pacheco, R.; Santos, T.; Monteiro, R.; Guerra, A.; Petrucci-Fonseca, F.; Brito, F.; et al. Antimicrobial resistance in faecal enterococci and Escherichia coli isolates recovered from Iberian wolf. Lett. Appl. Microbiol. 2013, 56, 268–274. [Google Scholar] [CrossRef]
- Zigo, F.; Takac, L.; Zigova, M.; Takacova, J.; Vasi, M. Occurrence of antibiotic-resistant bacterial strains isolated in carrier pigeons during the race season. J. Chem. Pharm. Sci. 2017, 10, 10–13. [Google Scholar]
- Kimpe, A.; Decostere, A.; Martel, A.; Haesebrouck, F.; Devriese, L.A. Prevalence of antimicrobial resistance among pigeon isolates of Streptococcus gallolyticus, Escherichia coli and Salmonella enterica serotype Typhimurium. Avian Pathol. 2002, 31, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Iroha, I.; Afiukwa, F.; Oji, A.; Ejikeugwu, P.; Nwakeze, E. Occurrence of extended spectrum beta lactamase producing Escherichia coli from human clinical and wild birds (pigeons, bats, parrots and ducks) samples from Ebonyi state, Nigeria. World J. Pharm. Sci. 2015, 4, 20–29. [Google Scholar]
- Borges, C.A.; Cardozo, M.V.; Beraldo, L.G.; Oliveira, E.S.; Maluta, R.P.; Barboza, K.B.; Werther, K.; Ávila, F.A. Wild birds and urban pigeons as reservoirs for diarrheagenic Escherichia coli with zoonotic potential. J. Microbiol. 2017, 55, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.E. The relationship between antimicrobial resistance and patient outcomes: Mortality, length of hospital stays, and health care costs. Clin. Infect. Dis. 2006, 42 (Suppl. S2), S82–S89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tumbarello, M.; Spanu, T.; Di Bidino, R.; Marchetti, M.; Ruggeri, M.; Trecarichi, E.M.; De Pascale, G.; Proli, E.M.; Cauda, R.; Cicchetti, A.; et al. Costs of bloodstream infections caused by Escherichia coli and influence of extended-spectrum-beta-lactamase production and inadequate initial antibiotic therapy. Antimicrob. Agents Chemother. 2010, 54, 4085–4091. [Google Scholar] [CrossRef] [Green Version]
- WHO. Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- OIE. OIE List of Antimicrobial Agents of Veterinary Importance; World Organization for Animal Health: Paris, France, 2018. [Google Scholar]
- Ishiguro, N.; Oka, C.; Sato, G. Isolation of citrate-positive variants of Escherichia coli from domestic pigeons, pigs, cattle, and horses. Appl. Environ. Microbiol. 1978, 36, 217–222. [Google Scholar]
- Guerra, B.; Junker, E.; Schroeter, A.; Malorny, B.; Lehmann, S.; Helmuth, R. Phenotypic and genotypic characterization of antimicrobial resistance in German Escherichia coli isolates from cattle, swine and poultry. J. Antimicrob. Chemother. 2003, 52, 489–492. [Google Scholar] [CrossRef]
- Costa, D.; Poeta, P.; Saenz, Y.; Vinue, L.; Rojo-Bezares, B.; Jouini, A.; Zarazaga, M.; Rodrigues, M.J.; Torres, C. Detection of Escherichia coli harbouring extended-spectrum beta-lactamases of the CTX-M, TEM and SHV classes in faecal samples of wild animals in Portugal. J. Antimicrob. Chemother. 2006, 58, 1311–1312. [Google Scholar] [CrossRef]
