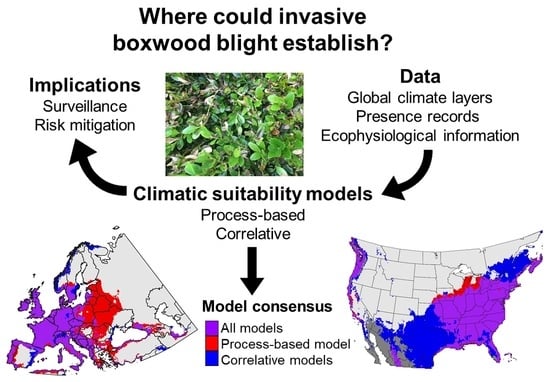
Potential Distribution of Invasive Boxwood Blight Pathogen (Calonectriapseudonaviculata) as Predicted by Process-Based and Correlative Models
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Modeling Overview
2.2. Boxwood Blight Presence Records
2.3. CLIMEX Model
2.3.1. Temperature and Moisture Index Parameters
2.3.2. Temperature and Moisture Stress Parameters
2.4. Correlative Models
2.4.1. Data Inputs and Pre-Processing
2.4.2. Model Fitting and Performance
2.4.3. Global Model Projections
2.5. Estimating the Potential Distribution
2.6. Model Validation
2.7. Correlative Models Based on Climate Data for the Invasion Time Period
3. Results
3.1. Correlative Model Evaluations and Variable Importance
3.2. Climatic Suitability for and Potential Distribution of Cps in Europe and Western Asia



3.3. Climatic Suitability for and Potential Distribution of Cps in North America
3.4. Global Climatic Suitability for and Potential Distribution of Cps


3.5. Validation of Predictions of the Potential Distribution
4. Discussion
4.1. Climatic Suitability for and Potential Distribution of Cps in Europe, Western Asia, and North America
4.2. Establishment Risk for Cps in Global Centers of Diversity for Buxus and Congeners
4.3. Potential Geographic Origin of Cps
4.4. Model Uncertainty
4.5. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thakur, M.P.; van der Putten, W.H.; Cobben, M.M.P.; van Kleunen, M.; Geisen, S. Microbial invasions in terrestrial ecosystems. Nat. Rev. Microbiol. 2019, 17, 621–631. [Google Scholar] [CrossRef]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef] [Green Version]
- Lovett, G.M.; Weiss, M.; Liebhold, A.M.; Holmes, T.P.; Leung, B.; Lambert, K.F.; Orwig, D.A.; Campbell, F.T.; Rosenthal, J.; McCullough, D.G.; et al. Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecol. Appl. 2016, 26, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Ricciardi, A.; Blackburn, T.M.; Carlton, J.T.; Dick, J.T.A.; Hulme, P.E.; Iacarella, J.C.; Jeschke, J.M.; Liebhold, A.M.; Lockwood, J.L.; MacIsaac, H.J.; et al. Invasion science: A horizon scan of emerging challenges and opportunities. Trends Ecol. Evol. 2017, 32, 464–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santini, A.; Ghelardini, L.; De Pace, C.; Desprez-Loustau, M.L.; Capretti, P.; Chandelier, A.; Cech, T.; Chira, D.; Diamandis, S.; Gaitniekis, T.; et al. Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol. 2013, 197, 238–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahrung, H.F.; Carnegie, A.J. Non-native forest insects and pathogens in Australia: Establishment, spread, and impact. Front. For. Glob. Chang. 2020, 1, e37. [Google Scholar] [CrossRef] [Green Version]
- Daughtrey, M.L. Boxwood blight: Threat to ornamentals. Annu. Rev. Phytopathol. 2019, 57, 189–209. [Google Scholar] [CrossRef]
- Hong, C. Saving American gardens from boxwood blight. Boxwood Bull. 2019, 58, 3–10. [Google Scholar]
- Batdorf, L.R. Boxwood Handbook: A Practical Guide to Knowing and Growing Boxwood, 3rd ed.; The American Boxwood Society: Boyce, VA, USA, 1995. [Google Scholar]
- Mitchell, R.; Chitanava, S.; Dbar, R.; Kramarets, V.; Lehtijärvi, A.; Matchutadze, I.; Mamadashvili, G.; Matsiakh, I.; Nacambo, S.; Papazova-Anakieva, I.; et al. Identifying the ecological and societal consequences of a decline in Buxus forests in Europe and the Caucasus. Biol. Invasions 2018, 20, 3605–3620. [Google Scholar] [CrossRef]
- Şimşek, S.A.; Katırcıoğlu, Y.Z.; Çakar, D.; Rigling, D.; Maden, S. Impact of fungal diseases on common box (Buxus sempervirens L.) vegetation in Turkey. Eur. J. Plant Pathol. 2019, 153, 1203–1220. [Google Scholar] [CrossRef]
- Matsiakh, I. Assessment of Forest Pests and Diseases in Native Boxwood Forests of Georgia: Final Report; European Neighborhood and Partnership Instrument East Countries Forest Law Enforcement and Governance II Program: Washington, DC, USA, 2016; Available online: https://www.enpi-fleg.org/docs/assessment-of-forest-pests-and-diseases-in-georgia/ (accessed on 14 April 2022).
