Postmortem and Antemortem Forensic Assessment of Pediatric Fracture Healing from Radiographs and Machine Learning Classification
Abstract
:Simple Summary
Abstract
1. Introduction
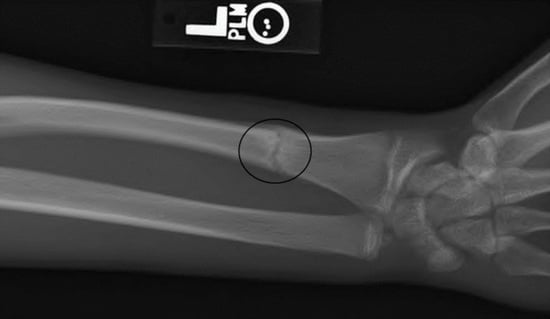
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyons, R.A.; Delahunty, A.; Kraus, D.; Heaven, M.; McCabe, M.; Allen, H.; Nash, P. Children’s fractures: A population based study. Inj. Prev. 1999, 5, 129–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiScala, C.; Sege, R.; Li, G.; Reece, R.M. Child abuse and unintentional injuries: A 10-year retrospective. Arch. Pediatr. Adolesc. Med. 2000, 154, 16–22. [Google Scholar] [PubMed]
- Frick, S.L.; Jones, E. Skeletal growth, development, and healing as related to pediatric trauma. In Green’s Skeletal Trauma in Children; Mencio, G.A., Swiontkowski, M.F., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2014; pp. 1–15. [Google Scholar]
- Baig, M. A review of epidemiological distribution of different types of fractures in paediatric age. Cureus 2017, 9, e1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Boer, H.H.; Van der Merwe, A.E.; Hammer, S.; Steyn, M.; Maat, G.J.R. Assessing posttraumatic time interval in human dry bone. Int. J. Osteoarchaeol. 2015, 25, 98–109. [Google Scholar] [CrossRef]
- Corrales, L.A.; Morshed, S.; Bhandari, M.; Miclau III, T. Variability in the assessment of fracture-healing in orthopaedic trauma studies. J. Bone Jt. Surg. 2008, 90, 1862–1868. [Google Scholar] [CrossRef]
- Johnson, K. Skeletal aspects of non-accidental injury. In Calcium and Bone Disorders in Children and Adolescents; Allgrove, J., Shaw, N., Eds.; Karger Publishers: Basel, Switzerland, 2009; Volume 16, pp. 233–245. [Google Scholar]
- Drury, A.; Cunningham, C. Determining when a fracture occurred: Does the method matter? Analysis of the similarity of three different methods for estimating time since fracture of juvenile long bones. J. Forensic Leg. Med. 2018, 53, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.S.; Kazam, J.J.; Fufa, D.; Bartolotta, R.J. Radiologic evaluation of fracture healing. Skeletal Radiol. 2019, 48, 349–361. [Google Scholar] [CrossRef]
- Prosser, I.; Maguire, S.; Harrison, S.K.; Mann, M.; Sibert, J.R.; Kemp, A.M. How old is this fracture? Radiologic dating of fractures in children: A systematic review. Am. J. Roentgenol. 2005, 184, 1282–1286. [Google Scholar] [CrossRef] [Green Version]
- Cappella, A.; de Boer, H.H.; Cammilli, P.; De Angelis, D.; Messina, C.; Sconfienza, L.M.; Sardanelli, F.; Sforza, C.; Cattaneo, C. Histologic and radiological analysis on bone fractures: Estimation of posttraumatic survival time in skeletal trauma. Forensic Sci. Int. 2019, 302, 109909. [Google Scholar] [CrossRef]
- Moraitis, K.; Spiliopoulou, C. Identification and differential diagnosis of perimortem blunt force trauma in tubular long bones. Forensic Sci. Med. Pathol. 2006, 2, 221–229. [Google Scholar] [CrossRef]
- Klotzbach, H.; Delling, G.; Richter, E.; Sperhake, J.; Püschel, K. Post-mortem diagnosis and age estimation of infants’ fractures. Int. J. Leg. Med. 2003, 117, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, P.K.; Marks Jr, S.C.; Spevak, M.R.; Belanger, P.L.; Richmond, J.M. Extension of growth-plate cartilage into the metaphysis: A sign of healing fracture in abused infants. Am. J. Roentgenol. 1991, 156, 775–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinman, P.K.; Walters, M.M. Dating fractures. In Diagnostic Imaging of Child Abuse, 3rd ed.; Kleinman, P.K., Ed.; Cambridge University Press: Cambridge, UK, 2015; pp. 208–216. [Google Scholar] [CrossRef]
- Bachim, A.; Harper, N.S. Fractures and skeletal injuries. In A Practical Guide to the Evaluation of Child Physical Abuse and Neglect, 3rd ed.; Giardino, A.P., Lyn, M.A., Giardino, E.R., Eds.; Springer: New York, NY, USA, 2009; pp. 133–194. [Google Scholar] [CrossRef]
- Carty, H. Fractures caused by child abuse. J. Bone Jt. Surg. Br. 1993, 75, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Paediatrics and Child Health. Child Protection Evidence—Systematic Review on Fractures. Available online: https://www.rcpch.ac.uk/sites/default/files/2020-10/Chapter%20Fractures_Update_280920.pdf (accessed on 18 April 2022).
