Fabrication of Piezoelectric ZnO Nanowires on Laser Textured Copper Substrate to Enhance Catalytic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
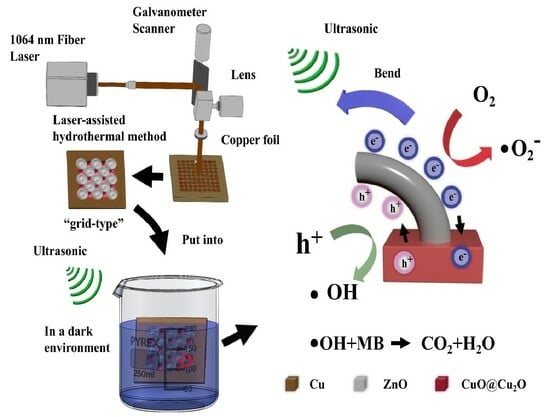
2.2. Preparation of Periodic 3D CuO-Cu2O-ZnO Heterojunction Array
2.3. Mechanical Stability of ZnO Nanowires on Laser Ablated Substrate
2.4. Piezocatalytic Performance Evaluation
2.5. Active Species Trapping
2.6. Characterization
3. Results and Discussion
3.1. Growth of ZnO Nanowires on Laser Textured Copper Substrate
3.2. Modulating the Morphology of ZnO Nanowires
3.3. Piezocatalytic Performance Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, J.; Ren, J.; Jia, Y.; Wu, Z.; Chen, L.; Haugen, N.O.; Huang, H.; Liu, Y. High efficiency bi-harvesting light/vibration energy using piezoelectric zinc oxide nanorods for dye decomposition. Nano Energy 2019, 62, 376–383. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, Z.; Wu, M.; Liu, H.; Zhang, X.; Wang, Z.; Wang Zhong, L.; Li, L. Enhanced Piezo-Photoelectric Catalysis with Oriented Carrier Migration in Asymmetric Au-ZnO Nanorod Array. Small 2020, 16, 1907603. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.; Zhao, B.; Thai, Q.; Lita, A.; Dalal, N.S.; Kroto, H.W.; Acquah, S.F.A. A synergistic approach to light-free catalysis using zinc oxide embedded multi-walled carbon nanotube paper. Carbon 2014, 77, 705–709. [Google Scholar] [CrossRef]
- Chen, L.; Jia, Y.; Zhao, J.; Ma, J.; Wu, Z.; Yuan, G.; Cui, X. Strong piezocatalysis in barium titanate/carbon hybrid nanocomposites for dye wastewater decomposition. J. Colloid Interface Sci. 2021, 586, 758–765. [Google Scholar] [CrossRef]
- Jia, S.; Su, Y.; Zhang, B.; Zhao, Z.; Li, S.; Zhang, Y.; Li, P.; Xu, M.; Ren, R. Few-layer MoS2 nanosheet-coated KNbO3 nanowire heterostructures: Piezo-photocatalytic effect enhanced hydrogen production and organic pollutant degradation. Nanoscale 2019, 11, 7690–7700. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jile, H.; Chen, Z.; Xu, D.; Yi, Z.; Chen, X.; Chen, J.; Yao, W.; Wu, P.; Yi, Y. Fabrication of ZnO@MoS2 Nanocomposite Heterojunction Arrays and Their Photoelectric Properties. Micromachines 2020, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Sun, Z.; Li, K.; Chen, Y.; Cao, Y.; Zhang, S.; Feng, L. Integrated oil separation and water purification by a double-layer TiO2-based mesh. Energy Environ. Sci. 2013, 6, 1147–1151. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Ruan, M.; Guo, Z.; E, L.; Zhao, W.; Zhao, D.; Wu, X.; Chen, D. Enhanced piezoelectric-effect-assisted photoelectrochemical performance in ZnO modified with dual cocatalysts. Appl. Catal. B-Environ. 2020, 262, 118279. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, S.; Lin, L.; Jing, Q.; Lin, Z.-H.; Niu, S.; Wu, Z.; Wang, Z.L. Rotary Triboelectric Nanogenerator Based on a Hybridized Mechanism for Harvesting Wind Energy. Acs Nano 2013, 7, 7119–7125. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, L.; Xu, Q.; Zheng, Y.; Qin, Y.; Wang, Z.L. Two dimensional woven nanogenerator. Nano Energy 2013, 2, 749–753. [Google Scholar] [CrossRef]
