Designed Antimicrobial Peptides for Topical Treatment of Antibiotic Resistant Acne Vulgaris
Abstract
:1. Introduction
2. Results
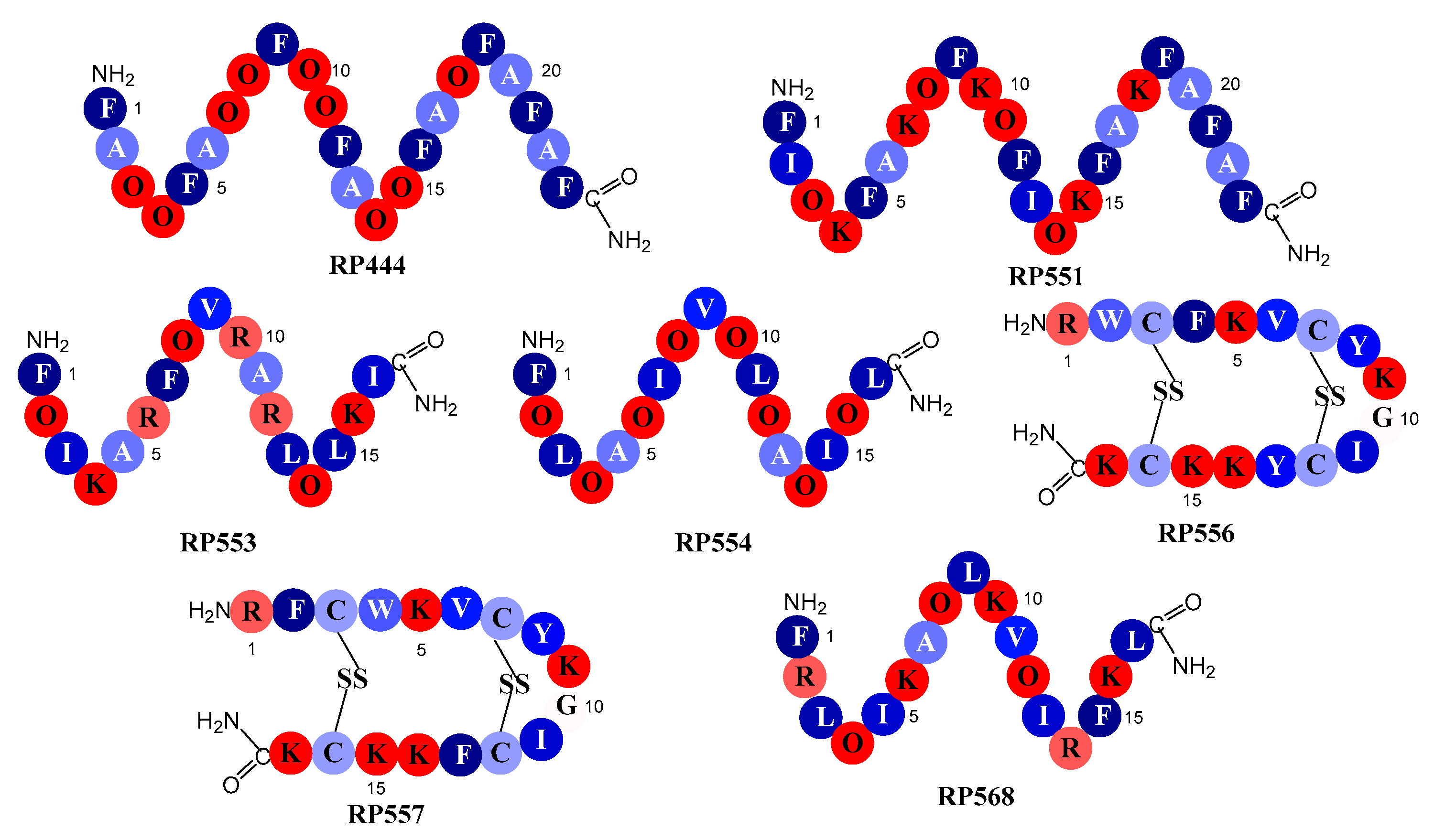
2.1. Designed Antimicrobial Peptides
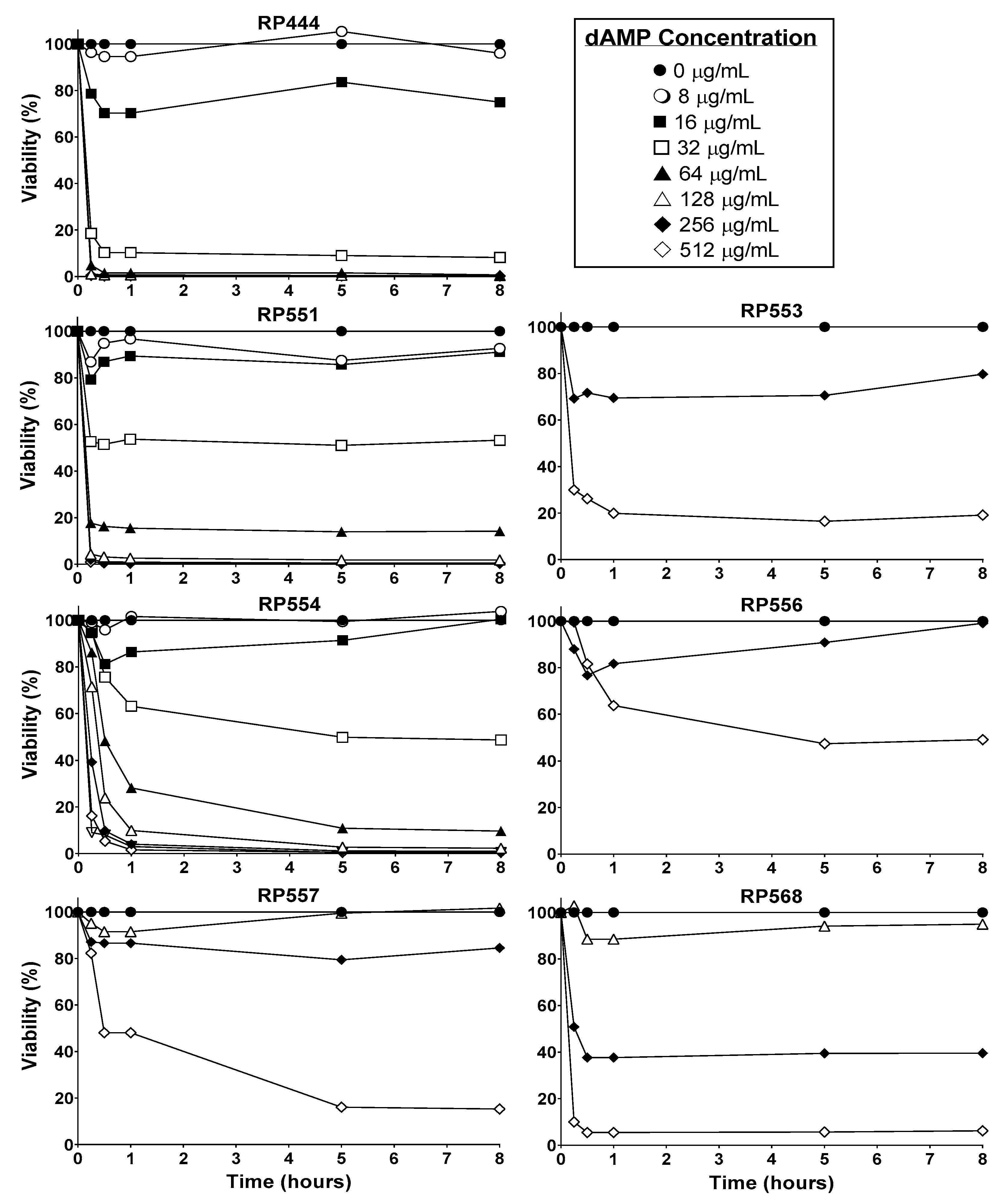
2.2. C. acnes In Vitro Antibacterial Activity
2.3. Limited Mammalian Cytotoxicity
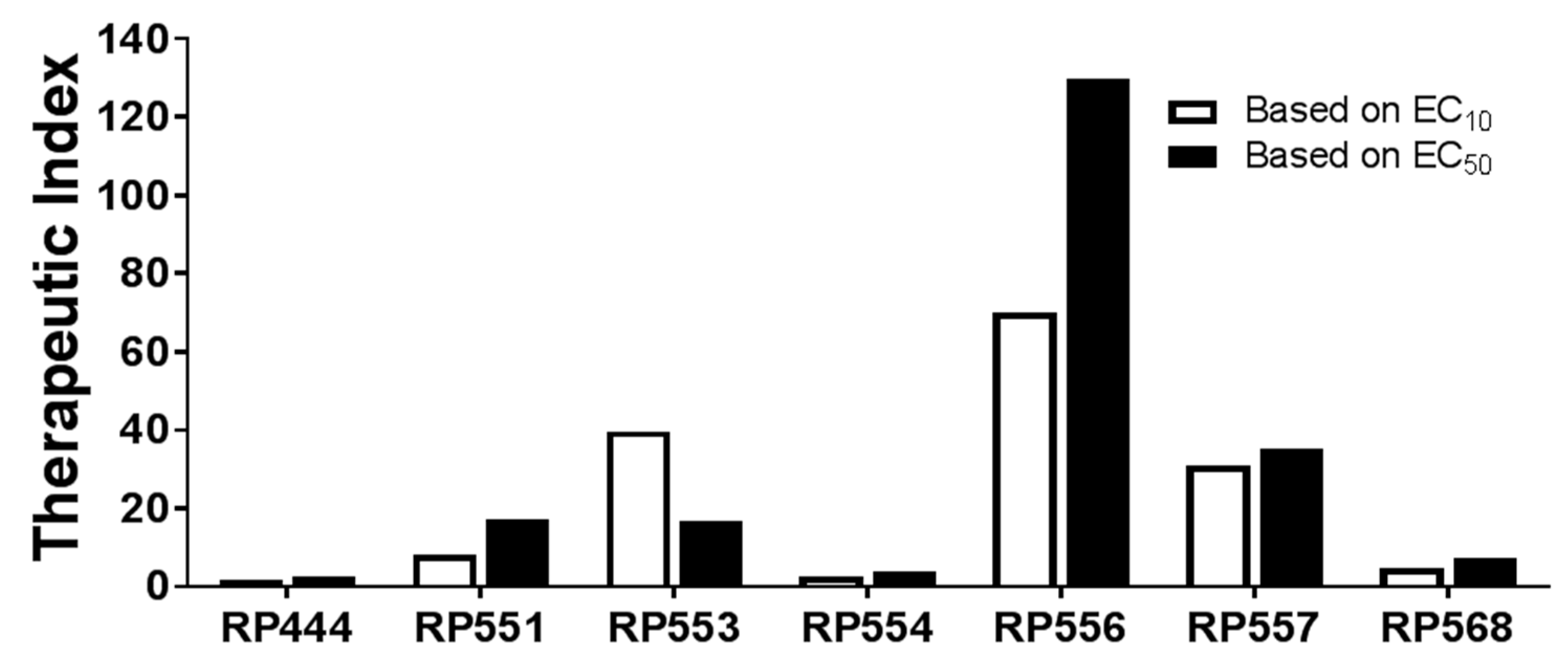
2.4. High Therapeutic Index
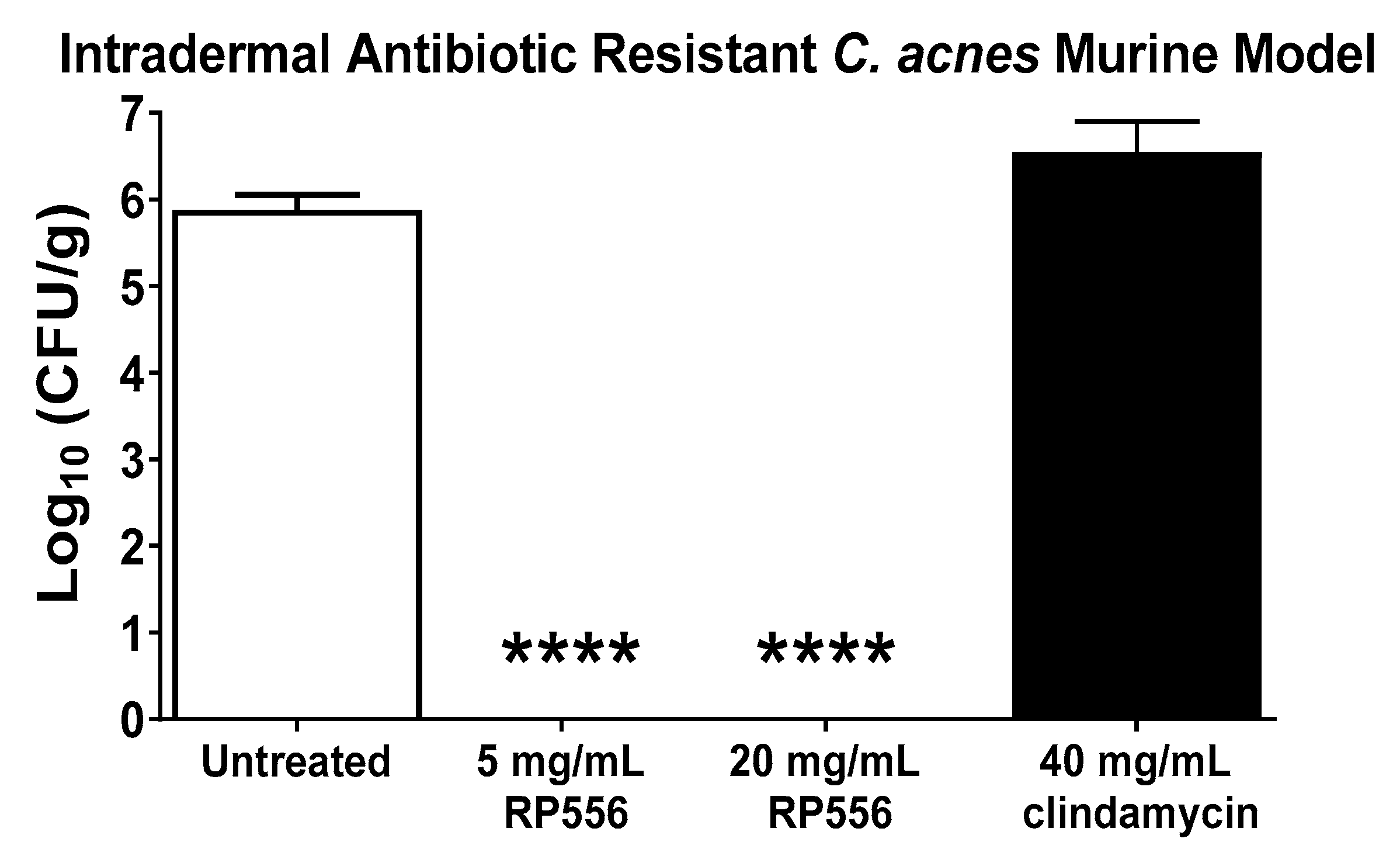
2.5. RP556 Activity in a Multidrug Resistant C. acnes Murine Model
3. Discussion
4. Materials and Methods
4.1. Designed Antimicrobial Peptides
4.2. Minimum Inhibitory Concentration (MIC)
4.3. Mammalian Cytotoxicity
4.4. Animals
4.5. In Vivo Efficacy Assessment of RP556 against Multidrug-Resistant HL043PA2 C. acnes
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Walsh, T.R.; Efthimiou, J.; Dréno, B. Systematic review of antibiotic resistance in acne: An increasing topical and oral threat. Lancet Infect. Dis. 2016, 16, e23–e33. [Google Scholar] [CrossRef] [Green Version]
- Tanghetti, E.A.; Popp, K.F. A current review of topical benzoyl peroxide: New perspectives on formulation and utilization. Dermatol. Clin. 2009, 27, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.M.; Dreno, B.; Gollnick, H.P.; Zouboulis, C.C. A review of the European Directive for prescribing systemic isotretinoin for acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 773–776. [Google Scholar] [CrossRef] [PubMed]