- Radhouani, H.; Poeta, P.; Igrejas, G.; Goncalves, A.; Vinue, L.; Torres, C. Antimicrobial resistance and phylogenetic groups in isolates of Escherichia coli from seagulls at the Berlengas nature reserve. Vet. Rec. 2009, 165, 138–142. [Google Scholar] [CrossRef]
- Radhouani, H.; Igrejas, G.; Gonçalves, A.; Pacheco, R.; Monteiro, R.; Sargo, R.; Brito, F.; Torres, V.; Poeta, P. Antimicrobial resistance and virulence genes in Escherichia coli and enterococci from red foxes (Vulpes vulpes). Anaerobe 2013, 23, 82–86. [Google Scholar] [CrossRef]
- Stenzel, T.; Bancerz-Kisiel, A.; Tykałowski, B.; Śmiałek, M.; Pestka, D.; Koncicki, A. Antimicrobial resistance in bacteria isolated from pigeons in Poland. Pol. J. Vet. Sci. 2014, 17, 169–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-Hernando, A.; Prieto, M.; García-Fernández, C.; Alonso-Calleja, C.; Capita, R. Increase over time in the prevalence of multiple antibiotic resistance among isolates of Listeria monocytogenes from poultry in Spain. Food Control 2012, 23, 37–41. [Google Scholar] [CrossRef]
- Álvarez-Fernández, E.; Cancelo, A.; Díaz-Vega, C.; Capita, R.; Alonso-Calleja, C. Antimicrobial resistance in E. coli isolates from conventionally and organically reared poultry: A comparison of agar disc diffusion and Sensi Test Gram-negative methods. Food Control 2013, 30, 227–234. [Google Scholar] [CrossRef]
- Guerrero-Ramos, E.; Molina-González, D.; Blanco-Morán, S.; Igrejas, G.; Poeta, P.; Alonso-Calleja, C.; Capita, R. Prevalence, antimicrobial resistance, and genotypic characterization of vancomycin-resistant enterococci in meat preparations. J. Food Protect. 2016, 79, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Ramos, E.; Cordero, J.; Molina-González, D.; Poeta, P.; Igrejas, G.; Alonso-Calleja, C.; Capita, R. Antimicrobial resistance and virulence genes in enterococci from wild game meat in Spain. Food Microbiol. 2016, 53, 156–164. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Capita, R.; Alonso-Calleja, C. Microbial loads and antibiotic resistance patterns of Staphylococcus aureus in different types of raw poultry-based meat preparations. Poult. Sci. 2017, 96, 4046–4052. [Google Scholar] [CrossRef]
- Cordero, J.; Alonso-Calleja, C.; García-Fernández, C.; Capita, R. Microbial load and antibiotic resistance patterns of Escherichia coli and Enterococcus faecalis isolates from the meat of wild and domestic pigeons. Foods 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.L.; Nicoli, J.R.; Nascimento, T.C.; Diniz, C.G. Diarrheagenic Escherichia coli strains recovered from urban pigeons (Columba livia) in Brazil and their antimicrobial susceptibility patterns. Curr. Microbiol. 2009, 59, 302–308. [Google Scholar] [CrossRef]
- Hasan, B.; Islam, K.; Ahsan, M.; Hossain, Z.; Rashid, M.; Talukder, B.; Ahmed, K.U.; Olsen, B.; Kashem, M.A. Fecal carriage of multi-drug resistant and extended spectrum beta-lactamases producing E. coli in household pigeons, Bangladesh. Vet. Microbiol. 2014, 168, 221–224. [Google Scholar] [CrossRef]