- Wang, X.; Song, J.; Liu, J.; Wang, Z.L. Direct-current nanogenerator driven by ultrasonic waves. Science 2007, 316, 102–105. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Wang, Z.; Zhou, Y.; Qin, Y.; Wang, Z.L. Piezotronic Effect Enhanced Photocatalysis in Strained Anisotropic ZnO/TiO2 Nanoplatelets via Thermal Stress. Acs Nano 2016, 10, 2636–2643. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pradel, K.C.; Jing, Q.; Wu, J.M.; Zhang, F.; Zhou, Y.; Zhang, Y.; Wang, Z.L. Thermoelectric Nanogenerators Based on Single Sb-Doped ZnO Micro/Nanobelts. Acs Nano 2012, 6, 6984–6989. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Qiu, Y.; Zhang, H.; Ji, J.; Lei, J.; Luo, Y.; Zhao, Y.; Hu, L. Piezoelectric nanogenerator with 3D-ZnO micro-thornyballs prepared by chemical vapour deposition. J. Mater. Sci. -Mater. Electron. 2015, 26, 742–746. [Google Scholar] [CrossRef]
- Xu, S.; Wei, Y.; Liu, J.; Yang, R.; Wang, Z.L. Integrated Multilayer Nanogenerator Fabricated Using Paired Nanotip-to-Nanowire Brushes. Nano Lett. 2008, 8, 4027–4032. [Google Scholar] [CrossRef]
- Zhang, F.; Ding, Y.; Zhang, Y.; Zhang, X.; Wang, Z.L. Piezo-phototronic Effect Enhanced Visible and Ultraviolet Photodetection Using a ZnO-CdS Core-Shell Micro/nanowire. Acs Nano 2012, 6, 9229–9236. [Google Scholar] [CrossRef]
- Xiong, D.; Deng, W.; Tian, G.; Gao, Y.; Chu, X.; Yan, C.; Jin, L.; Su, Y.; Yan, W.; Yang, W. A piezo-phototronic enhanced serrate-structured ZnO-based heterojunction photodetector for optical communication. Nanoscale 2019, 11, 3021–3027. [Google Scholar] [CrossRef]
- Hong, K.-S.; Xu, H.; Konishi, H.; Li, X. Direct Water Splitting Through Vibrating Piezoelectric Microfibers in Water. J. Phys. Chem. Lett. 2010, 1, 997–1002. [Google Scholar] [CrossRef]
- Wang, P.; Tang, Q.; Zhang, L.; Xu, M.; Sun, L.; Sun, S.; Zhang, J.; Wang, S.; Liang, X. Ultrasmall Barium Titanate Nanoparticles for Highly Efficient Hypoxic Tumor Therapy via Ultrasound Triggered Piezocatalysis and Water Splitting. Acs Nano 2021, 15, 11326–11340. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jia, Y.; Xiao, L.; Wu, Z. Strong vibration-catalysis of ZnO nanorods for dye wastewater decolorization via piezo-electro-chemical coupling. Chemosphere 2018, 193, 1143–1148. [Google Scholar] [CrossRef]
- Hong, D.; Zang, W.; Guo, X.; Fu, Y.; He, H.; Sun, J.; Xing, L.; Liu, B.; Xue, X. High Piezo-photocatalytic Efficiency of CuS/ZnO Nanowires Using Both Solar and Mechanical Energy for Degrading Organic Dye. ACS Appl. Mater. Interfaces 2016, 8, 21302–21314. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, M.; Gao, Y.; Bao, B.; Wang, S. ZnO-Zn/CNT hybrid film as light-free nanocatalyst for degradation reaction. Nano Energy 2013, 2, 1329–1336. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y. Facile Synthesis and Enhanced Photocatalytic Performance of Flower-like ZnO Hierarchical Microstructures. J. Phys. Chem. C 2010, 114, 890–896. [Google Scholar] [CrossRef]
- Alenezi, M.R.; Henley, S.J.; Emerson, N.G.; Silva, S.R.P. From 1D and 2D ZnO nanostructures to 3D hierarchical structures with enhanced gas sensing properties. Nanoscale 2014, 6, 235–247. [Google Scholar] [CrossRef]
- Xu, P.; Wang, P.; Wang, Q.; Wei, R.; Li, Y.; Xin, Y.; Zheng, T.; Hu, L.; Wang, X.; Zhang, G. Facile synthesis of Ag2O/ZnO/rGO heterojunction with enhanced photocatalytic activity under simulated solar light: Kinetics and mechanism. J. Hazard. Mater. 2021, 403, 124011. [Google Scholar] [CrossRef]
- Huan, H.; Jile, H.; Tang, Y.; Li, X.; Yi, Z.; Gao, X.; Chen, X.; Chen, J.; Wu, P. Fabrication of ZnO@Ag@Ag3PO4 Ternary Heterojunction: Superhydrophilic Properties, Antireflection and Photocatalytic Properties. Micromachines 2020, 11, 309. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Huang, X.; Zhang, L.; Gao, F.; Lei, R.; Jiang, C.; Feng, W.; Liu, P. Tuning piezoelectric field for optimizing the coupling effect of piezo-photocatalysis. Appl. Catal. B-Environ. 2020, 278, 119291. [Google Scholar] [CrossRef]