- James, K.A.; Burkhart, C.N.; Morrell, D.S. Emerging drugs for acne. Expert Opin. Emerg. Drugs 2009, 14, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Zaenglein, A.L.; Pathy, A.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, O., Jr.; Thornsberry, C.; Cardin, C.W.; Smiles, K.A.; Leyden, J.J. Bacterial resistance and therapeutic outcome following three months of topical acne therapy with 2% erythromycin gel versus its vehicle. Acta Derm. Venereol. 2002, 82, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Guidos, R.; Gilbert, D.; Bradley, J.; Boucher, H.W.; Scheld, W.M.; Bartlett, J.G.; Edwards, J., Jr. Infectious Diseases Society of America (2008) The epidemic of antibiotic-resistant infections: A call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Clemens, L.E.; Jaynes, J.; Lim, E.; Kolar, S.S.; Reins, R.Y.; Baidouri, H.; Hanlon, S.; McDermott, A.M.; Woodburn, K.W. Designed host defense peptides for the treatment of bacterial keratitis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6273–6281. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, K.W.; Jaynes, J.M.; Clemens, L.E. Evaluation of the antimicrobial peptide, RP557, for the broad-spectrum treatment of wound pathogens and biofilm. Front. Microbiol. 2019, 10, 1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodburn, K.W.; Clemens, L.E.; Jaynes, J.; Joubert, L.M.; Botha, A.; Nazik, H.; Stevens, D.A. Designed antimicrobial peptides for recurrent vulvovaginal candidiasis treatment. Antimicrob. Agents Chemother. 2019, 63, e02690-18, Print 2019 Nov. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Berthold, N.; Czihal, P.; Fritsche, S.; Sauer, U.; Schiffer, G.; Knappe, D.; Alber, G.; Hoffmann, R. Novel apidaecin 1b analogs with superior serum stabilities for treatment of infections by gram-negative pathogens. Antimicrob. Agents Chemother. 2013, 57, 402–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyden, M.; Freites, J.A.; Ulmschneider, M.B.; White, S.H.; Tobias, D.J. Assembley and stability of α-helical membrane proteins. Soft Matter 2012, 8, 7742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, A.; Barnard, E.; Nagy, I.; Gao, A.; Tomida, S.; Li, H.; Eady, A.; Cove, J.; Nord, C.E.; Patrick, S.; et al. An expanded multilocus sequence typing scheme for Propionibacterium acnes: Investigation of ‘pathogenic’, ‘commensal’ and antibiotic resistant