- Santos, T.; Silva, N.; Igrejas, G.; Rodrigues, P.; Micael, J.; Rodrigues, T.; Resendes, R.; Goncalves, A.; Marinho, C.; Goncalves, D.; et al. Dissemination of antibiotic resistant Enterococcus spp. and Escherichia coli from wild birds of Azores Archipelago. Anaerobe 2013, 24, 25–31. [Google Scholar] [CrossRef]
- Hata, A.; Shibahara, T.; Yamamoto, H.; Fujitani, N. Antimicrobial resistance of Enterobacteriaceae in feral pigeons living in the Kanto region of Japan. Int. J. Anal. Bio-Sci. 2018, 6, 10–18. [Google Scholar]
- Álvarez-Fernández, E.; Alonso-Calleja, C.; García-Fernández, C.; Capita, R. Prevalence and antimicrobial resistance of Salmonella serotypes isolated from poultry in Spain: Comparison between 1993 and 2006. Int. J. Food Microbiol. 2012, 153, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, N.; Casas-Díaz, E.; Porrero, C.M.; Mateos, A.; Domínguez, L.; Lavín, S.; Serrano, E. Food-borne zoonotic pathogens and antimicrobial resistance of indicator bacteria in urban wild boars in Barcelona, Spain. Vet. Microbiol. 2013, 167, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Radhouani, H.; Silva, N.; Poeta, P.; Torres, C.; Correia, S.; Igrejas, G. Potential impact of antimicrobial resistance in wildlife, environment and human health. Front. Microbiol. 2014, 5, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Doming, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Machado, E.; Coque, T.M.; Cantón, R.; Sousa, J.C.; Peixe, L. Antibiotic resistance integrons and extended-spectrum β-lactamases among Enterobacteriaceae isolates recovered from chickens and swine in Portugal. J. Antimicrob. Chemother. 2008, 62, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poeta, P.; Radhouani, H.; Pinto, L.; Martinho, A.; Rego, V.; Rodrigues, R.; Gonçalves, A.; Rodrigues, J.; Estepa, V.; Torres, C.; et al. Wild boars as reservoirs of extended-spectrum beta-lactamase (ESBL) producing Escherichia coli of different phylogenetic groups. J. Basic Microbiol. 2009, 49, 584–588. [Google Scholar] [CrossRef]
- Carlos, C.; Pires, M.M.; Stoppe, N.C.; Hachich, E.M.; Sato, M.I.Z.; Gomes, T.A.T.; Amaral, L.A.; Ottoboni, L.M.M. Escherichia coli phylogenetic group determination and its application in the identification of the major animal source of fecal contamination. BMC Microbiol. 2010, 10, 161. [Google Scholar] [CrossRef] [Green Version]
- Radhouani, H.; Poeta, P.; Goncalves, A.; Pacheco, R.; Sargo, R.; Igrejas, G. Wild birds as biological indicators of environmental pollution: Antimicrobial resistance patterns of Escherichia coli and enterococci isolated from common buzzards (Buteo buteo). J. Med. Microbiol. 2012, 61, 837–843. [Google Scholar] [CrossRef]
- Chakraborty, A.; Saralaya, V.; Adhikari, P.; Shenoy, S.; Baliga, S.; Hegde, A. Characterization of Escherichia coli phylogenetic groups associated with extraintestinal infections in South Indian population. Ann. Med. Health Sci. Res. 2015, 5, 241–246. [Google Scholar]