- Kolganikhina, G.B. Year dynamics of the Colchis box health status and Cylindrocladium box blight development in the Sochi national park. Russ. J. Ecol. 2014, 6, 202–209. [Google Scholar]
- Lombard, L.; Crous, P.W.; Wingfield, B.D.; Wingfield, M.J. Systematics of Calonectria: A genus of root, shoot and foliar pathogens. Stud. Mycol. 2010, 66, 1–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehesquière, B.; Crouch, J.A.; Marra, R.E.; Van Poucke, K.; Rys, F.; Maes, M.; Gobin, B.; Höfte, M.; Heungens, K. Characterization and taxonomic reassessment of the box blight pathogen Calonectria pseudonaviculata, introducing Calonectria henricotiae sp. nov. Plant Pathol. 2016, 65, 37–52. [Google Scholar] [CrossRef]
- EPPO Calonectria pseudonaviculata (CYLDBU). Available online: https://gd.eppo.int/taxon/CYLDBU/distribution (accessed on 14 April 2022).
- Castroagudín, V.L.; Weiland, J.E.; Baysal-Gurel, F.; Cubeta, M.A.; Daughtrey, M.L.; Gauthier, N.W.; LaMondia, J.; Luster, D.G.; Hand, F.P.; Shishkoff, N.; et al. One clonal lineage of Calonectria pseudonaviculata is primarily responsible for the boxwood blight epidemic in the United States. Phytopathology 2020, 110, 1845–1853. [Google Scholar] [CrossRef]
- Gehesquière, B. Cylindrocladiumbuxicola Nom. Cons. Prop. (syn. Calonectria pseudonaviculata) on Buxus: Molecular Characterization, Epidemiology, Host Resistance and Fungicide Control. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2014. [Google Scholar]
- LeBlanc, N.; Salgado-Salazar, C.; Crouch, J.A. Boxwood blight: An ongoing threat to ornamental and native boxwood. Appl. Microbiol. Biotechnol. 2018, 102, 4371–4380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehesquière, B.; D’Haeyer, S.; Pham, K.T.K.; Van Kuik, A.J.; Maes, M.; Höfte, M.; Heungens, K. QPCR assays for the detection of Cylindrocladium buxicola in plant, water, and air samples. Plant Dis. 2013, 97, 1082–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henricot, B.; Pérez Sierra, A.; Prior, C. A new blight disease on Buxus in the UK caused by the fungus Cylindrocladium. Plant Pathol. 2000, 49, 805. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z.; Hill, C.F. Cylindrocladiumpseudonaviculatum sp. nov. from New Zealand, and new Cylindrocladium records from Vietnam. Sydowia 2002, 54, 23–34. [Google Scholar]
- Palmer, C.L.; Shishkoff, N. Boxwood blight: A new scourge, a new paradigm for collaborative research. Outlooks Pest Manag. 2014, 25, 230–236. [Google Scholar] [CrossRef]
- Akili, S.; Katircioglu, Y.Z.; Zor, K.; Maden, S. First report of blight on Buxus spp. caused by Cylindrocladium pseudonaviculatum in the Eastern Black Sea region of Turkey. Plant Dis. 2012, 87, 1539. [Google Scholar] [CrossRef]
- Gorgiladze, L.; Meparishvili, G.; Sikharulidze, Z.; Natsarishvili, K.; Davitadze, R. First report of Cylindrocladium buxicola in Georgia. Plant Dis. 2011, 23, 24. [Google Scholar] [CrossRef] [Green Version]
- Gasich, E.L.; Kazartsev, I.A.; Gannibal, P.B.; Koval, A.G.; Shipilova, N.P.; Khlopunova, L.B.; Ovsyannikova, E.I. Calonectriapseudonaviculata—A new for Abkhazia species, the causal agent of boxwood blight. Mikol. I Fitopatol. 2013, 47, 129–131. [Google Scholar]
- Mirabolfathy, M. Outbreak of boxwood tree leaf drop in Guilan and Mazandaran forests. In Proceedings of the 1st Iranian Mycological Congress, Rasht, Iran, 3–5 September 2013; University of Guilan: Rasht, Iran, 2013; p. 8. [Google Scholar]
- Rezaee, S.; Kia-Daliri, H.; Sharifi, K.; Ahangaran, Y.; Hajmansoor, S. Boxwood blight caused by Cylindrocladium buxicola in Tonekabon forest. Appl. Entomol. Phytopathol. 2013, 80, 197–198. [Google Scholar]
- Khazaeli, P.; Rezaee, S.; Mirabolfathy, M.; Zamanizade, H.; Kiadaliri, H. Genetic and phenotypic variation of Calonectria pseudonaviculata isolates causing boxwood blight disease in the Hyrcanian forest of Iran. Agric. Res. Technol. Open Access J. 2018, 19, 556081. [Google Scholar] [CrossRef] [Green Version]
- Ivors, K.L.; Lacey, L.W.; Milks, D.C.; Douglas, S.M.; Inman, M.K.; Marra, R.E.; LaMondia, J.A. First report of boxwood blight caused by Cylindrocladium pseudonaviculatum in the United States. Plant Dis. 2012, 96, 1070. [Google Scholar] [CrossRef]
- Douglas, S.M. Boxwood Blight—A New Threat to Boxwood in the U.S. CNLA/CGGA Winter Symposium: Plantsville, CT, USA, 2012; Available online: https://nationalplantboard.org/wp-content/uploads/docs/2012_meeting/npb_2012_bwb.pdf (accessed on 27 July 2021).