- Vannabouathong, C.; Sprague, S.; Bhandari, M. Guidelines for fracture healing assessments in clinical trials. Part I: Definitions and endpoint committees. Injury 2011, 42, 314–316. [Google Scholar] [CrossRef]
- Firoozabadi, R.; Morshed, S.; Engelke, K.; Prevrhal, S.; Fierlinger, A.; Miclau III, T.; Genant, H.K. Qualitative and quantitative assessment of bone fragility and fracture healing using conventional radiography and advanced imaging technologies—focus on wrist fractures. J. Orthop. Trauma 2008, 22, S83–S90. [Google Scholar] [CrossRef]
- Kooistra, B.W.; Dijkman, B.G.; Busse, J.W.; Sprague, S.; Schemitsch, E.H.; Bhandari, M. The radiographic union scale in tibial fractures: Reliability and validity. J. Orthop. Trauma 2010, 24, S81–S86. [Google Scholar] [CrossRef]
- Cekic, E.; Alici, E.; Yesil, M. Reliability of the radiographic union score for tibial fractures. Acta Orthop. Traumatol. Turc. 2014, 48, 533–540. [Google Scholar] [CrossRef]
- Whelan, D.; Bhandari, M.; McKee, M.; Guyatt, G.; Kreder, H.; Stephen, D.; Schemitsch, E. Interobserver and intraobserver variation in the assessment of the healing of tibial fractures after intramedullary fixation. J. Bone Jt. Surg. Br. 2002, 84, 15–18. [Google Scholar] [CrossRef]
- Patel, S.P.; Anthony, S.G.; Zurakowski, D.; Didolkar, M.M.; Kim, P.S.; Wu, J.S.; Kung, J.W.; Dolan, M.; Rozental, T.D. Radiographic scoring system to evaluate union of distal radius fractures. J. Hand Surg. 2014, 39, 1471–1479. [Google Scholar] [CrossRef]
- Prosser, I.; Lawson, Z.; Evans, A.; Harrison, S.; Morris, S.; Maguire, S.; Kemp, A.M. A timetable for the radiologic features of fracture healing in young children. Am. J. Roentgenol. 2012, 198, 1014–1020. [Google Scholar] [CrossRef]
- Calori, G.M.; Albisetti, W.; Agus, A.; Iori, S.; Tagliabue, L. Risk factors contributing to fracture non-unions. Inj. Int. J. Care Inj. 2007, 38, S11–S18. [Google Scholar] [CrossRef]
- Chapman, S. The radiological dating of injuries. Arch. Dis. Child. 1992, 67, 1063–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaston, M.S.; Simpson, A.H.R.W. Inhibition of fracture healing. J. Bone Jt. Surg. 2007, 89, 1553–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malone, C.A.; Sauer, N.J.; Fenton, T.W. A radiographic assessment of pediatric fracture healing and time since injury. J. Forensic Sci. 2011, 56, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Perren, S.M. Evolution of the internal fixation of long bone fractures. J. Bone Jt. Surg. 2002, 84, 1093–1110. [Google Scholar] [CrossRef]
- Kayal, R.; Tsatsas, D.; Bauer, M.; Allen, B.; Al-Sebaei, M.O.; Kakar, S.; Leone, C.W.; Morgan, E.F.; Gerstenfeld, L.C.; Einhorn, T.A.; et al. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J. Bone Miner. Res. 2007, 22, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Perkins, R.; Skirving, A.P. Callus formation and the rate of healing of femoral fractures in patients with head injuries. J. Bone Jt. Surg. 1987, 69, 521–524. [Google Scholar] [CrossRef] [Green Version]