- Sáenz, Y. Caracterización Fenotípica y Genotípica de la Resistencia a Antibióticos en Cepas de Escherichia coli no Patógenas de Alimentos y de la Microflora Intestinal de Humanos y Animales; Tesis Doctoral, Universidad de La Rioja: Logroño, Spain, 2004. [Google Scholar]
- Gonçalves, A.; Igrejas, G.; Radhouani, H.; Estepa, V.; Pacheco, R.; Monteiro, R.; Brito, F.; Guerra, A.; Petrucci-Fonseca, F.; Torres, C.; et al. Iberian wolf as a reservoir of extended-spectrum beta-lactamase-producing Escherichia coli of the TEM, SHV, and CTX-M groups. Microb. Drug Res. 2012, 18, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Mehrotra, M.; Ghimire, S.; Adewoye, L. Beta-Lactam resistance and beta-lactamases in bacteria of animal origin. Vet. Microbiol. 2007, 121, 97–214. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A. Animal reservoirs for extended spectrum beta-lactamase producers. Clin. Microbiol. Infect. 2008, 14, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.; Aoki, T. Sequence analysis of the florfenicol resistance gene encoded in the transferable R-plasmid of a fish pathogen, Pasteurella piscicida. Microbiol. Immunol. 1996, 40, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, K.M.; White, D.G.; Hume, M.E.; Poole, T.L.; Nisbet, D.J. The chloramphenicol resistance gene cmlA is disseminated on transferable plasmids that confer multiple-drug resistance in swine Escherichia coli. FEMS Microbiol. Lett. 2005, 243, 285–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momtaz, H.; Rahimi, E.; Moshkelani, S. Molecular detection of antimicrobial resistance genes in E. coli isolated from slaughtered commercial chickens in Iran. Vet. Med. 2012, 57, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Sousa, J.C.F.; Peixe, L.V. Antibióticos Antibacterianos. In Microbiologia; Ferreira, W.F.C., Sousa, J.C.F., Lima, N., Eds.; LIDEL—Edições Técnicas, Lda: Lisboa, Portugal, 2000; pp. 453–469. [Google Scholar]
- Altalhi, A.D.; Gherbawy, Y.A.; Hassan, S.A. Antibiotic resistance in Escherichia coli isolated from retail raw chicken meat in Taif, Saudi Arabia. Foodborne Pathog. Dis. 2000, 7, 281–285. [Google Scholar] [CrossRef]
- Literak, I.; Dolejska, M.; Janoszowska, D.; Hrusakova, J.; Meissner, W.; Rzyska, H.; Bzoma, S.; Cizek, A. Antibiotic-resistant Escherichia coli bacteria, including strains with genes encoding the extended-spectrum beta-lactamase and QnrS, in waterbirds on the Baltic Sea Coast of Poland. Appl. Environ. Microbiol. 2010, 76, 8126–8134. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.M. Elementos genéticos móveis. In Microbiologia; Ferreira, W.F.C., Sousa, J.C.F., Lima, N., Eds.; LIDEL—Edições Técnicas, Lda: Lisboa, Portugal, 2010; pp. 261–282. [Google Scholar]
- Perreten, V.; Boerlin, P. A new sulfonamide resistance gene (sul3) in Escherichia coli is widespread in the pig population of Switzerland. Antimicrob. Agents Chemother. 2003, 47, 1169–1172. [Google Scholar] [CrossRef] [Green Version]
