Synergistic Antimicrobial Activity of Supplemented Medical-Grade Honey against Pseudomonas aeruginosa Biofilm Formation and Eradication
Abstract
:1. Introduction
2. Results
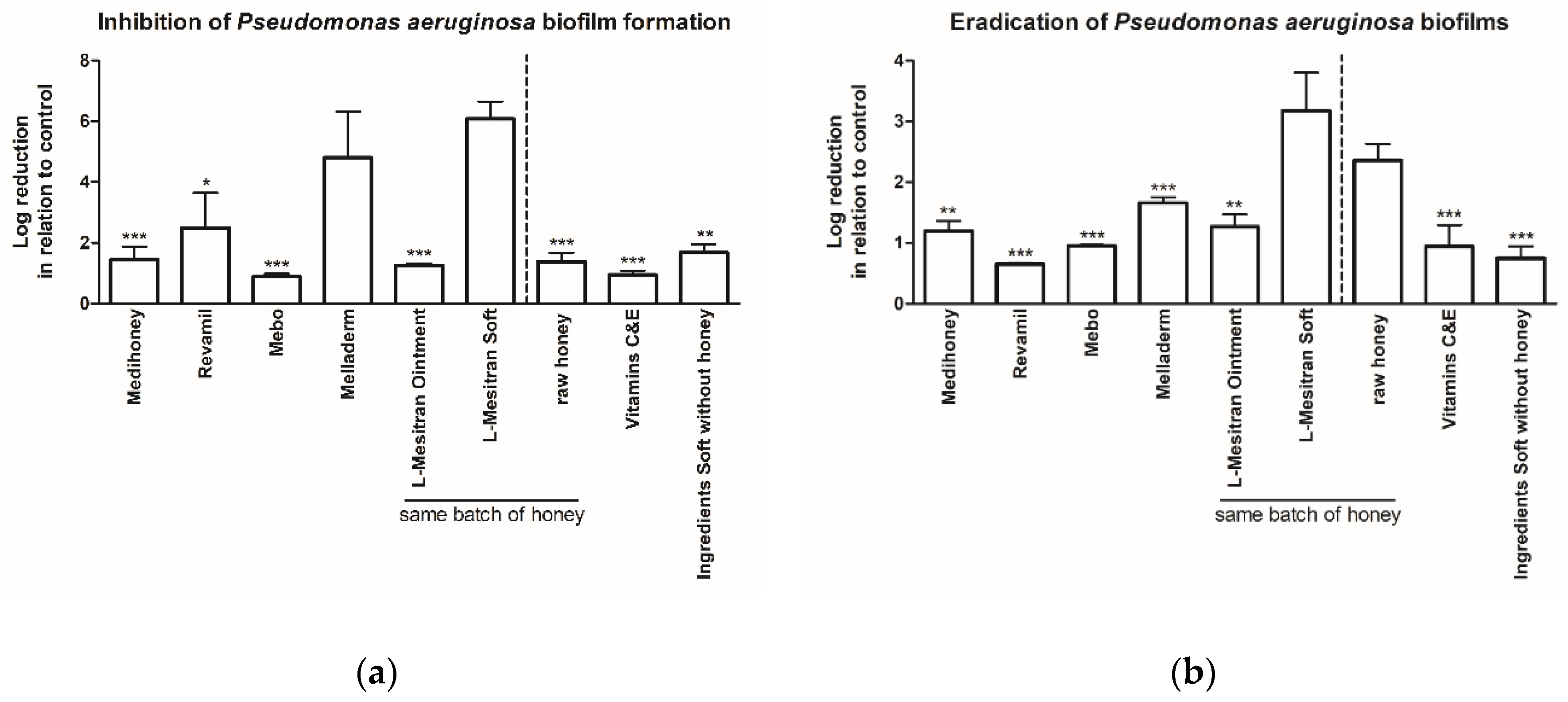
2.1. MGH Strongly Inhibits Pseudomonas aeruginosa Biofilm Formation, Which Can Further Be Enhanced by Supplements
2.2. MGH Eradicates Existing Pseudomonas aeruginosa Biofilms, with Increased Efficacy by Supplements
2.3. L-Mesitran Soft Has also the Strongest Antibiofilm Activity against Staphylococcus aureus
2.4. Case Reports Illustrating the Antibiofilm Activity in the Clinic
2.4.1. Case 1: Infected Diabetic Ulcer
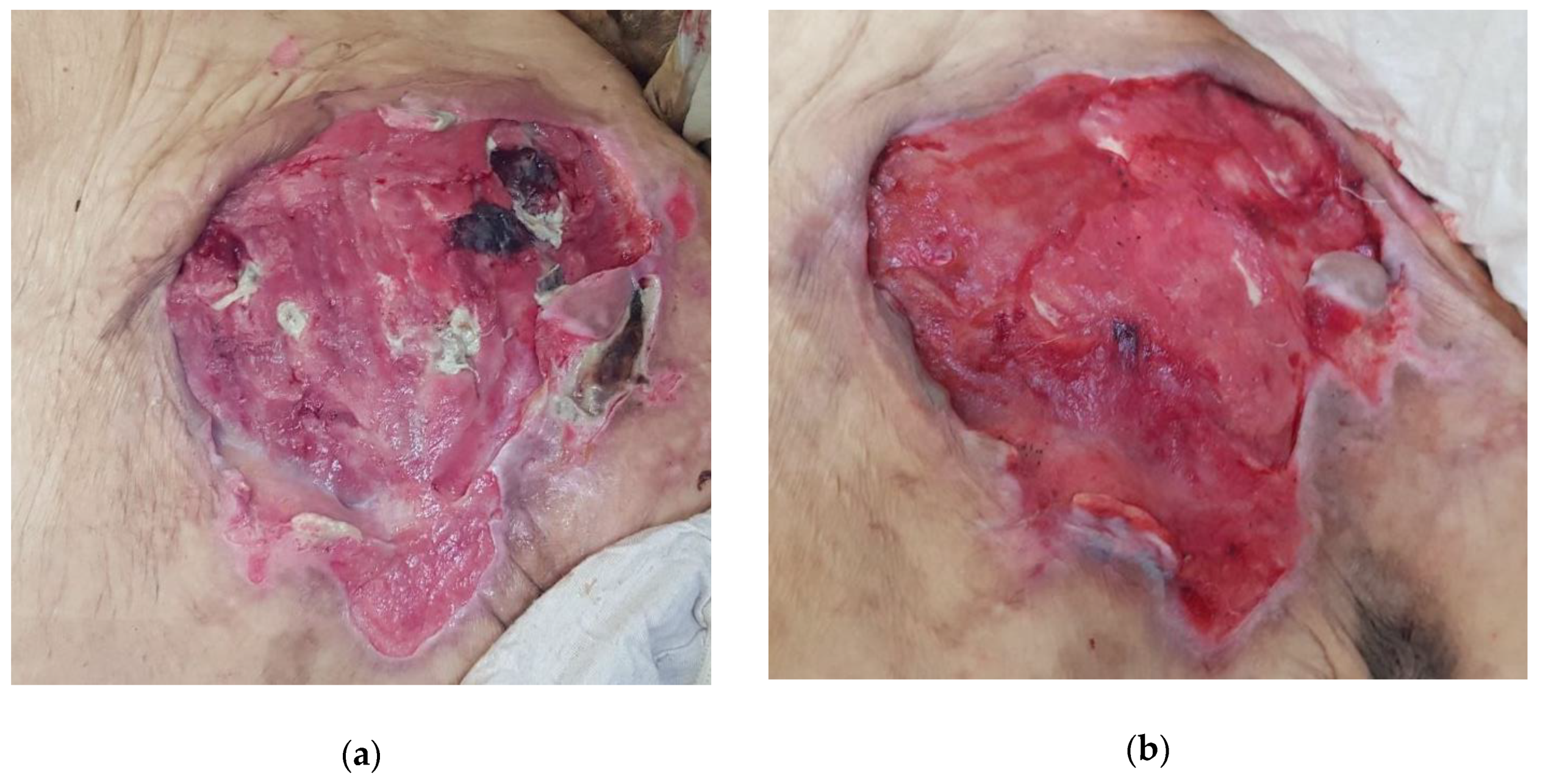
2.4.2. Case 2: Massive Abscess Infected with Pseudomonas aeruginosa and Staphylococcus aureus
2.4.3. Case 3: Pressure Ulcer
2.4.4. Case 4: Heavily Infected Wound on the Ankle
3. Discussion
4. Materials and Methods
4.1. Evaluation of Biofilm Inhibitory and Eradicating Activity of MGH-Containing Wound Care Products Using an In Vitro Wound Model
4.2. Honey Samples and Other Test Conditions
4.3. Case Series
4.4. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Y.K.; Cheng, N.C.; Cheng, C.M. Biofilms in Chronic Wounds: Pathogenesis and Diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef]
- Lu, J.; Turnbull, L.; Burke, C.M.; Liu, M.; Carter, D.A.; Schlothauer, R.C.; Whitchurch, C.B.; Harry, E.J. Manuka-type honeys can eradicate biofilms produced by Staphylococcus aureus strains with different biofilm-forming abilities. PeerJ 2014, 2, e326. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, T.; Wolcott, R.D.; Peghetti, A.; Leaper, D.; Cutting, K.; Polignano, R.; Rosa Rita, Z.; Moscatelli, A.; Greco, A.; Romanelli, M.; et al. Recommendations for the management of biofilm: A consensus document. J. Wound Care 2016, 25, 305–317. [Google Scholar] [CrossRef]
- Olsson, M.; Jarbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leaper, D.; Assadian, O.; Edmiston, C.E. Approach to chronic wound infections. Br. J. Dermatol. 2015, 173, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Halstead, F.D.; Webber, M.A.; Oppenheim, B.A. Use of an engineered honey to eradicate preformed biofilms of important wound pathogens: An in vitro study. J. Wound Care 2017, 26, 442–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burmolle, M.; Thomsen, T.R.; Fazli, M.; Dige, I.; Christensen, L.; Homoe, P.; Tvede, M.; Nyvad, B.; Tolker-Nielsen, T.; Givskov, M.; et al. Biofilms in chronic infections—A matter of opportunity—monospecies biofilms in multispecies infections. FEMS Immunol. Med. Microbiol. 2010, 59, 324–336. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Cokcetin, N.N.; Burke, C.M.; Turnbull, L.; Liu, M.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Honey can inhibit and eliminate biofilms produced by Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 18160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Gjodsbol, K.; Christensen, J.J.; Karlsmark, T.; Jorgensen, B.; Klein, B.M.; Krogfelt, K.A. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 2006, 3, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazli, M.; Bjarnsholt, T.; Kirketerp-Moller, K.; Jorgensen, B.; Andersen, A.S.; Krogfelt, K.A.; Givskov, M.; Tolker-Nielsen, T. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J. Clin. Microbiol. 2009, 47, 4084–4089. [Google Scholar] [CrossRef] [Green Version]