- Pickett, T.A. The challenges of accurately estimating time of long bone injury in children. J. Forensic Leg. Med. 2015, 33, 105–110. [Google Scholar] [CrossRef]
- Rang, M.; Wenger, D.R. Children are not just small adults. In Rang’s Children’s Fractures, 3rd ed.; Rang, M., Pring, M.E., Wenger, D.R., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 1–10. [Google Scholar]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Cunha, E.; Pinheiro, J. Bone Pathology and Antemortem Trauma. In Forensic Anthropology; Houck, M.M., Ed.; Academic Press: London, UK, 2017; pp. 177–184. [Google Scholar]
- Islam, O.; Soboleski, D.; Symons, S.; Davidson, L.K.; Ashworth, M.A.; Babyn, P. Development and duration of radiographic signs of bone healing in children. Am. J. Roentgenol. 2000, 175, 75–78. [Google Scholar] [CrossRef]
- Sanchez, T.R.; Nguyen, H.; Palacios, W.; Doherty, M.; Coulter, K. Retrospective evaluation and dating of non-accidental rib fractures in infants. Clin. Radiol. 2013, 68, e467–e471. [Google Scholar] [CrossRef] [PubMed]
- Skak, S.V.; Jensen, T.T. Femoral shaft fracture in 265 children: Log-normal correlation with age of speed of healing. Acta Orthop. Scand. 1988, 59, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Tritella, S.; Obertová, Z.; Sconfienza, L.M.; Collini, F.; Cristini, E.; Amadasi, A.; Ciprandi, B.; Spairani, R.; Albano, D.; Viero, A.; et al. Multi-rater agreement using the adapted fracture healing scale (AFHS) for the assessment of tubular bones on conventional radiographs: Preliminary study. J. Forensic Sci. 2020, 65, 2112–2116. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, R.W. Fracture Healing. In Radiology of Skeletal Trauma, 3rd ed.; Rogers, L.F., Ed.; Churchill Livingstone: Philadelphia, PA, USA, 2002; Volume 1, pp. 203–230. [Google Scholar]
- Leventhal, J.M.; Thomas, S.A.; Rosenfield, N.S.; Markowitz, R.I. Fractures in young children: Distinguishing child abuse from unintentional injuries. Am. J. Dis. Child. 1993, 147, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Messer, D.L.; Adler, B.H.; Brink, F.W.; Xiang, H.; Agnew, A.M. Radiographic timelines for pediatric healing fractures: A systematic review. Pediatr. Radiol. 2020, 50, 1041–1048. [Google Scholar] [CrossRef]
- Ousley, S.D. Patricia (Pediatric Radiology Interactive Atlas). Available online: http://math.mercyhurst.edu/~sousley/databases/radiographic_database/ (accessed on 8 June 2019).
- Rivara, F.P.; Parish, R.A.; Mueller, B.A. Extremity injuries in children: Predictive value of clinical findings. Pediatrics 1986, 78, 803–807. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wright, M.N.; Ziegler, A. Ranger: A fast implementation of random forests for high dimensional data in C++ and R. J. Stat. Softw. 2017, 77, 1–17. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Random Forests; University of Cincinnati: Cincinnati, OH, USA, 2018. Available online: https://uc-r.github.io/random_forests (accessed on 15 May 2019).
- Ridgeway, G. Generalized Boosted Models: A Guide to the Gbm Package. 2020. Available online: https://pbil.univ-lyon1.fr/CRAN/web/packages/gbm/vignettes/gbm.pdf (accessed on 15 May 2019).