- Appelberg, R. Virulência Microbiana. In Microbiologia; Ferreira, W.F.C., Sousa, J.C.F., Lima, N., Eds.; Edições Técnicas, Lda: Lisboa, Portugal, 2010; pp. 261–282. [Google Scholar]
- Warner, P.J.; Williams, P.H.; Bindereif, A.; Neilands, J.B. ColV plasmid-specific aerobactin synthesis by invasive strains of Escherichia coli. Infect. Immun. 1981, 33, 540–545. [Google Scholar]
- Gado, I.; Milch, H.; Czirok, E.; Herpay, M. The frequency of aerobactin production and its effect on the pathogenicity of human Escherichia coli strains. Acta Microbiol. Hung. 1989, 36, 51–60. [Google Scholar] [PubMed]
- Suárez, S.; Celemin, C.; Zdunczyr, E.; Medina, E.; Williams, P.H. Aerobactin production by enterotoxigenic Escherichia coli of porcine intestine. Vet. Microbiol. 1995, 47, 229–233. [Google Scholar] [CrossRef]
- Tarchouna, M.; Ferjani, A.; Ben-Selma, W.; Boukadida, J. Distribution of uropathogenic virulence genes in Escherichia coli isolated from patients with urinary tract infection. Int. J. Infect. Dis. 2013, 17, e450–e453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, A.; Igrejas, G.; Radhouani, H.; Estepa, V.; Alcaide, E.; Zorrilla, I.; Serra, R.; Torres, C.; Poeta, P. Detection of extended-spectrum beta-lactamase-producing Escherichia coli isolates in faecal samples of Iberian lynx. Lett. Appl. Microbiol. 2012, 54, 73–77. [Google Scholar] [CrossRef]
- Flores, C.E.; Loureiro, L.; Bessa, L.J.; Martins Da Costa, P. Presence of multidrug-resistant E. coli, Enterococcus spp. and Salmonella spp. in lakes and fountains of Porto, Portugal. J. Water Res. Prot. 2013, 5, 1117–1126. [Google Scholar] [CrossRef] [Green Version]
- Stenske, K.A.; Bemis, D.A.; Gillespie, B.E.; Oliver, S.P.; Draughon, F.A.; Matteson, K.J.; Bartges, J.W. Prevalence of urovirulence genes cnf, hlyD, sfa/foc, and papGIII in fecal Escherichia coli from healthy dogs and their owners. Am. J. Vet. Res. 2009, 70, 1401–1406. [Google Scholar] [CrossRef] [Green Version]
- Norgren, M.; Bága, M.; Tennent, J.M.; Normark, S. Nucleotide sequence, regulation and functional analysis of the papC gene required for cell surface localization of Pap pili of uropathogenic Escherichia coli. Mol. Microbiol. 1987, 1, 169–178. [Google Scholar] [CrossRef]
- CLSI. M100 Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- Cormican, M.G.; Marshall, S.A.; Jones, R.N. Detection of extended-spectrum beta-lactamase (ESBL)-producing strains by the Etest ESBL screen. J. Clin. Microbiol. 1996, 34, 1880–1884. [Google Scholar]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [Green Version]
- Oram, M.; Fisher, L.M. 4-Quinolone resistance mutations in the DNA gyrase of Escherichia coli clinical isolates identified by using the polymerase chain reaction. Antimicrob. Agents Chemother. 1991, 35, 387–389. [Google Scholar] [CrossRef] [Green Version]