- Guo, X.; Fu, Y.; Hong, D.; Yu, B.; He, H.; Wang, Q.; Xing, L.; Xue, X. High-efficiency sono-solar-induced degradation of organic dye by the piezophototronic/photocatalytic coupling effect of FeS/ZnO nanoarrays. Nanotechnology 2016, 27, 375704. [Google Scholar] [CrossRef] [PubMed]
- Sahay, R.; Sundaramurthy, J.; Kumar, P.S.; Thavasi, V.; Mhaisalkar, S.G.; Ramakrishna, S. Synthesis and characterization of CuO nanofibers, and investigation for its suitability as blocking layer in ZnO NPs based dye sensitized solar cell and as photocatalyst in organic dye degradation. J. Solid State Chem. 2012, 186, 261–267. [Google Scholar] [CrossRef]
- Li, P.; Zhou, Y.; Zhao, Z.; Xu, Q.; Wang, X.; Xiao, M.; Zou, Z. Hexahedron Prism-Anchored Octahedronal CeO2: Crystal Facet-Based Homojunction Promoting Efficient Solar Fuel Synthesis. J. Am. Chem. Soc. 2015, 137, 9547–9550. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Zhao, Y.; Ding, Y.; Zhang, Z.; Jiang, T.; Wang, Z.L.; Li, L. Piezo-phototronic effect boosted catalysis in plasmonic bimetallic ZnO heterostructure with guided fermi level alignment. Mater. Today Nano 2022, 18, 375704. [Google Scholar] [CrossRef]
- Ma, W.; Lv, M.; Cao, F.; Fang, Z.; Feng, Y.; Zhang, G.; Yang, Y.; Liu, H. Synthesis and characterization of ZnO-GO composites with their piezoelectric catalytic and antibacterial properties. J. Environ. Chem. Eng. 2022, 10, 107840. [Google Scholar] [CrossRef]
- Fageria, P.; Gangopadhyay, S.; Pande, S. Synthesis of ZnO/Au and ZnO/Ag nanoparticles and their photocatalytic application using UV and visible light. RSC Adv. 2014, 4, 24962–24972. [Google Scholar] [CrossRef]
- Guo, M.; Diao, P.; Cai, S.M. Hydrothermal growth of well-aligned ZnO nanorod arrays: Dependence of morphology and alignment ordering upon preparing conditions. J. Solid State Chem. 2005, 178, 1864–1873. [Google Scholar] [CrossRef]
- Pauporte, T.; Lincot, D. Heteroepitaxial electrodeposition of zinc oxide films on gallium nitride. Appl. Phys. Lett. 1999, 75, 3817–3819. [Google Scholar] [CrossRef]
- Scarpellini, D.; Paoloni, S.; Medaglia, P.G.; Pizzoferrato, R.; Orsini, A.; Falconi, C. Structural and optical properties of dense vertically aligned ZnO Mark nanorods grown onto silver and gold thin films by galvanic effect with iron contamination. Mater. Res. Bull. 2015, 65, 231–237. [Google Scholar] [CrossRef]
- Zheng, Z.; Lim, Z.S.; Peng, Y.; You, L.; Chen, L.; Wang, J. General Route to ZnO Nanorod Arrays on Conducting Substrates via Galvanic-cell-based approach. Sci. Rep. 2013, 3, 2434. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Yang, Q.; Guo, C.; Chen, F.; Hou, X. A review of femtosecond laser-structured superhydrophobic or underwater superoleophobic porous surfaces/materials for efficient oil/water separation. RSC Adv. 2019, 9, 12470–12495. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, C.; Chen, Y.; Nie, Z. Research Progress on the Preparation and Applications of Laser-Induced Graphene Technology. Nanomaterials 2022, 12, 2336. [Google Scholar] [CrossRef]
- Tour, J. Laser-induced graphene. Abstr. Pap. Am. Chem. Soc. 2019, 257, 1609–1620. [Google Scholar]
- Wu, M.L.; Ren, C.Z.; Xu, H.Z.; Zhou, C.L. Fabrication of a bionic microstructure on a C/SiC brake lining surface: Positive applications of surface defects for surface wetting control. Appl. Surf. Sci. 2018, 440, 669–679. [Google Scholar] [CrossRef]