- Dryden, M.S. Complicated skin and soft tissue infection. J. Antimicrob. Chemother. 2010, 65 (Suppl. S3), iii35–iii44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veesenmeyer, J.L.; Hauser, A.R.; Lisboa, T.; Rello, J. Pseudomonas aeruginosa virulence and therapy: Evolving translational strategies. Crit. Care Med. 2009, 37, 1777–1786. [Google Scholar] [CrossRef] [Green Version]
- Drenkard, E. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect. 2003, 5, 1213–1219. [Google Scholar] [CrossRef]
- Stover, C.K.; Pham, X.Q.; Erwin, A.L.; Mizoguchi, S.D.; Warrener, P.; Hickey, M.J.; Brinkman, F.S.; Hufnagle, W.O.; Kowalik, D.J.; Lagrou, M.; et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 2000, 406, 959–964. [Google Scholar] [CrossRef]
- Klockgether, J.; Munder, A.; Neugebauer, J.; Davenport, C.F.; Stanke, F.; Larbig, K.D.; Heeb, S.; Schock, U.; Pohl, T.M.; Wiehlmann, L.; et al. Genome diversity of Pseudomonas aeruginosa PAO1 laboratory strains. J. Bacteriol. 2010, 192, 1113–1121. [Google Scholar] [CrossRef] [Green Version]
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.D.T.G.; Cremers, N.A.J. Defining the standards for medical grade honey. J. Apic. Res. 2020, 59, 125–135. [Google Scholar] [CrossRef]
- Smaropoulos, E.; Cremers, N.A. Medical grade honey for the treatment of paediatric abdominal wounds: A case series. J. Wound Care 2020, 29, 94–99. [Google Scholar] [CrossRef]
- Smaropoulos, E.; Cremers, N.A.J. The pro-healing effects of medical grade honey supported by a pediatric case series. Complement. Ther. Med. 2019, 45, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Smaropoulos, E.; Cremers, N.A.J. Medical grade honey for the treatment of extravasation-induced injuries in preterm neonates—A case series. Adv. Neonatal Care 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Smaropoulos, E.; Cremers, N.A.J. Treating severe wounds in pediatrics with medical grade honey: A case series. Clin. Case Rep. 2020, 8, 469–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremers, N.; Belas, A.; Santos Costa, S.; Couto, I.; de Rooster, H.; Pomba, C. In vitro antimicrobial efficacy of two medical grade honey formulations against common high-risk meticillin-resistant staphylococci and Pseudomonas spp. pathogens. Vet. Dermatol. 2020, 31, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.C.; Aygin, D. Honey Dressing in Wound Treatment: A Systematic Review. Complement. Ther. Med. 2020, 51, 102388. [Google Scholar] [CrossRef] [PubMed]
- Merckoll, P.; Jonassen, T.O.; Vad, M.E.; Jeansson, S.L.; Melby, K.K. Bacteria, biofilm and honey: A study of the effects of honey on ‘planktonic’ and biofilm-embedded chronic wound bacteria. Scand. J. Infect. Dis. 2009, 41, 341–347. [Google Scholar] [CrossRef]
- Vallabha, T.; Ragate, A.; Sindagikar, V.; Deshpande, H.; Narasanagi, B. Is Honey an Answer for Eradication of Biofilms? Indian J. Surg. 2018. [Google Scholar] [CrossRef]
- Chatzoulis, G.; Chatzoulis, K.; Spyridopoulos, P.; Pappas, P.; Ploumis, A. Salvage of an infected titanium mesh in a large incisional ventral hernia using medicinal honey and vacuum-assisted closure: A case report and literature review. Hernia 2012, 16, 475–479. [Google Scholar] [CrossRef]