- Swanson, L.; Griffin, D.; Bogard, L.; VonFeldt, E. New Plant Disease—Boxwood Blight; Oregon State University Extension; Springer: Tillamook, OR, USA, 2012. [Google Scholar]
- Elmhirst, J.F.; Auxier, B.E.; Wegener, L.A. First report of box blight caused by Cylindrocladium pseudonaviculatum in British Columbia, Canada. Plant Dis. 2013, 97, 559. [Google Scholar] [CrossRef]
- Castroagudín, V.L.; Yang, X.; Daughtrey, M.L.; Luster, D.G.; Pscheidt, J.W.; Weiland, J.E.; Crouch, J.A. Boxwood blight disease: A diagnostic guide. Plant Health Prog. 2020, 21, 291–300. [Google Scholar] [CrossRef]
- Hall, C.R.; Hong, C.; Gouker, F.E.; Daughtrey, M. Analyzing the structural shifts in U.S. boxwood production due to boxwood blight. J. Environ. Hortic. 2021, 39, 91–99. [Google Scholar] [CrossRef]
- USDA National Agricultural Statistics Service Census of Horticultural Specialties. 2020. Available online: https://www.nass.usda.gov/Publications/AgCensus/2017/Online_Resources/Census_of_Horticulture_Specialties/ (accessed on 9 September 2021).
- LaMondia, J.A. Management of Calonectria pseudonaviculata in boxwood with fungicides and less susceptible host species and varieties. Plant Dis. 2015, 99, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Lehtijärvi, A.; Doğmuş-Lehtijärvi, H.T.; Oskay, F. Boxwood blight in Turkey: Impact on natural boxwood populations and management challenges. Balt. For. 2017, 23, 274–278. [Google Scholar]
- Mirabolfathy, M.; Ahangaran, Y.; Lombard, L.; Crous, P.W. Leaf blight of Buxus sempervirens in northern forests of Iran caused by Calonectria pseudonaviculata. Plant Dis. 2013, 97, 1121. [Google Scholar] [CrossRef]
- Henricot, B.; Gorton, C.; Denton, G.; Denton, J. Studies on the control of Cylindrocladium buxicola using fungicides and host resistance. Plant Dis. 2008, 92, 1273–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shishkoff, N.; Daughtrey, M.; Aker, S.; Olsen, R.T.; Disease, U.F.; Science, W.; Daughtrey, M.; Island, L. Evaluating boxwood susceptibility to Calonectria pseudonaviculata using cuttings from the national boxwood collection. Plant Health Prog. 2015, 16, 11–15. [Google Scholar] [CrossRef]
- LaMondia, J.A.; Shishkoff, N. Susceptibility of boxwood accessions from the national boxwood collection to boxwood blight and potential for differences between Calonectria pseudonaviculata and C. henricotiae. HortScience 2017, 52, 873–879. [Google Scholar] [CrossRef]
- Malapi-Wight, M.; Salgado-Salazar, C.; Demers, J.E.; Clement, D.L.; Rane, K.K.; Crouch, J.A. Sarcococca blight: Use of whole-genome sequencing for fungal plant disease diagnosis. Plant Dis. 2016, 100, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Ryan, C.; Williams-Woodward, J.; Zhang, D.L. Susceptibility of Sarcococca taxa to boxwood blight by Calonectria pseudonaviculata. In Proceedings of the Southern Nursery Association Research Conference; Baysal-Gurel, F., Ed.; Southern Nursery Association: Acworth, GA, USA, 2018; Volume 62, pp. 64–67. [Google Scholar]
- LaMondia, J.A.; Li, D.W.; Marra, R.E.; Douglas, S.M. First report of Cylindrocladium pseudonaviculatum causing leaf spot of Pachysandra terminalis. Plant Dis. 2012, 96, 1069. [Google Scholar] [CrossRef]
- Kong, P.; Likins, T.M.; Hong, C.X. First report of Pachysandra terminalis leaf spot by Calonectria pseudonaviculata in Virginia. Plant Dis. 2017, 101, 509. [Google Scholar] [CrossRef]
- LaMondia, J.A.; Li, D.W. Calonectriapseudonaviculata can cause leaf spot and stem blight of Pachysandra procumbens. Plant Health Prog. 2013, 14, 58. [Google Scholar] [CrossRef] [Green Version]
- Richardson, P.A.; Daughtrey, M.; Hong, C. Indications of susceptibility to Calonectria pseudonaviculata in some common groundcovers and boxwood companion plants. Plant Dis. 2020, 104, 1127–1132. [Google Scholar] [CrossRef] [Green Version]
- Hong, C. Fighting plant pathogens together. Science 2019, 365, 229. [Google Scholar] [CrossRef]
- Magarey, R.D.; Fowler, G.A.; Borchert, D.M.; Sutton, T.B.; Colunga-Garcia, M.; Simpson, J.A.; Health, P.; Service, I.; Colunga-Garcia, M. NAPPFAST: An internet system for the weather-based mapping of plant pathogens. Plant Dis. 2007, 91, 336–345. [Google Scholar] [CrossRef] [Green Version]