- Kisala, J.; Gnilitskyi, I.; Cieniek, B.; Krzeminski, P.; Marchewka, M.; Barylyak, A.; Bobitski, Y. Synthesis of Micro-Spikes and Herringbones Structures by Femtosecond Laser Pulses on a Titanium Plate-A New Material for Water Organic Pollutants Degradation. Materials 2021, 14, 5556. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Shen, F.; Cui, J.; Zhang, Y.; Yan, H.; Carlos, S.S.J. Electrophoretic Deposition of Graphene Oxide on Laser-Ablated Copper Mesh for Enhanced Oil/Water Separation. Coatings 2019, 9, 157. [Google Scholar] [CrossRef]
- Zhou, R.; Lin, S.; Shen, F.; Khew, S.Y.; Hong, M. A universal copper mesh with on-demand wettability fabricated by pulsed laser ablation for oil/water separation. Surf. Coat. Technol. 2018, 348, 73–80. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Wu, J.M. Synergistically catalytic activities of BiFeO3/TiO2 core-shell nanocomposites for degradation of organic dye molecule through piezophototronic effect. Nano Energy 2019, 56, 74–81. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Feoktistov, D.V.; Orlova, E.G.; Batishcheva, K.; Ilenok, S.S. Unification of the textures formed on aluminum after laser treatment. Appl. Surf. Sci. 2019, 469, 974–982. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, C.; Zhou, R.; Ji, R.; Hong, M. Hybrid micro/nano-structure formation by angular laser texturing of Si surface for surface enhanced Raman scattering. Opt. Express 2016, 24, 10352–10358. [Google Scholar] [CrossRef]
- Tian, J.-H.; Hu, J.; Li, S.-S.; Zhang, F.; Liu, J.; Shi, J.; Li, X.; Tian, Z.-Q.; Chen, Y. Improved seedless hydrothermal synthesis of dense and ultralong ZnO nanowires. Nanotechnology 2011, 22, 245601. [Google Scholar] [CrossRef]
- Weintraub, B.; Deng, Y.; Wang, Z.L. Position-controlled seedless growth of ZnO nanorod arrays on a polymer substrate via wet chemical synthesis. J. Phys. Chem. C 2007, 111, 10162–10165. [Google Scholar] [CrossRef]
- Pham Van, T.; Le Thi Quynh, N.; Hong Hanh, M.; Nguyen Viet, T.; Sai Cong, D.; Nguyen Canh, V.; Do Trung, K. Zinc Oxide Nanorods Grown on Printed Circuit Board for Extended-Gate Field-Effect Transistor pH Sensor. J. Electron. Mater. 2017, 46, 3732–3737. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Yeom, J. A Floatable Piezo-Photocatalytic Platform Based on Semi-Embedded ZnO Nanowire Array for High-Performance Water Decontamination. Nano-Micro Lett. 2019, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Weng, Z.; Cao, L.; Li, L.; Liang, K.; Lian, Z.; Xu, J.; Wang, Y.; Zhang, Y.; Song, Z.; et al. Fabrication of oil-water separation stainless steel mesh via direct laser interference lithography, candle soot deposition, and thermal treatment. J. Laser Appl. 2019, 31, 012003. [Google Scholar] [CrossRef]
- Khew, S.Y.; Tan, C.F.; Yan, H.; Lin, S.; Thian, E.S.; Zhou, R.; Hong, M. Nanosecond laser ablation for enhanced adhesion of CuO nanowires on copper substrate and its application for oil-water separation. Appl. Surf. Sci. 2019, 465, 995–1002. [Google Scholar] [CrossRef]
- Yan, H.; Xiao, X.; Chen, Z.; Chen, Y.; Zhou, R.; Wang, Z.; Hong, M. Realization of adhesion enhancement of CuO nanowires growth on copper substrate by laser texturing. Opt. Laser Technol. 2019, 119, 012003. [Google Scholar] [CrossRef]
- He, Y.; Yanagida, T.; Nagashima, K.; Zhuge, F.; Meng, G.; Xu, B.; Klamchuen, A.; Rahong, S.; Kanai, M.; Li, X.; et al. Crystal-Plane Dependence of Critical Concentration for Nucleation on Hydrothermal ZnO Nanowires. J. Phys. Chem. C 2013, 117, 1197–1203. [Google Scholar] [CrossRef]