- Vila, J.; Ruiz, J.; Goni, P.; De Anta, M.T. Detection of mutations in parC in quinolone-resistant clinical isolates of Escherichia coli. Antimicrob. Agents Chemother. 1996, 40, 491–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stapleton, P.D.; Shannon, K.P.; French, G.L. Carbapenem resistance in Escherichia coli associated with plasmid-determined CMY-4 beta-lactamase production and loss of an outer membrane protein. Antimicrob. Agents Chemother. 1999, 43, 1206–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sáenz, Y.; Briñas, L.; Domínguez, E.; Ruiz, J.; Zarazaga, M.; Vila, J.; Torres, C. Mechanisms of resistance in multiple-antibiotic-resistant Escherichia coli strains of human, animal, and food origins. Antimicrob. Agents Chemother. 2004, 48, 3996–4001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollingshead, S.; Vapnek, D. Nucleotide sequence analysis of the gene encoding a streptomycin/spectinomycin adenylyltransferase. Plasmid 1985, 13, 17–30. [Google Scholar] [CrossRef]
- Guardabassi, L.; Dijkshoorn, L.; Collard, J.M.; Olsen, J.E.; Dalsgaard, A. Distribution and in-vitro transfer of tetracycline resistance determinants in clinical and aquatic Acinetobacter strains. J. Med. Microbiol. 2000, 49, 929–936. [Google Scholar] [CrossRef] [Green Version]
- Mazel, D.; Dychinco, B.; Webb, V.A.; Davies, J. Antibiotic resistance in the ECOR collection: Integrons and identification of a novel aad gene. Antimicrob. Agents Chemother. 2000, 44, 1568–1574. [Google Scholar] [CrossRef] [Green Version]
- Maynard, C.; Fairbrother, J.M.; Bekal, S.; Sanschagrin, F.; Levesque, R.C.; Brousseau, R.; Masson, L.; Lariviere, S.; Harel, J. Antimicrobial resistance genes in enterotoxigenic Escherichia coli O149:K91 isolates obtained over a 23-year period from pigs. Antimicrob. Agents Chemother. 2003, 47, 3214–3221. [Google Scholar] [CrossRef] [Green Version]
- Levesque, C.; Roy, P.H. PCR Analysis of integrons. In Diagnostic Molecular Microbiology; Persing, D.H., Smith, T.F., Tenover, C.F., Whith, T.J., Eds.; American Society for Microbiology: Washington, DC, USA, 1993; pp. 590–594. [Google Scholar]
- White, P.A.; McIver, C.J.; Rawlinson, W.D. Integrons and gene cassettes in the enterobacteriaceae. Antimicrob. Agents Chemother. 2001, 45, 2658–2661. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Terai, A.; Yuri, K.; Kurazono, H.; Takeda, Y.; Yoshida, O. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immunol. Med. Microbiol. 1995, 12, 85–90. [Google Scholar] [CrossRef]
- Ruiz, J.; Simon, K.; Horcajada, J.P.; Velasco, M.; Barranco, M.; Roig, G.; Moreno-Martinez, A.; Martinez, J.A.; Jimenez De Anta, T.; Mensa, J.; et al. Differences in virulence factors among clinical isolates of Escherichia coli causing cystitis and pyelonephritis in women and prostatitis in men. J. Clin. Microbiol. 2002, 40, 4445–4449. [Google Scholar] [CrossRef] [Green Version]
- Paton, J.C.; Paton, A.W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 1998, 11, 450–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Strain Number | Origin | Resistance Pattern |
|---|---|---|
| 22 | Domestic pigeon | AMP/ATM/STR |
| 19 | Domestic pigeon | AMP/CAZ/STR |