- Mancuso, E.; Tonda-Turo, C.; Ceresa, C.; Pensabene, V.; Connell, S.D.; Fracchia, L.; Gentile, P. Potential of Manuka Honey as a Natural Polyelectrolyte to Develop Biomimetic Nanostructured Meshes with Antimicrobial Properties. Front. Bioeng. Biotechnol. 2019, 7, 344. [Google Scholar] [CrossRef]
- Mandel, H.H.; Sutton, G.A.; Abu, E.; Kelmer, G. Intralesional application of medical grade honey improves healing of surgically treated lacerations in horses. Equine Vet. J. 2020, 52, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Brackman, G.; Garcia-Fernandez, M.J.; Lenoir, J.; De Meyer, L.; Remon, J.P.; De Beer, T.; Concheiro, A.; Alvarez-Lorenzo, C.; Coenye, T. Dressings Loaded with Cyclodextrin-Hamamelitannin Complexes Increase Staphylococcus aureus Susceptibility Toward Antibiotics Both in Single as well as in Mixed Biofilm Communities. Macromol. Biosci. 2016, 16, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Avison, M.B.; Bennett, P.M.; Howe, R.A.; Walsh, T.R. Preliminary analysis of the genetic basis for vancomycin resistance in Staphylococcus aureus strain Mu50. J. Antimicrob. Chemother. 2002, 49, 255–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyibo, S.O.; Jude, E.B.; Tarawneh, I.; Nguyen, H.C.; Harkless, L.B.; Boulton, A.J. A comparison of two diabetic foot ulcer classification systems: The Wagner and the University of Texas wound classification systems. Diabetes Care 2001, 24, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutluoglu, M.; Uzun, G. Pseudomonas infection in a postoperative foot wound. CMAJ 2011, 183, E499. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.C.; Chan, W.W.; Metelitsa, A.I.; Fiorillo, L.; Lin, A.N. Pseudomonas skin infection: Clinical features, epidemiology, and management. Am. J. Clin. Dermatol. 2011, 12, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. S11), S240–S252. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, R.; Cremers, N.A.J.; Leeming, J.P.; van der Werf, E.T. Sweet Relief: Determining the Antimicrobial Activity of Medical Grade Honey Against Vaginal Isolates of Candida albicans. J. Fungi 2019, 5, 85. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.M.P.; Devesa, J.S.P.; Hill, P.B. In vitro efficacy of a honey-based gel against canine clinical isolates of Staphylococcus pseudintermedius and Malassezia pachydermatis. Vet. Dermatol. 2018, 29, 180-e165. [Google Scholar] [CrossRef]
- Burgess, C. Topical vitamins. J. Drugs Dermatol. 2008, 7, s2–s6. [Google Scholar]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef]
- Burke, K.E. Interaction of vitamins C and E as better cosmeceuticals. Dermatol. Ther. 2007, 20, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.H.; Lin, J.Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Selim, M.A.; Monteiro-Riviere, N.A.; Grichnik, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J. Investig. Dermatol. 2005, 125, 826–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinno, S.; Lee, D.S.; Khachemoune, A. Vitamins and cutaneous wound healing. J. Wound Care 2011, 20, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.A. Honey for wound healing, ulcers, and burns; data supporting its use in clinical practice. ScientificWorldJournal 2011, 11, 766–787. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.C.; Pereira, A.P.; Silva, J.R.; Oliveira, L.S.; Resck, M.C.; Grechi, C.O.; Bernardes, M.T.; Olimpio, F.M.; Santos, A.M.; Incerpi, E.K.; et al. Ascorbic acid for the healing of skin wounds in rats. Braz. J. Biol. 2009, 69, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- Schencke, C.; Vasconcellos, A.; Sandoval, C.; Torres, P.; Acevedo, F.; Del Sol, M. Morphometric evaluation of wound healing in burns treated with Ulmo (Eucryphia cordifolia) honey alone and supplemented with ascorbic acid in guinea pig (Cavia porcellus). Burns Trauma 2016, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Moores, J. Vitamin C: A wound healing perspective. Br. J. Community Nurs. 2013, 18 (Suppl. S6), S8–S11. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Ward, S.; Wayne, J.S.; Brophy, D.F.; Fowler, A.A., 3rd; Yager, D.R.; Natarajan, R. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int. Wound J. 2016, 13, 572–584. [Google Scholar] [CrossRef]
- Dickerson, J.W. Ascorbic acid, zinc and wound healing. J. Wound Care 1993, 2, 350–353. [Google Scholar] [CrossRef]
- Majtan, J.; Sojka, M.; Palenikova, H.; Bucekova, M.; Majtan, V. Vitamin C Enhances the Antibacterial Activity of Honey against Planktonic and Biofilm-Embedded Bacteria. Molecules 2020, 25, 992. [Google Scholar] [CrossRef] [Green Version]
- Myrvik, Q.N.; Volk, W.A. Comparative study of the antibacterial properties of ascorbic acid and reductogenic compounds. J. Bacteriol. 1954, 68, 622–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stacpoole, P.W. Role of vitamin C in infectious disease and allergic reactions. Med. Hypotheses 1975, 1, 43–45. [Google Scholar] [CrossRef]
- Rawal, B.D. Bactericidal action of ascorbic acid on Psuedomonas aeruginosa: Alteration of cell surface as a possible mechanism. Chemotherapy 1978, 24, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.; Ashraf, M.A.; Sajid, M.; Shahzad, A.; Rafique, A.; Mahmood, M.S. Evaluation of synergistic antimicrobial effect of vitamins (A, B1, B2, B6, B12, C, D, E and K) with antibiotics against resistant bacterial strains. J. Glob. Antimicrob. Resist. 2018, 13, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Tabrez, E.; Peela, J.; Honnavar, P.D.; Tabrez, S.S.M. Vitamin C: A Preventative, Therapeutic Agent Against Helicobacter pylori. Cureus 2018, 10, e3062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierpaoli, E.; Cirioni, O.; Barucca, A.; Orlando, F.; Silvestri, C.; Giacometti, A.; Provinciali, M. Vitamin E supplementation in old mice induces antimicrobial activity and improves the efficacy of daptomycin in an animal model of wounds infected with methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2011, 66, 2184–2185. [Google Scholar] [CrossRef] [Green Version]
- Provinciali, M.; Cirioni, O.; Orlando, F.; Pierpaoli, E.; Barucca, A.; Silvestri, C.; Ghiselli, R.; Scalise, A.; Brescini, L.; Guerrieri, M.; et al. Vitamin E improves the in vivo efficacy of tigecycline and daptomycin in an animal model of wounds infected with meticillin-resistant Staphylococcus aureus. J. Med. Microbiol. 2011, 60, 1806–1812. [Google Scholar] [CrossRef] [Green Version]