- Singh, J.; Juneja, S.; Soni, R.K.; Bhattacharya, J. Sunlight mediated enhanced photocatalytic activity of TiO2 nanoparticles functionalized CuO-Cu2O nanorods for removal of methylene blue and oxytetracycline hydrochloride. J. Colloid Interface Sci. 2021, 590, 60–71. [Google Scholar] [CrossRef]
- Shu, X.; Zheng, H.; Xu, G.; Zhao, J.; Cui, L.; Cui, J.; Qin, Y.; Wang, Y.; Zhang, Y.; Wu, Y. The anodization synthesis of copper oxide nanosheet arrays and their photoelectrochemical properties. Appl. Surf. Sci. 2017, 412, 505–516. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, X.; Song, X.; Liang, S.; Wang, L.; Yang, Z. Bottom-up assembly of hierarchical Cu2O nanospheres: Controllable synthesis, formation mechanism and enhanced photochemical activities. Crystengcomm 2012, 14, 3545–3553. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Y.; Chen, C.; Yang, S. One-dimensional ZnO micro/nanostructures: Deep insight into the growth mechanism and fine control of the microscopic morphology. Dalton Trans. 2021, 50, 3011–3019. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yao, B.; Zhang, W.; He, Y.; Yu, Y.; Niu, J. Fabrication of PVDF-based piezocatalytic active membrane with enhanced oxytetracycline degradation efficiency through embedding few-layer E-MoS2 nanosheets. Chem. Eng. J. 2021, 415, 129000. [Google Scholar] [CrossRef]
- Zhao, Y.; Low, Z.-X.; Pan, Y.; Zhong, Z.; Gao, G. Universal water disinfection by piezoelectret aluminium oxide-based electroporation and generation of reactive oxygen species. Nano Energy 2022, 92, 106749. [Google Scholar] [CrossRef]
- Wan, L.; Tian, W.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. Hydrophilic porous PVDF membrane embedded with BaTiO3 featuring controlled oxygen vacancies for piezocatalytic water cleaning. Nano Energy 2022, 94, 106930. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Dong, S.; Wang, P.; Chen, W.; Lu, Z.; Ye, D.; Pan, B.; Wu, D.; Vecitis, C.D.; et al. Ultrasonic activation of inert poly(tetrafluoroethylene) enables piezocatalytic generation of reactive oxygen species. Nat. Commun. 2021, 12, 3508. [Google Scholar] [CrossRef]
- Ruan, S.; Huang, W.; Zhao, M.; Song, H.; Gao, Z. A Z-scheme mechanism of the novel ZnO/CuO n-n heterojunction for photocatalytic degradation of Acid Orange 7. Mater. Sci. Semicond. Process. 2020, 107, 104835. [Google Scholar] [CrossRef]
- Siripala, W.; Ivanovskaya, A.; Jaramillo, T.F.; Baeck, S.H.; McFarland, E.W. A Cu2O/TiO2 heterojunction thin film cathode for photoelectrocatalysis. Sol. Energy Mater. Sol. Cells 2003, 77, 229–237. [Google Scholar] [CrossRef]
- Xue, X.; Zang, W.; Deng, P.; Wang, Q.; Xing, L.; Zhang, Y.; Wang, Z.L. Piezo-potential enhanced photocatalytic degradation of organic dye using ZnO nanowires. Nano Energy 2015, 13, 414–422. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhou, R.; Yan, H.; Liu, H. Fabrication of Piezoelectric ZnO Nanowires on Laser Textured Copper Substrate to Enhance Catalytic Properties. Coatings 2023, 13, 1963. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings13111963
Wang H, Zhou R, Yan H, Liu H. Fabrication of Piezoelectric ZnO Nanowires on Laser Textured Copper Substrate to Enhance Catalytic Properties. Coatings. 2023; 13(11):1963. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings13111963
Chicago/Turabian StyleWang, Hongbin, Rui Zhou, Huangping Yan, and Hongjun Liu. 2023. "Fabrication of Piezoelectric ZnO Nanowires on Laser Textured Copper Substrate to Enhance Catalytic Properties" Coatings 13, no. 11: 1963. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings13111963