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Greenwell, B.; Boehmke, B.; Cunningham, J.; GBM Developers. gbm: Generalized Boosted Regression Models, R Package Version 2.1.8. 2020. Available online: https://CRAN.R-project.org/package=gbm (accessed on 15 May 2019).
- Gradient Boosting Machines. In UC Business Analytics R Programming Guide; University of Cincinnati: Cincinnati, OH, USA, 2018; Available online: http://uc-r.github.io/gbm_regression (accessed on 15 May 2019).
- NIST. What are outliers in the data? In e-Handbook of Statistical Methods; NIST: Gaithersburg, MD, USA, 2012. [Google Scholar] [CrossRef]
- Cappella, A.; Amadasi, A.; Gaudio, D.; Gibelli, D.; Borgonovo, S.; Di Giancamillo, M.; Cattaneo, C. The application of cone-beam CT in the aging of bone calluses: A new perspective? Int. J. Leg. Med. 2013, 127, 1139–1144. [Google Scholar] [CrossRef]
- Love, J.C.; Derrick, S.M.; Wiersema, J.M. Skeletal examination method. In Skeletal Atlas of Child Abuse; Humana Press: Totowa, NJ, USA, 2011; pp. 1–8. [Google Scholar]
- Messer, D.; Adler, B.; Brink, F.; Xiang, H.; Agnew, A. The influence of age on pediatric fracture healing: A radiographic approach. Pediatr. Radiol. 2018, 48, 5262. [Google Scholar]
- Franklin, D. Forensic age estimation in human skeletal remains: Current concepts and future directions. Leg. Med. 2010, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Krishan, K.; Chatterjee, P.M.; Kanchan, T.; Kaur, S.; Baryah, N.; Singh, R.K. A review of sex estimation techniques during examination of skeletal remains in forensic anthropology casework. Forensic Sci. Int. 2016, 261, 165.e1–165.e8. [Google Scholar] [CrossRef] [PubMed]
- Stull, K.E.; Cirillo, L.E.; Cole, S.J.; Hulse, C.N. Subadult sex estimation and KidStats. In Sex Estimation of the Human Skeleton; Klales, A.R., Ed.; Academic Press: London, UK, 2020; pp. 219–242. [Google Scholar] [CrossRef]
- Kowal-Vern, A.; Paxton, T.P.; Ros, S.P.; Lietz, H.; Fitzgerald, M.; Gamelli, R.L. Fractures in the under-3-year-old age cohort. Clin. Pediatr. 1992, 31, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Uhthoff, H.K.; Poitras, P.; Backman, D.S. Internal plate fixation of fractures: Short history and recent developments. J. Orthop. Sci. 2006, 11, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Ousley, S.; Daly, S.; Frazee, K.; Stull, K. A Radiographic Database for Estimating Biological Parameters in Modern Subadults; National Institute of Justice: Washington, DC, USA; Department of Anthropology/Archaeology, Mercyhurst University: Erie, PA, USA, 2013; p. 59. [Google Scholar]






| Stage | Stage Description | Mean Healing Time (Days) | Range | SD |
|---|---|---|---|---|
| 1 | No healing: sharp fracture lines, absence of bridging and callus formation | 3.3 | 0–14 | 3.4 |
| 2 | Granulation: beginning of resorption along fracture line, “fluffy” callus formation, blurring of fracture line, absence of a complete mature callus | 21 | 4–50 | 10.5 |
| 3 | Callus: mature callus formation around fracture site; callus bulging over site and demonstrating a radiopaque appearance, fracture line visible but may be blurred | 38.4 | 15–75 | 13.4 |
| 4 | Bridging: fracture gap is connected across the fracture site in some, but not all areas (<50%), blurring of the fracture line, callus may still be present | 43.9 | 24–93 | 15.2 |
| 5 | Clinical Union: fracture line is significantly blurred; fracture line is connected in most areas (more than 50%), callus presence minimal | 65.2 | 24–156 | 48.2 |
| 6 | Completion: no evidence of fracture line, callus presence minimal or not observable | 313.3 | 42–750 | 235.7 |