- Kallio, J.; Jaakkola, M.; Maki, M.; Kilpelainen, P.; Virtanen, V. Vitamin C inhibits staphylococcus aureus growth and enhances the inhibitory effect of quercetin on growth of Escherichia coli in vitro. Planta Med. 2012, 78, 1824–1830. [Google Scholar] [CrossRef] [Green Version]
- Verghese, R.; Mathew, S.; David, A. Antimicrobial Activity of Vitamin C Demonstrated on Uropathogenic Escherichia coli and Klebsiella pneumoniae. J. Curr. Res. Sci. Med. 2017, 3, 88. [Google Scholar]
- Avci, P.; Freire, F.; Banvolgyi, A.; Mylonakis, E.; Wikonkal, N.M.; Hamblin, M.R. Sodium ascorbate kills Candida albicans in vitro via iron-catalyzed Fenton reaction: Importance of oxygenation and metabolism. Future Microbiol. 2016, 11, 1535–1547. [Google Scholar] [CrossRef] [Green Version]
- Helgadóttir, S.; Pandit, S.; Mokkapati, V.R.S.S.; Westerlund, F.; Apell, P.; Mijakovic, I. Vitamin C Pretreatment Enhances the Antibacterial Effect of Cold Atmospheric Plasma. Front. Cell. Infect. Microbiol. 2017, 7, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, C.; Fraunfelder, F.T.; Cable, M.; Hardberger, R.E. The effect of ophthalmic ointments on corneal wound healing. Am. J. Ophthalmol. 1973, 76, 193–200. [Google Scholar] [CrossRef]
- Naguib, M.M.; Valvano, M.A. Vitamin E Increases Antimicrobial Sensitivity by Inhibiting Bacterial Lipocalin Antibiotic Binding. mSphere 2018, 3, e00564-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Immunomodulatory and Antimicrobial Effects of Vitamin C. Eur. J. Microbiol. Immunol. 2019, 9, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Ravikumar, V.; Abdel-Haleem, A.M.; Derouiche, A.; Mokkapati, V.; Sihlbom, C.; Mineta, K.; Gojobori, T.; Gao, X.; Westerlund, F.; et al. Low Concentrations of Vitamin C Reduce the Synthesis of Extracellular Polymers and Destabilize Bacterial Biofilms. Front. Microbiol. 2017, 8, 2599. [Google Scholar] [CrossRef] [Green Version]
- Ali Mirani, Z.; Khan, M.N.; Siddiqui, A.; Khan, F.; Aziz, M.; Naz, S.; Ahmed, A.; Khan, S.I. Ascorbic acid augments colony spreading by reducing biofilm formation of methicillin-resistant Staphylococcus aureus. Iran. J. Basic Med. Sci. 2018, 21, 175–180. [Google Scholar] [CrossRef]
- Ong, K.S.; Mawang, C.I.; Daniel-Jambun, D.; Lim, Y.Y.; Lee, S.M. Current anti-biofilm strategies and potential of antioxidants in biofilm control. Expert Rev. Anti-Infect. Ther. 2018, 16, 855–864. [Google Scholar] [CrossRef]
- Chvapil, M.; Gaines, J.A.; Gilman, T. Lanolin and epidermal growth factor in healing of partial-thickness pig wounds. J. Burn. Care Rehabil. 1988, 9, 279–284. [Google Scholar] [CrossRef]
- Brent, N.; Rudy, S.J.; Redd, B.; Rudy, T.E.; Roth, L.A. Sore nipples in breast-feeding women: A clinical trial of wound dressings vs conventional care. Arch. Pediatr. Adolesc. Med. 1998, 152, 1077–1082. [Google Scholar] [CrossRef]
- Dennis, C.L.; Schottle, N.; Hodnett, E.; McQueen, K. An all-purpose nipple ointment versus lanolin in treating painful damaged nipples in breastfeeding women: A randomized controlled trial. Breastfeed. Med. 2012, 7, 473–479. [Google Scholar] [CrossRef]
- Huml, S. Sore nipples. A new look at an old problem through the eyes of a dermatologist. Pract. Midwife 1999, 2, 28–31. [Google Scholar] [PubMed]