- Lantschner, M.V.; de la Vega, G.; Corley, J.C. Predicting the distribution of harmful species and their natural enemies in agricultural, livestock and forestry systems: An overview. Int. J. Pest Manag. 2019, 65, 190–206. [Google Scholar] [CrossRef]
- Avenot, H.F.; King, C.; Edwards, T.P.; Baudoin, A.; Hong, C.X. Effects of inoculum dose, temperature, cultivar, and interrupted leaf wetness period on infection of boxwood by Calonectria pseudonaviculata. Plant Dis. 2017, 101, 866–873. [Google Scholar] [CrossRef] [Green Version]
- Avenot, H.F.; Baudoin, A.; Hong, C. Conidial production and viability of Calonectria pseudonaviculata on infected boxwood leaves as affected by temperature, wetness, and dryness periods. Plant Pathol. 2022, 71, 696–701. [Google Scholar] [CrossRef]
- Zhu, G.P.; Peterson, A.T. Do consensus models outperform individual models? Transferability evaluations of diverse modeling approaches for an invasive moth. Biol. Invasions 2017, 19, 2519–2532. [Google Scholar] [CrossRef]
- Watling, J.I.; Brandt, L.A.; Bucklin, D.N.; Fujisaki, I.; Mazzotti, F.J.; Romañach, S.S.; Speroterra, C. Performance metrics and variance partitioning reveal sources of uncertainty in species distribution models. Ecol. Model. 2015, 309–310, 48–59. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.R.; Phillips, S.J. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Maywald, G.F.; Yonow, T.; Zurcher, E.J.; Herrmann, N.; Sutherst, R.W. CLIMEX Version 4: Exploring the Effects of Climate on Plants, Animals and Diseases; CSIRO: Canberra, Australia, 2016. [Google Scholar]
- Sutherst, R.W.; Maywald, G.F. A computerised system for matching climates in ecology. Agric. Ecosyst. Environ. 1985, 13, 281–299. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H. Do they? How do they? WHY do they differ? On finding reasons for differing performances of species distribution models. Ecography 2009, 32, 66–77. [Google Scholar] [CrossRef]
- Dormann, C.F.; Schymanski, S.J.; Cabral, J.; Chuine, I.; Graham, C.; Hartig, F.; Kearney, M.R.; Morin, X.; Römermann, C.; Schröder, B.; et al. Correlation and process in species distribution models: Bridging a dichotomy. J. Biogeogr. 2012, 39, 2119–2131. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papeş, M.; Soberón, J. Mechanistic and correlative models of ecological niches. Eur. J. Ecol. 2015, 1, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Kearney, M.R.; Porter, W. Mechanistic niche modelling: Combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 2009, 12, 334–350. [Google Scholar] [CrossRef]
- Charney, N.D.; Record, S.; Gerstner, B.E.; Merow, C.; Zarnetske, P.L.; Enquist, B.J. A test of species distribution model transferability across environmental and geographic space for 108 western North American tree species. Front. Ecol. Evol. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Owens, H.L.; Campbell, L.P.; Dornak, L.L.; Saupe, E.E.; Barve, N.; Soberón, J.; Ingenloff, K.; Lira-Noriega, A.; Hensz, C.M.; Myers, C.E.; et al. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol. Modell. 2013, 263, 10–18. [Google Scholar] [CrossRef]
- Sutherst, R.W. Pest species distribution modelling: Origins and lessons from history. Biol. Invasions 2014, 16, 239–256. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Webber, B.L.; Leriche, A.; Ota, N.; Macadam, I.; Bathols, J.; Scott, J.K. CliMond: Global high-resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods Ecol. Evol. 2012, 3, 53–64. [Google Scholar] [CrossRef]
- Dart, N.; Hong, C.; Craig, C.A.; Fry, J.T.; Hu, X. Soil inoculum production, survival, and infectivity of the boxwood blight pathogen, Calonectria pseudonaviculata. Plant Dis. 2015, 99, 1689–1694. [Google Scholar] [CrossRef] [Green Version]
- Henricot, B.B.; Culham, A. Cylindrocladium buxicola, a new species affecting Buxus spp., and its phylogenetic status. Mycologia 2002, 94, 980. [Google Scholar] [CrossRef]
- Pfender, W.F.; Gent, D.H.; Mahaffee, W.F. Sensitivity of disease management decision aids to temperature input errors associated with sampling interval and out-of-canopy sensor placement. Plant Dis. 2012, 96, 726–736. [Google Scholar] [CrossRef] [Green Version]
- United States Department of Agriculture. Growing Boxwoods; U.S. Department of Agriculture: Washington, DC, USA, 1976.