| 12 | Domestic pigeon | AMC/AMP/CAZ/STR |
| 13 | Domestic pigeon | AMC/AMP/CAZ/STR |
| 18 | Domestic pigeon | AMC/AMP/CAZ/STR |
| 4 | Domestic pigeon | AMC/AMP/STR/TE |
| 24 | Domestic pigeon | AMP/ATM/ CAZ/CTX |
| 14 | Domestic pigeon | AMP/CAZ/CN/STR |
| 16 | Domestic pigeon | AMP/CAZ/AK/STR |
| 7 | Domestic pigeon | AMP/CAZ/STR/TE |
| 9 | Domestic pigeon | AMP/CAZ/STR/TE |
| 1 | Domestic pigeon | AMC/AMP/CAZ/STR/TE |
| 5 | Domestic pigeon | AMC/AMP/CAZ/STR/TE |
| 6 | Domestic pigeon | AMC/AMP/CAZ/STR/TE |
| 10 | Domestic pigeon | AMC/AMP/CAZ/STR/TE |
| 11 | Domestic pigeon | AMC/AMP/CAZ/STR/TE |
| 21 | Domestic pigeon | AMC/AMP/CAZ/STR/TE |
| 23 | Domestic pigeon | AMC/AMP/CAZ/STR/TE |
| 15 | Domestic pigeon | AMP/CAZ/C/AK/STR |
| 20 | Domestic pigeon | AMP/CAZ/AK/STR/TOB |
| 3 | Domestic pigeon | AMP/CAZ/CTX/STR/TOB/TE |
| 2 | Domestic pigeon | NA/AMC/AMP/CAZ/STR/TOB/TE/SXT |
| 17 | Domestic pigeon | CIP/AMC/AMP/CAZ/C/CN/AK/STR |
| 8 | Domestic pigeon | AMC/AMP/ FOX/CAZ/C/AK/TOB/TE |
| 28 | Wild pigeon | AMP/CAZ |
| 25 | Wild pigeon | AMP/CAZ/STR |
| 32 | Wild pigeon | AMP/CAZ/STR |
| 37 | Wild pigeon | AMP/CAZ/TE |
| 34 | Wild pigeon | NA/AMP/CAZ/TE |
| 30 | Wild pigeon | AMC/AMP/CAZ/STR |
| 33 | Wild pigeon | AMC/AMP/CAZ/STR |
| 27 | Wild pigeon | AMC/AMP/CAZ/STR |
| 31 | Wild pigeon | AMP/CAZ/STR/TE |
| 36 | Wild pigeon | AMP/CAZ/STR/SXT |
| 29 | Wild pigeon | AMP/FOX/CAZ/CTX/TE |
| 35 | Wild pigeon | AMP/ FOX/CAZ/ STR/TOB/TE |
| 26 | Wild pigeon | AMC/AMP/CAZ/C/ CN/STR/TE |
| Virulence Factors | Number of Strains |
|---|---|
| fimA | 4 |
| papGIII | 1 |
| aer/fimA | 14 |
| cnf1/fimA | 1 |
| fimA/hly | 4 |
| cnf1/fimA/hly | 4 |
| aer/fimA/hly/papGIII | 5 |
| cnf1/fimA/hly/papGIII | 1 |
| aer/cnf1/fimA/hly/papGIII | 2 |
| None | 1 |
| Target Gene | Primer Name | Sequence (5′-3′) | Annealing Temperature (°C) (Amplicon Size, bp) | Reference |
|---|---|---|---|---|
| chuaA | chuaA-F | GACGAACCAACGGTCAGGAT | 55 (279) | [68] |
| chuaA-R | TGCCGCCAGTACCAAAGACA | |||
| tspE4.C2 | tspE4.C2-F | GAGTAATGTCGGGGCATTCA | 55 (152) | [68] |
| tspE4.C2-R | CGCGCCAACAAAGTATTACG | |||
| yjaA | yjaA-F | TGAAGTGTCAGGAGACGCTG | 55 (211) | [68] |
| yjaA-R | ATGGAGAATGCGTTCCTCAAC |
| Target Gene | Primer Name | Sequence (5′-3′) | Annealing Temperature (°C) (Amplicon Size, bp) | Reference |
|---|---|---|---|---|
| gyrA | gyrA-F | TACACCGGTCAACATTGAGG | 64 (648) | [69] |
| gyrA-R | TTAATGATTGCCGCCGTCGG | |||
| parC | parC-F | AAACCTGTTCAGCGCCGCATT | 55 (395) | [70] |
| parC-R | GTGGTGCCGTTAAGCAAA | |||
| ampC | ampC-F | AATGGGTTTTCTACGGTCTG | 57 (1800) | [71] |
| ampC-R | GGGCAGCAAATGTGGAGCAA | |||
| cmlA | cmlA-F | TGTCATTTACGGCATACTCG | 55 (455) | [72] |
| cmlA-R | ATCAGGCATCCCATTCCCAT | |||
| aadA | aadA-F | GCAGCGCAATGACATTCTTG | 60 (282) | [73] |
| aadA-R | ATCCTTCGGCGCGATTTTG | |||
| aac(3)II | aac(3)II-F | ACTGTGATGGGATACGCGTC | 60 (237) | [72] |
| aac(3)II-R | CTCCGTCAGCGTTTCAGCTA | |||
| aac(3)IV | aac(3)IV-F | CTTCAGGATGGCAAGTTGGT | 60 (286) | [72] |
| aac(3)IV-R | TCATCTCGTTCTCCGCTCAT | |||