- Abou-Dakn, M.; Fluhr, J.W.; Gensch, M.; Wockel, A. Positive effect of HPA lanolin versus expressed breastmilk on painful and damaged nipples during lactation. Skin Pharmacol. Physiol. 2011, 24, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Behera, J. Comprehensive view on chemistry, manufacturing & applications of lanolin extracted from wool pretreatment. Am. J. Eng. Res. 2014, 3, 33–43. [Google Scholar]
- Clark, E.W.; Steel, I. Investigations into biomechanisms of the moisturizing function of lanolin. J. Cosmet. Sci. 1993, 44, 181–195. [Google Scholar]
- Stone, L. Medilan: A hypoallergenic lanolin for emollient therapy. Br. J. Nurs. 2000, 9, 54–57. [Google Scholar] [CrossRef] [Green Version]
- Biondi, O.; Motta, S.; Mosesso, P. Low molecular weight polyethylene glycol induces chromosome aberrations in Chinese hamster cells cultured in vitro. Mutagenesis 2002, 17, 261–264. [Google Scholar] [CrossRef] [Green Version]
- Klonne, D.R.; Dodd, D.E.; Losco, P.E.; Troup, C.M.; Tyler, T.R. Two-week aerosol inhalation study on polyethylene glycol (PEG) 3350 in F-344 rats. Drug Chem. Toxicol. 1989, 12, 39–48. [Google Scholar] [CrossRef]
- Subrahmanyam, N. Addition of antioxidants and polyethylene glycol 4000 enhances the healing properties of honey in burns. Ann. Burns Fire Dis. 1996, 9, 2. [Google Scholar]
- Nalawade, T.M.; Bhat, K.; Sogi, S.H. Bactericidal activity of propylene glycol, glycerine, polyethylene glycol 400, and polyethylene glycol 1000 against selected microorganisms. J. Int. Soc. Prev. Community Dent. 2015, 5, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Leibson, T.; Davies, P.; Nickel, C.; Koren, G. Hyperosmolar metabolic acidosis in burn patients exposed to glycol based topical antimicrobials-A systematic review. Burns 2018, 44, 776–783. [Google Scholar] [CrossRef]
- Kinnunen, T.; Koskela, M. Antibacterial and antifungal properties of propylene glycol, hexylene glycol, and 1,3-butylene glycol in vitro. Acta Derm. Venereol. 1991, 71, 148–150. [Google Scholar] [PubMed]
- Henriques, A.F.; Jenkins, R.E.; Burton, N.F.; Cooper, R.A. The effect of manuka honey on the structure of Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 167–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, A.E.L.; Maddocks, S.E.; Cooper, R.A. Manuka honey is bactericidal against Pseudomonas aeruginosa and results in differential expression of oprF and algD. Microbiology 2012, 158, 3005–3013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouzo, D.; Cokcetin, N.N.; Li, L.; Ballerin, G.; Bottomley, A.L.; Lazenby, J.; Whitchurch, C.B.; Paulsen, I.T.; Hassan, K.A.; Harry, E.J. Characterizing the Mechanism of Action of an Ancient Antimicrobial, Manuka Honey, against Pseudomonas aeruginosa Using Modern Transcriptomics. mSystems 2020, 5, e00106-20. [Google Scholar] [CrossRef] [PubMed]
- Kronda, J.M.; Cooper, R.A.; Maddocks, S.E. Manuka honey inhibits siderophore production in Pseudomonas aeruginosa. J. Appl. Microbiol. 2013, 115, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, S.E.; Jenkins, R.E.; Rowlands, R.S.; Purdy, K.J.; Cooper, R.A. Manuka honey inhibits adhesion and invasion of medically important wound bacteria in vitro. Future Microbiol. 2013, 8, 1523–1536. [Google Scholar] [CrossRef] [Green Version]