- Shishkoff, N.; Camp, M.J. The effect of different temperatures and moisture levels on survival of Calonectria pseudonaviculata in boxwood leaves and twigs and as microsclerotia produced in culture. Plant Dis. 2016, 100, 2018–2024. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Hong, C. Microsclerotial enumeration, size, and survival of Calonectria pseudonaviculata. Plant Dis. 2018, 102, 983–990. [Google Scholar] [CrossRef] [Green Version]
- Salvesen, P.H.; Kanz, B. Boxwood Cultivars in Old Gardens in Norway. Plants and Culture: Seeds of the Cultural Heritage of Europe; Morel, J.-P., Mercuri, A.M., Eds.; Edipuglia: Puglia, Italy, 2009; pp. 247–262. [Google Scholar]
- Khazaeli, P.; Rezaee, S.; Mirabolfathy, M.; Zamanizadeh, H.; Kia-daliri, S.K. Report of boxwood blight extension to Golestan province forests. Appl. Entomol. Phytopathol. 2015, 83, 85–86. [Google Scholar]
- Hagan, A.K.; Conner, K. Boxwood blight—A New Disease of Boxwood in the Nursery and Landscape in Alabama; No. PP-737; Alabama Cooperative Extension: Auburn, AL, USA, 2013. [Google Scholar]
- Hūšang, A. Boxtree. In Encyclopedia Iranica; Yarshater, E., Ed.; Routledge: Oxfordshire, UK, 1989; Volume 4, pp. 418–420. [Google Scholar]
- de Andrade, A.F.A.; Velazco, S.J.E.; De Marco Júnior, P. ENMTML: An R package for a straightforward construction of complex ecological niche models. Environ. Model. Softw. 2020, 125, 104615. [Google Scholar] [CrossRef]
- R Development Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: http://www.R-project.org (accessed on 10 March 2022).
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Package “dismo”. R Package Version 1.3-5. CRAN. Available online: http://cran.r-project.org/web/packages/dismo/index.html (accessed on 3 January 2022). R Package Version 1.3-5.
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M.; et al. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- Veloz, S.D. Spatially autocorrelated sampling falsely inflates measures of accuracy for presence-only niche models. J. Biogeogr. 2009, 36, 2290–2299. [Google Scholar] [CrossRef]
- Evans, J. SpatialEco. R package Version 1.3-6. Available online: https://github.com/jeffreyevans/spatialEco (accessed on 22 March 2022).
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- De Marco, P.; Nóbrega, C.C. Evaluating collinearity effects on species distribution models: An approach based on virtual species simulation. PLoS ONE 2018, 13, e0202403. [Google Scholar] [CrossRef] [PubMed]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Petitpierre, B.; Broennimann, O.; Kueffer, C.; Daehler, C.; Guisan, A. Selecting predictors to maximize the transferability of species distribution models: Lessons from cross-continental plant invasions. Glob. Ecol. Biogeogr. 2017, 26, 275–287. [Google Scholar] [CrossRef]
- Leutner, B.; Horning, N. Rstoolbox: Tools for Remote Sensing Data Analysis. R Package Version 0.2.6. Available online: https://cran.r-project.org/web/packages/RStoolbox/index.html (accessed on 22 March 2022).
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef]
- Golding, N.; Purse, B.V. Fast and flexible Bayesian species distribution modelling using Gaussian processes. Methods Ecol. Evol. 2016, 7, 598–608. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Prasad, A.M.; Iverson, L.R.; Liaw, A. Newer classification and regression tree techniques: Bagging and random forests for ecological prediction. Ecosystems 2006, 9, 181–199. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, C.; Work, T.; Candau, J.N.; Desrochers, A.; Kneeshaw, D. Application of machine-learning methods in forest ecology: Recent progress and future challenges. Environ. Rev. 2018, 26, 339–350. [Google Scholar] [CrossRef] [Green Version]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Probst, P.; Wright, M.N.; Boulesteix, A.L. Hyperparameters and tuning strategies for random forest. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2019, 9, e1301. [Google Scholar] [CrossRef] [Green Version]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Leroy, B.; Delsol, R.; Hugueny, B.; Meynard, C.N.; Barhoumi, C.; Barbet-Massin, M.; Bellard, C. Without quality presence–absence data, discrimination metrics such as TSS can be misleading measures of model performance. J. Biogeogr. 2018, 45, 1994–2002. [Google Scholar] [CrossRef]
- Li, W.; Guo, Q. How to assess the prediction accuracy of species presence-absence models without absence data? Ecography 2013, 36, 788–799. [Google Scholar] [CrossRef]
- Zhu, G.; Fan, J.; Peterson, A.T. Cautions in weighting individual ecological niche models in ensemble forecasting. Ecol. Model. 2021, 448, 109502. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Testing whether ensemble modelling is advantageous for maximising predictive performance of species distribution models. Ecography 2020, 43, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Cobos, M.E.; Townsend Peterson, A.; Barve, N.; Osorio-Olvera, L. Kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef] [Green Version]
- Walker, K. Package “Tigris”: Load Census TIGER/Line Shapefiles. R Package Version 1.6. Available online: https://github.com/walkerke/tigris (accessed on 11 April 2022).
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- USGCRP. Impacts, Risks, and Adaptation in the United States: Fourth National Climate Assessment, Volume II; Reidmiller, D.R., Avery, C.W., Easterling, D.R., Kunkel, K.E., Lewis, K.L.M., Maycock, T.K., Stewar, B.C., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2018. [Google Scholar]
- Cornes, R.C.; van der Schrier, G.; van den Besselaar, E.J.; Jones, P.D. Van Den An ensemble version of the E-OBS temperature and precipitation data sets. J. Geophys. Res. 2018, 123, 9391–9409. [Google Scholar] [CrossRef] [Green Version]
- Henricot, B. Box blight rampages onwards. Plantsman 2006, 5, 153–157. [Google Scholar]
- Iriarte, F.; Paret, M.; Knox, G.; Schubert, T.; Jeyaprakash, A.; Davison, D. First report of boxwood blight caused by Calonectria pseudonaviculata in Florida. Plant Health Prog. 2016, 17, 229–231. [Google Scholar] [CrossRef] [Green Version]
- Hall, C. Observations regarding the value of boxwood sales from 2014 to 2019. Boxwood Blight Insight Gr. Newsl. 2021, 2, 1–2. [Google Scholar]
- Bush, E.; Hansen, M.A.; Dart, N.; Hong, C.; Bordas, A.; Likins, T.M. Best Management Practices for Boxwood Blight in the Virginia Home Landscape; No. PPWS-85NP; Virginia Cooperative Extension: Blacksburg, VA, USA, 2016; Available online: https://www.pubs.ext.vt.edu/content/dam/pubs_ext_vt_edu/PPWS/PPWS-29/PPWS-29-pdf.pdf (accessed on 14 April 2022).