strains. PLoS ONE 2012, 7, e41480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannis, M.J. The use of antimicrobial peptides in ophthalmology: An experimental study in corneal preservation and the management of bacterial keratitis. Trans. Am. Ophthalmol. Soc. 2002, 100, 243–271. [Google Scholar] [PubMed]
- Hamblin, M.R.; Zahra, T.; Contag, C.H.; McManus, A.T.; Hasan, T. Optical monitoring and treatment of potentially lethal wound infections in vivo. J. Infect. Dis. 2003, 187, 1717–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| C. acnes | Antibiotic Sensitivity | dAMPs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tetra # | Ery # | Clind # | RP444 | RP551 | RP553 | RP554 | RP556 | RP557 | RP568 | |
| ATCC6919 | S | S | S | 2 | 2 | 8 | 4 | 4 | 8 | >32 |
| ATCC11827 | S | S | S | 8 | 2–4 | 16 | 8 | 8 | 8 | >32 |
| HL007PA1 | R | R | R | 4 | 2 | 16 | 4 | 4 | 8 | >32 |
| HL013PA1 | R | R | R | 4 | 2 | 8 | 2–4 | 2 | 2 | >32 |
| HL038PA1 | R | R | R | 4 | 2 | 16 | 8 | 4 | 8 | >32 |
| HL043PA1 | R | R | R | 4 | 2 | 16 | 4 | 4 | 8 | >32 |
| HL043PA2 | R | R | R | 8 | 2 | 16 | 8 | 4 | 8 | >32 |
| HL045PA1 | R | R | R | 4 | 2 | 16 | 4 | 4 | 4 | >32 |
| HL053PA1 | R | R | R | 4 | 2 | 16 | 4 | 4 | 4 | >32 |
| HL056PA1 | R | R | R | 4 | 2 | 16 | 4 | 2 | 4 | >32 |