| Criterion | Score | Description |
|---|---|---|
| 1 | No visible fracture callus | |
| Callus appearance | 2 | Fracture callus is visible, but is not the same radiodensity throughout and appears wispy, patchy, or hollow in areas |
| 3 | Fracture callus is the same radiodensity throughout, but is radiolucent compared to the unaffected bone cortex | |
| 4 | Fracture callus and unaffected bone cortex are the same radiodensity, callus is still clearly visible | |
| Fracture discontinuity | 1 | Fracture discontinuity is clearly visible |
| (nondisplaced torus/buckle fractures) | 2 | Fracture discontinuity is not visible |
| Fracture gap bridging | 1 | No bridging of the fracture gap |
| (displaced fractures) | 2 | Fracture gap is bridging or bridged, but still visible |
| 3 | Fracture gap is not visible | |
| 1 | No visible sclerosis | |
| Sclerosis | 2 | Sclerosis is visible above and/or below the fracture site as a thin, roughly linear band |
| 3 | Small patchy areas of sclerosis visible above and/or below the fracture site | |
| 4 | Widespread sclerosis above and/or below the fracture site |
| Malone Stage | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Displaced fractures | ||||||
| median observed in training set (predicted, n = 651) | 8 | 25 | 41 | 54 | 65 | 58 |
| median observed in test set (n = 278) | 8 | 21 | 41 | 57 | 92 | 51 |
| bias of prediction via median 2 | 0 | −4 | 0 | 3 | 27 | −7 |
| mean observed in training set (predicted, n = 651) 3 | 10 (8) | 26 (14) | 45 (22) | 63 (48) | 94 (97) | 103 (121) |
| mean observed in test set (n = 278) 3 | 11 (10) | 22 (12) 4 | 42 (20) | 58 (24) | 118 (76) 4 | 99 (138) |
| bias of prediction via mean 2 | 1 | −4 | −3 | −5 | 24 | −4 |
| Buckle fractures | ||||||
| median observed in training set (predicted, n = 623) | 8 | 22 | 28 | 28 | 28 | 22 |
| median observed in test set (n = 265) | 8 | 21 | 34 | 27 | 34 | 21 |
| bias of prediction via median 2 | 0 | −1 | 6 | −1 | 6 | −1 |
| mean observed in training set (predicted, n = 623) 3 | 10 (5) | 36 (82) | 29 (10) | 32 (14) | 35 (20) | 40 (80) |
| mean observed in test set (n = 265) 3 | 39 (135) 4 | 30 (41) | 77 (190) 4 | 28 (11) 4 | 47 (52) 4 | 39 (72) |
| bias of prediction via mean 2 | 29 | −6 | 48 | −4 | 12 | −1 |
| Fracture Type | Malone Scale | Proposed Scale | |
|---|---|---|---|
| GBM | Random Forest | ||
| Model * | Model * | ||
| Displaced (test set, n = 278) | |||
| median difference | 4.1 | 0.7 | 0.6 |
| mean difference | 0.3 | −8.7 | −9.6 |
| standard deviation | 56.3 | 52.3 | 55.0 |
| Buckle (test set, n = 265) | |||
| median difference | 10.9 | −0.4 | −0.1 |
| mean difference | −5.6 | −14.3 | −13.6 |
| standard deviation | 82.1 | 78.2 | 76.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyllonen, K.M.; Monson, K.L.; Smith, M.A. Postmortem and Antemortem Forensic Assessment of Pediatric Fracture Healing from Radiographs and Machine Learning Classification. Biology 2022, 11, 749. https://0-doi-org.brum.beds.ac.uk/10.3390/biology11050749
Kyllonen KM, Monson KL, Smith MA. Postmortem and Antemortem Forensic Assessment of Pediatric Fracture Healing from Radiographs and Machine Learning Classification. Biology. 2022; 11(5):749. https://0-doi-org.brum.beds.ac.uk/10.3390/biology11050749
Chicago/Turabian StyleKyllonen, Kelsey M., Keith L. Monson, and Michael A. Smith. 2022. "Postmortem and Antemortem Forensic Assessment of Pediatric Fracture Healing from Radiographs and Machine Learning Classification" Biology 11, no. 5: 749. https://0-doi-org.brum.beds.ac.uk/10.3390/biology11050749