- Miller, M.E.; Shishkoff, N.; Cubeta, M.A. Thermal sensitivity of Calonectria henricotiae and Calonectria pseudonaviculata conidia and microsclerotia. Mycologia 2018, 110, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Pintos Varela, C.; Penalta, B.G.; Vázquez, J.P.M.; Casal, O.A. First report of Cylindrocladium buxicola on Buxus sempervirens in Spain. Plant Dis. 2009, 93, 670. [Google Scholar] [CrossRef] [PubMed]
- Saurat, C.; Fourrier, C.; Ioos, R. First report of blight disease on Buxus caused by Cylindrocladium buxicola in France. Plant Dis. 2012, 96, 1069. [Google Scholar] [CrossRef]
- Saracchi, M.; Rocchi, F.; Pizzatti, C.; Cortesi, P. Box blight, a new disease of Buxus in Italy caused by Cylindrocladium buxicola. J. Plant Pathol. 2008, 90, 581–584. [Google Scholar] [CrossRef]
- Cech, T.; Diminic, D.; Heungens, K. Cylindrocladiumbuxicola causes common box blight in Croatia. Plant Pathol. 2010, 59, 1169. [Google Scholar] [CrossRef]
- Di Domenico, F.; Lucchese, F.; Magri, D. Buxus in Europe: Late Quaternary dynamics and modern vulnerability. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 354–362. [Google Scholar] [CrossRef]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Br. 2017, 12, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Bartíková, M.; Brand, T.; Beltz, H.; Šafránková, I. Host susceptibility and microclimatic conditions influencing the development of blight diseases caused by Calonectria henricotiae. Eur. J. Plant Pathol. 2020, 157, 103–117. [Google Scholar] [CrossRef]
- Blomquist, C.L.; Kosta, K.L.; Santos, P.F.; Rooney-Latham, S. First report of boxwood blight caused by Calonectria pseudonaviculata in California. Plant Dis. 2018, 102, 2379. [Google Scholar] [CrossRef]
- Bartíková, M.; Holková, L.; Šafránková, I. Occurrence of boxwood blight (Calonectria pseudonaviculata and C. henricotiae) in historical gardens in the Czech Republic. Eur. J. Plant Pathol. 2020, 158, 135–142. [Google Scholar] [CrossRef]
- Dart, N.; Hong, C.; Bordas, A.; Bush, E.; Hansen, M.; Likins, T. Best Management Practices for Boxwood Blight in Virginia Retail Nurseries WITHOUT Boxwood Blight; No. PPWS-35NP; Virginia Cooperative Extension: Blacksburg, VA, USA, 2016. [Google Scholar]
- Safránková, I.; Kmoch, M.; Holková, L. First report of Cylindrocladium buxicola on box in Czech Republic. New Dis. Reports 2012, 25, 5. [Google Scholar] [CrossRef] [Green Version]
- Köhler, E.; Brückner, P. The genus Buxus (Buxaceae): Aspects of its differentiation in space and time. Plant Syst. Evol. 1989, 162, 267–283. [Google Scholar] [CrossRef]
- Balthazar, M.; von Endress, P.K.; Qiu, Y.L. Phylogenetic relationships in Buxaceae based on nuclear internal transcribed spacers and plastid ndhF sequences. Int. J. Plant Sci. 2000, 161, 785–792. [Google Scholar] [CrossRef]
- Köhler, E. Buxaceae. In Flora de la República de Cuba. Fasciculo 19(1); Greute, W., Rankin-Rodríguez, R., Eds.; Koeltz Scientific Books: Oberreifenberg, Germany, 2014; pp. 1–124. [Google Scholar]
- Gutiérrez, P.A.G. Evolution and Biogeography of Buxus L. (Buxaceae) in Cuba and the Caribbean. Ph.D. Thesis, Free University of Berlin, Berlin, Germany, 2014. [Google Scholar]
- Schatz, G.E.; Lowry, P.P.I. A synoptic revision of the genus Buxus L. (Buxaceae) in Madagascar and the Comoro Islands. Adansonia 2002, 24, 179–196. [Google Scholar]
- Friss, I. A synopsis of the Buxaceae in Africa south of the Sahara. Kew Bull. 1989, 44, 293–299. [Google Scholar] [CrossRef]
- Patarkalashvili, T. Forest biodiversity of Georgia and endangered plant species. Ann. Agrar. Sci. 2017, 15, 349–351. [Google Scholar] [CrossRef]
- Panahi, P.; Jamzad, Z.; Jalili, A.; Sagheb Talebi, K.; Pourhashemi, M. The role of the National Botanical Garden of Iran in ex situ conservation of Buxus hyrcana Pojark.; An endangered species. Urban For. Urban Green. 2021, 57, 126951. [Google Scholar] [CrossRef]
- Matsiakh, I.; Kramarets, V.; Mamadashvili, G. Box tree moth Cydalima perspectalis as a threat to the native populations of Buxus colchica in Republic of Georgia. J. Entomol. Res. Soc. 2018, 20, 29–42. [Google Scholar]
- Kong, P.; Hong, C. Host responses and impact on the boxwood blight pathogen, Calonectria pseudonaviculata. Planta 2019, 249, 831–838. [Google Scholar] [CrossRef]
- LeBlanc, N.; Cubeta, M.A.; Crouch, J.A. Population genomics trace clonal diversification and intercontinental migration of an emerging fungal pathogen of boxwood. Phytopathology 2021, 111, 184–193. [Google Scholar] [CrossRef]
- EPPO. EPPO Study on the Risk of Imports of Plants for Planting. EPPO Technol. Doc. No. 1061. 2012. Available online: http://www.eppo.int/QUARANTINE/EPPO_Study_on_Plants_for_planting.pdf (accessed on 14 April 2022).