- Roberts, A.E.; Maddocks, S.E.; Cooper, R.A. Manuka honey reduces the motility of Pseudomonas aeruginosa by suppression of flagella-associated genes. J. Antimicrob. Chemother. 2015, 70, 716–725. [Google Scholar] [CrossRef] [Green Version]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Molan, P.C. Re-introducing honey in the management of wounds and ulcers—Theory and practice. Ostomy Wound Manag. 2002, 48, 28–40. [Google Scholar]
- Grego, E.; Robino, P.; Tramuta, C.; Giusto, G.; Boi, M.; Colombo, R.; Serra, G.; Chiado-Cutin, S.; Gandini, M.; Nebbia, P. Evaluation of antimicrobial activity of Italian honey for wound healing application in veterinary medicine. Schweiz Arch. Tierheilkd 2016, 158, 521–527. [Google Scholar] [CrossRef]
- Lusby, P.E.; Coombes, A.L.; Wilkinson, J.M. Bactericidal activity of different honeys against pathogenic bacteria. Arch. Med. Res. 2005, 36, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, O.; Dolan, A.; Athman, R.; Power, A.; Gethin, G.; Cowman, S.; Humphreys, H. Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complementary Altern. Med. 2010, 10, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kus, P.M.; Szweda, P.; Jerkovic, I.; Tuberoso, C.I. Activity of Polish unifloral honeys against pathogenic bacteria and its correlation with colour, phenolic content, antioxidant capacity and other parameters. Lett. Appl. Microbiol. 2016, 62, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Bohova, J.; Prochazka, E.; Klaudiny, J. Methylglyoxal may affect hydrogen peroxide accumulation in manuka honey through the inhibition of glucose oxidase. J. Med. Food 2014, 17, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Sindi, A.; Chawn, M.V.B.; Hernandez, M.E.; Green, K.; Islam, M.K.; Locher, C.; Hammer, K. Anti-biofilm effects and characterisation of the hydrogen peroxide activity of a range of Western Australian honeys compared to Manuka and multifloral honeys. Sci. Rep. 2019, 9, 17666. [Google Scholar] [CrossRef]
- Molan, P.; Rhodes, T. Honey: A Biologic Wound Dressing. Wounds 2015, 27, 141–151. [Google Scholar]
- Jeddar, A.; Kharsany, A.; Ramsaroop, U.G.; Bhamjee, A.; Haffejee, I.E.; Moosa, A. The antibacterial action of honey. An in vitro study. S. Afr. Med. J. 1985, 67, 257–258. [Google Scholar]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 2464507. [Google Scholar] [CrossRef]
- Abbas, H.A. Comparative Antibacterial and Antibiofilm Activities of Manuka Honey and Egyptian Clover Honey. Asian J. Appl. Sci. 2014, 2, 110–115. [Google Scholar]
- Gambogou, B.; Khadimallah, H.; Bouacha, M.; Ameyapoh, Y.A. Antibacterial activity of various honey monofloral and polyfloral from different regions of Algeria against uropathogenic Gram-Negative Bacilli. J. Apither. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Minden-Birkenmaier, B.A.; Bowlin, G.L. Honey-Based Templates in Wound Healing and Tissue Engineering. Bioengineering 2018, 5, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossiter, K.; Cooper, A.J.; Voegeli, D.; Lwaleed, B.A. Honey promotes angiogeneic activity in the rat aortic ring assay. J. Wound Care 2010, 19, 440, 442–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aron, M.; Victoria Akinpelu, O.; Dorion, D.; Daniel, S. Otologic safety of manuka honey. J. Otolaryngol. Head Neck Surg. 2012, 41 (Suppl. S1), S21–S30. [Google Scholar]