| HL072PA1 | R | R | R | 8 | 2–4 | 16 | 8 | 8 | 8 | >32 |
| HL082PA2 | R | R | S | 2 | 2 | 16 | 8 | 2 | 2 | >32 |
| dAMP | MIC (μg/mL) | EC10 (μg/mL) | Therapeutic Index | EC50 (μg/mL) | Therapeutic Index |
|---|---|---|---|---|---|
| RP444 | 8 | 13 | 1.63 | 20 | 2.5 |
| RP551 | 2 | 16.6 | 8.3 | 34.3 | 17.2 |
| RP553 | 16 | 238 | 39.7 | 268 | 16.8 |
| RP554 | 8 | 19 | 2.38 | 31 | 3.88 |
| RP556 | 4 | 276 | 70 | 519 | 130 |
| RP557 | 8 | 246 | 30.8 | 281 | 35.1 |
| RP568 | >32 | 146 | 4.56 | 229 | 7.16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woodburn, K.W.; Jaynes, J.; Clemens, L.E. Designed Antimicrobial Peptides for Topical Treatment of Antibiotic Resistant Acne Vulgaris. Antibiotics 2020, 9, 23. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9010023
Woodburn KW, Jaynes J, Clemens LE. Designed Antimicrobial Peptides for Topical Treatment of Antibiotic Resistant Acne Vulgaris. Antibiotics. 2020; 9(1):23. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9010023
Chicago/Turabian StyleWoodburn, Kathryn W., Jesse Jaynes, and L. Edward Clemens. 2020. "Designed Antimicrobial Peptides for Topical Treatment of Antibiotic Resistant Acne Vulgaris" Antibiotics 9, no. 1: 23. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9010023