- Van der Straten, M.J.; Muus, T.S.T. The box tree pyralid, Glyphodes perspectalis (Lepidoptera: Crambidae), an invasive alien moth ruining box trees. Proc. Neth. Entomol. Soc. Meet. 2010, 21, 107–111. [Google Scholar]
- CABI Cydalima perspectalis (box tree moth). Invasive Species Copendium. Wallingford, UK: CAB International. Available online: www.cabi.org/isc (accessed on 31 August 2021).
- Vale, C.G.; Tarroso, P.; Brito, J.C. Predicting species distribution at range margins: Testing the effects of study area extent, resolution and threshold selection in the Sahara-Sahel transition zone. Divers. Distrib. 2014, 20, 20–33. [Google Scholar] [CrossRef]
- Paeth, H.; Vogt, G.; Paxian, A.; Hertig, E.; Seubert, S.; Jacobeit, J. Quantifying the evidence of climate change in the light of uncertainty exemplified by the Mediterranean hot spot region. Glob. Planet. Chang. 2017, 151, 144–151. [Google Scholar] [CrossRef]
- Dukes, J.S.; Pontius, J.; Orwig, D.; Jeffrey, R.G.; Vikki, L.R.; Brazee, N.; Cooke, B.; Kathleen, A.T.; Erik, E.S.; Harrington, R.; et al. Responses of insect pests, pathogens, and invasive plant species to climate change in the forests of northeastern North America: What can we predict? Can. J. For. Res. 2009, 39, 231–248. [Google Scholar] [CrossRef]
- Gardner, A.S.; Gaston, K.J.; Maclean, I.M.D. Accounting for inter-annual variability alters long-term estimates of climate suitability. J. Biogeogr. 2021, 48, 1960–1971. [Google Scholar] [CrossRef]





| Parameter | Description | Value |
|---|---|---|
| Temperature index | ||
| DV0 | Limiting low temperature (°C) | 8 |
| DV1 | Lower optimal temperature (°C) | 21 |
| DV2 | Upper optimal temperature (°C) | 25 |
| DV3 | Limiting high temperature (°C) | 29 |
| Moisture index | ||
| SM0 | Limiting low moisture | 0.2 |
| SM1 | Lower optimal moisture | 0.7 |
| SM2 | Upper optimal moisture | 1.7 |
| SM3 | Limiting high moisture | 3.0 |
| Cold stress | ||
| TTCS | Cold stress temperature threshold (°C) | −10 |
| TCCS | Cold stress temperature rate (week−1) | −0.005 |
| Heat stress | ||
| TTHS | Heat stress temperature threshold (°C) | 32 |
| THHS | Heat stress temperature rate (week−1) | 0.01 |
| Dry stress | ||
| SMDS | Dry stress threshold | 0.2 |
| HDS | Dry stress rate (week−1) | −0.001 |
| Wet stress | ||
| SMWS | Wet stress threshold | 3.0 |
| HWS | Wet stress rate (week−1) | 0.005 |
| Variables and Proportion of Variance | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 |
|---|---|---|---|---|---|---|
| Variable | ||||||
| Annual mean temperature (bio1) | 0.10 | 0.26 | 0.06 | 0.29 | −0.05 | 0.05 |
| Mean diurnal temperature range (bio2) | 0.19 | −0.10 | 0.31 | −0.03 | −0.03 | 0.26 |
| Isothermality (bio3) | 0.04 | 0.35 | 0.11 | −0.10 | −0.04 | 0.10 |
| Temperature seasonality (bio4) | 0.11 | −0.45 | 0.06 | 0.11 | −0.07 | 0.05 |
| Max temperature of warmest week (bio5) | 0.22 | −0.02 | 0.13 | 0.31 | −0.09 | 0.12 |
| Min temperature of coldest week (bio6) | 0.01 | 0.41 | −0.02 | 0.15 | −0.02 | −0.02 |
| Temperature annual range (bio7) | 0.15 | −0.44 | 0.12 | 0.07 | −0.05 | 0.11 |
| Mean temperature of wettest quarter (bio8) | −0.22 | −0.07 | −0.15 | 0.75 | 0.16 | −0.14 |
| Mean temperature of driest quarter (bio9) | 0.21 | 0.25 | 0.08 | −0.06 | −0.06 | 0.11 |
| Mean temperature of warmest quarter (bio10) | 0.19 | 0.04 | 0.10 | 0.37 | −0.09 | 0.08 |
| Mean temperature of coldest quarter (bio11) | 0.03 | 0.39 | 0.01 | 0.15 | −0.01 | 0.01 |