- Grecka, K.; Kus, P.M.; Worobo, R.W.; Szweda, P. Study of the Anti-Staphylococcal Potential of Honeys Produced in Northern Poland. Molecules 2018, 23, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Products (Manufacturer) | Ingredients | Remark |
|---|---|---|
| Untreated control | Not applicable | No product |
| Medihoney (Derma Sciences, Inc., Toronto, Canada) | 100% Manuka (Leptospermum scoparium) honey | Tube, Lot#: 1937 Expiry date: 2022-09 |
| Revamil (BFactory Health Products B.V., Rhenen, the Netherlands) | 100% pure medicinal honey | Tube, Lot#: AFR Expiry date: 2022-09 |
| MEBO (Moist Exposed Burn Ointment) (Gulf Pharmaceutical Industries, Ras Al Khaimah, United Arab Emirates) | The base of the ointment is composed of beeswax and sesame oil. The main active ingredient is 0.25% beta-sitosterol. Other ingredients include eighteen amino acids, four major fatty acids, vitamins, trace elements, and polysaccharides. | Unknown |
| Melladerm Plus (SanoMed Manufacturing bv, Oostburg, the Netherlands) | 45% honey, glycerine, propylene glycol, and PEG 4000 | Tube, Lot#D8250311 Expiry date: 2023-04 |
| L-Mesitran Ointment (Theo Manufacturing B.V., Maastricht, the Netherlands) | 48% MGH, vitamins C and E, medical-grade hypoallergenic lanolin, sunflower oil, cod liver oil, Calendula officinalis, Aloe barbadensis, and zinc oxide | Tube, Lot#: 0020H Expiry date: 2021-12 |
| L-Mesitran Soft (Theo Manufacturing B.V., Maastricht, the Netherlands) | 40% MGH, vitamins C and E, medical-grade hypoallergenic lanolin, PEG 4000, and propylene glycol | Tube, Lot#: 201908X Expiry date: 2022-07 |
| Raw honey | 40% raw MGH, obtained from the same batch as L-Mesitran Ointment and L-Mesitran Soft (the concentration is similar as in L-Mesitran Soft) | Provided by Triticum Exploitatie BV |
| Vitamins C and E | Used at the same dose as present in L-Mesitran Soft | Provided by Triticum Exploitatie BV |
| Ingredients L-Mesitran Soft without the MGH | All ingredients at the same dose as used in L-Mesitran Soft, excluding MGH | Provided by Triticum Exploitatie BV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleeging, C.C.F.; Coenye, T.; Mossialos, D.; de Rooster, H.; Chrysostomou, D.; Wagener, F.A.D.T.G.; Cremers, N.A.J. Synergistic Antimicrobial Activity of Supplemented Medical-Grade Honey against Pseudomonas aeruginosa Biofilm Formation and Eradication. Antibiotics 2020, 9, 866. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9120866
Pleeging CCF, Coenye T, Mossialos D, de Rooster H, Chrysostomou D, Wagener FADTG, Cremers NAJ. Synergistic Antimicrobial Activity of Supplemented Medical-Grade Honey against Pseudomonas aeruginosa Biofilm Formation and Eradication. Antibiotics. 2020; 9(12):866. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9120866
Chicago/Turabian StylePleeging, Carlos C. F., Tom Coenye, Dimitris Mossialos, Hilde de Rooster, Daniela Chrysostomou, Frank A. D. T. G. Wagener, and Niels A. J. Cremers. 2020. "Synergistic Antimicrobial Activity of Supplemented Medical-Grade Honey against Pseudomonas aeruginosa Biofilm Formation and Eradication" Antibiotics 9, no. 12: 866. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9120866