| tetA | tetA-F | GTAATTCTGAGCACTGTCGC | 62 (937) | [74] |
| tetA-R | CTGCCTGGACAACATTGCTT | |||
| tetB | tetB-F | CTCAGTATTCCAAGCCTTTG | 57 (416) | [74] |
| tetB-R | CTAAGCACTTGTCTCCTGTT | |||
| tetC | tetC-F | TCTAACAATGCGCTCATCGT | 62 (570) | [74] |
| tetC-R | GGTTGAAGGCTCTCAAGGGC | |||
| sul1 | sul1-F | TGGTGACGGTGTTCGGCATTC | 63 (789) | [75] |
| sul1-R | GCGAGGGTTTCCGAGAAGGTG | |||
| sul2 | sul2-F | CGGCATCGTCAACATAACC | 50 (722) | [76] |
| sul2-R | GTGTGCGGATGAAGTCAG | |||
| sul3 | sul3-F | CATTCTAGAAAACAGTCGTAGTTCG | 51 (990) | [56] |
| sul3-R | CATCTGCAGCTAACCTAGGGCTTTGGA | |||
| intl1 | intl1-F | GGGTCAAGGATCTGGATTTCG | 62 (483) | [75] |
| intl1-R | ACATGGGTGTAAATCATCGTC | |||
| rvintl1 | rvintl1-F | GGCATCCAAGCAGCAAG | 55 (variable) | [77] |
| rvintl1-R | AAGCAGACTTGACCTGA | |||
| qacEΔ1 | qacEΔ1-F | GGCTGGCTTTTTCTTGTTATCG | 60 (287) | [75] |
| qacEΔ1-R | TGAGCCCCATACCTACAAAGC | |||
| intl2 | intl2-F | CACGGATATGCGACAAAAAGGT | 62 (788) | [75] |
| intl2-R | GTAGCAAACGAGTGACGAAATG | |||
| rvintl2 | rvintl2-F | CGGGATCCCGGACGGCATGCACGATTTGTA | 60 (variable) | [78] |
| rvintl2-R | GATGCCATCGCAAGTACGAG |
| Target Gene | Primer Name | Sequence (5′-3′) | Annealing Temperature (°C) (Amplicon Size, bp) | Reference |
|---|---|---|---|---|
| aer | aer-F | TACCGGATTGTCATATGCAGACCGT | 63 (602) | [79] |
| aer-R | AATATCTTCCTCCAGTCCGGAGAAG | |||
| cnf1 | cnf1-F | AAGATGGAGTTTCCTATGCAGGAG | 63 (498) | [79] |
| cnf1-R | CATTCAGAGTCCTGCCCTCATTATT | |||
| fimA | fimA-F | GTTGTTCTGTCGGCTCTGTC | 55 (447) | [80] |
| fimA-R | ATGGTGTTGGTTCCGTTATTC | |||
| hly | hly-F | AACAAGGATAAGCACTGTTCTGGCT | 63 (1177) | [79] |
| hly-R | ACCATATAAGCGGTCATTCCCGTCA | |||
| papC | papC-F | GACGGCTGTACTGCAGGGTGTGGCG | 63 (328) | [79] |
| papC-R | ATATCCTTTCTGCAGGGATGCAATA | |||
| papGIII | papGIII-F | CATTTATCGTCCTCAACTTAG | 55 (482) | [80] |
| papGIII-R | AAGAAGGGATTTTGTAGCGTC | |||
| stx1 | stx1-R | ATAAATCGCCATTCGTTGACTAC | 65-60 (180) | [81] |
| stx1-R | AGAACGCCCACTGAGATCATC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capita, R.; Cordero, J.; Molina-González, D.; Igrejas, G.; Poeta, P.; Alonso-Calleja, C. Phylogenetic Diversity, Antimicrobial Susceptibility and Virulence Characteristics of Escherichia coli Isolates from Pigeon Meat. Antibiotics 2019, 8, 259. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8040259
Capita R, Cordero J, Molina-González D, Igrejas G, Poeta P, Alonso-Calleja C. Phylogenetic Diversity, Antimicrobial Susceptibility and Virulence Characteristics of Escherichia coli Isolates from Pigeon Meat. Antibiotics. 2019; 8(4):259. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8040259
Chicago/Turabian StyleCapita, Rosa, Jorge Cordero, Diana Molina-González, Gilberto Igrejas, Patrícia Poeta, and Carlos Alonso-Calleja. 2019. "Phylogenetic Diversity, Antimicrobial Susceptibility and Virulence Characteristics of Escherichia coli Isolates from Pigeon Meat" Antibiotics 8, no. 4: 259. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8040259