| Annual precipitation (bio12) | −0.07 | 0.00 | −0.05 | 0.02 | −0.19 | 0.33 |
| Precipitation of wettest week (bio13) | −0.10 | −0.01 | −0.05 | 0.06 | 0.06 | 0.46 |
| Precipitation of driest week (bio14) | −0.11 | 0.01 | 0.02 | 0.02 | −0.45 | 0.10 |
| Precipitation seasonality (bio15) | −0.06 | 0.04 | 0.08 | −0.03 | 0.67 | 0.38 |
| Precipitation of wettest quarter (bio16) | −0.11 | 0.00 | −0.05 | 0.05 | 0.04 | 0.44 |
| Precipitation of driest quarter (bio17) | −0.09 | 0.01 | 0.00 | 0.03 | −0.45 | 0.11 |
| Precipitation of warmest quarter (bio18) | −0.33 | −0.09 | 0.02 | 0.15 | −0.09 | 0.15 |
| Precipitation of coldest quarter (bio19) | 0.14 | 0.06 | −0.11 | −0.07 | −0.17 | 0.34 |
| Annual mean moisture index (bio28) | −0.13 | 0.00 | −0.26 | −0.07 | −0.02 | 0.05 |
| Highest weekly moisture index (bio29) | 0.10 | −0.02 | −0.47 | −0.03 | 0.02 | 0.08 |
| Lowest weekly moisture index (bio30) | −0.34 | 0.01 | 0.02 | −0.04 | −0.03 | 0.06 |
| Moisture index seasonality (bio31) | 0.41 | −0.02 | −0.11 | 0.01 | 0.05 | 0.09 |
| Mean moisture index of wettest quarter (bio32) | 0.09 | −0.02 | −0.48 | −0.03 | 0.01 | 0.06 |
| Mean moisture index of driest quarter (bio33) | −0.33 | 0.02 | −0.01 | −0.06 | −0.04 | 0.04 |
| Mean moisture index of warmest quarter (bio34) | −0.35 | 0.02 | 0.02 | −0.06 | −0.01 | 0.07 |
| Mean moisture index of coldest quarter (bio35) | 0.12 | −0.02 | −0.51 | 0.04 | −0.05 | 0.03 |
| Proportion of variance | ||||||
| Proportion explained by each PC (%) | 49.3 | 27.3 | 8 | 4.6 | 3.7 | 3.1 |
| Accumulated proportion explained by PCs (%) | 49.3 | 76.6 | 84.6 | 89.2 | 92.9 | 96 |
| Algorithm | AUC | TSS | Sørensen | Fpb |
|---|---|---|---|---|
| Boosted regression trees | 0.68 | 0.37 | 0.74 | 1.18 |
| Gaussian process | 0.70 | 0.42 | 0.75 | 1.21 |
| Maxent (“simple”) | 0.72 | 0.44 | 0.75 | 1.20 |
| Random forest | 0.71 | 0.44 | 0.75 | 1.21 |
| Ensemble | 0.72 | 0.48 | 0.76 | 1.22 |
| Variable | Climatic Relevance | BRT | GAU | MXS | RDF | Avg. (%) |
|---|---|---|---|---|---|---|
| PC1 | Warm season precipitation and soil moisture, soil moisture seasonality | 26.6 | 38.4 | 24.7 | 19.5 | 27.3 |
| PC2 | Cold season temperatures, temperature seasonality | 18.2 | 11.7 | 9.3 | 16.8 | 14 |
| PC3 | Cold and wet season soil moisture | 23.2 | 24.6 | 27.1 | 18.4 | 23.3 |
| PC4 | Warm and wet season temperature | 11.2 | 11.6 | 18.5 | 15.6 | 14.2 |
| PC5 | Dry season precipitation, precipitation seasonality | 11.8 | 3.7 | 3.5 | 15.4 | 8.6 |
| PC6 | Cold and wet season precipitation, annual precipitation | 9.1 | 10 | 16.9 | 14.3 | 12.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barker, B.S.; Coop, L.; Hong, C. Potential Distribution of Invasive Boxwood Blight Pathogen (Calonectriapseudonaviculata) as Predicted by Process-Based and Correlative Models. Biology 2022, 11, 849. https://0-doi-org.brum.beds.ac.uk/10.3390/biology11060849
Barker BS, Coop L, Hong C. Potential Distribution of Invasive Boxwood Blight Pathogen (Calonectriapseudonaviculata) as Predicted by Process-Based and Correlative Models. Biology. 2022; 11(6):849. https://0-doi-org.brum.beds.ac.uk/10.3390/biology11060849
Chicago/Turabian StyleBarker, Brittany S., Leonard Coop, and Chuanxue Hong. 2022. "Potential Distribution of Invasive Boxwood Blight Pathogen (Calonectriapseudonaviculata) as Predicted by Process-Based and Correlative Models" Biology 11, no. 6: 849. https://0-doi-org.brum.beds.ac.uk/10.3390/biology11060849