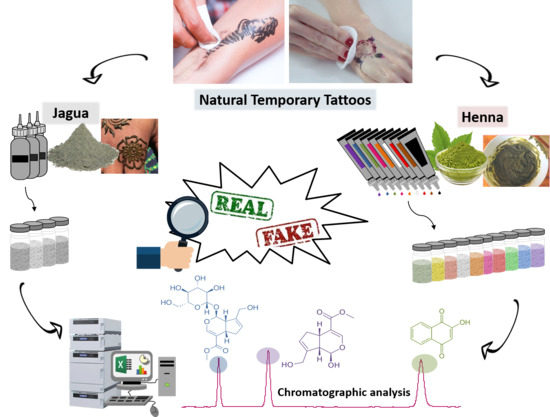
Monitoring of Natural Pigments in Henna and Jagua Tattoos for Fake Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Tattoo Samples
2.3. Sample Preparation
2.4. Liquid Chromatography (LC) with Diode-Array Detector (DAD)
2.5. Ultra High Performance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (UHPLC-QOF-MS)
3. Results and Discussion
3.1. Solubility Studies
3.2. Chromatographic Analysis
3.3. Application to Real Samples
4. Conclusions and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manual of the Working Group on Cosmetic Products (Sub-Group on Borderline Products) on the Scope of Application of the Cosmetics Regulation (EC) No 1223/2009 (Art. 2(1)(A)) Version 3.1. 2017. Available online: https://ec.europa.eu/docsroom/documents/29002 (accessed on 23 September 2020).
- The European Parliament and The Council of The European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council. Off. J. Eur. Union L 2009, 342, 59. [Google Scholar]
- The European Parliament and The Council of The European Union. Directive 2009/48/EC of the European Parliament and of the Council of 18 June 2009. On the safety of toys. Off. J. Eur. Union L 2009, 170, 1–37. [Google Scholar]
- Lores, M.; Celeiro, M.; Rubio, L.; Llompart, M.; Garcia-Jares, C. Extreme cosmetics and borderline products: An analytical-based survey of European regulation compliance. Anal. Bioanal. Chem. 2018, 410, 7085–7102. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.C. Side-effects of henna and semi-permanent ‘black henna’ tattoos: A full review. Contact Dermat. 2013, 69, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Le Coz, C.J.; Lefebvre, C.; Keller, F.; Grosshans, E. Allergic contact dermatitis caused by skin painting pseudotattooing with black henna, a mixture of henna and p-phenylenediamine and its derivatives. Arch. Dermatol. 2000, 136, 1515–1517. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Consumer Safety Opinion on Lawsonia inermis (Henna) COLIPA n_ C169, 19 September 2013 SCCS/1511/13. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_140.pdf (accessed on 23 September 2020).
- Kazandjieva, J.; Grozdev, L.; Tsankov, N. Temporary henna tattoos. Clin. Dermatol. 2007, 25, 383–387. [Google Scholar] [CrossRef]
- Almeida, P.J.; Borrego, L.; Pulido-Melian, E.; Gonzalez-Diaz, O. Quantification of p-phenylenediamine and 2-hydroxy-1,4-naphthoquinone in henna tattoos. Contact Dermat. 2012, 66, 33–37. [Google Scholar] [CrossRef]
- Aktas Sukuroglu, A.; Battal, D.; Burgaz, S. Monitoring of lawsone, p-phenylenediamine and heavy metals in commercial temporary black henna tattoos sold in Turkey. Contact Dermat. 2017, 76, 89–95. [Google Scholar] [CrossRef]
- Rubio, L.; Guerra, E.; Garcia-Jares, C.; Lores, M. Body-decorating products: Ingredients of permanent and temporary tattoos from analytical and european regulatory perspectives. Anal. Chim. Acta 2019, 1079, 59–72. [Google Scholar] [CrossRef]
- Waton, J.; Brault, F.; Laveine, E. A putative case of allergic contact dermatitis caused by a jagua tattoo. Contact Dermat. 2017, 76, 296–321. [Google Scholar] [CrossRef]
- Maarouf, M.; Saberian, C.; Segal, R.J.; Shi, V.Y. A new era for tattoos, with new potential complications. J. Clin. Aesthet. Dermatol. 2019, 12, 37–38. [Google Scholar] [PubMed]
- Bircher, A.J.; Sigg, R.; Hofmeier, K.S.; Schlegel, U.; Hauri, U. Allergic contact dermatitis caused by a new temporary blue-black tattoo dye- sensitization to genitin from jagua (Genipa Americana L.) fruit extract. Contact Dermat. 2017, 77, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Zhao, X.; Wei, L.; Wang, H.; Xiao, C.; Zheng, X. Determination of Jasminoidin in rabbit plasma for the pharmacokinetic investigation after single dose oral administration of Gardenia jasminoides Ellis and Gardenia jasminoides Ellis coupling Coptis chinensis franch extracts. Chromatographia 2010, 71, 1031–1037. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, L.; Li, Q.; Li, X.Q.; Chen, X.H.; Bi, K.S. Determination of geniposide in rats plasma. Application to pharmacokinetic studies of sendeng-4 decoction. Chromatographia 2006, 63, 493–497. [Google Scholar] [CrossRef]
- Tseng, T.-Y.; Tsai, T.-H. Measurement of unbound geniposide in blood, liver, brain and bile of anesthetized rats: An application of pharmacokinetic study and its influence on acupuncture. Anal. Chim. Acta 2004, 517, 47–52. [Google Scholar] [CrossRef]
- Nathia-Neves, G.; Nogueira, G.C.; Vardanega, R.; Meireles, M.A.d.A. Identification and quantification of genipin and geniposide from Genipa americana L. by HPLC-DAD using a fused-core column. Food. Sci. Technol. 2018, 38, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Bentes, A.d.S.; Mercadante, A.Z. Influence of the stage of ripeness on the composition of iridoids and phenolic compounds in genipap (Genipa americana L.). J. Agric. Food. Chem. 2014, 62, 10800–10808. [Google Scholar] [CrossRef]
- Bergonzi, M.C.; Righeschi, C.; Isacchi, B.; Bilia, A.R. Identification and quantification of constituents of Gardenia jasminoides Ellis (Zhizi) by HPLC-DAD-ESI-MS. Food. Chem. 2012, 134, 1199–1204. [Google Scholar] [CrossRef]
- Lee, E.J.; Hong, J.K.; Whang, W.K. Simultaneous determination of bioactive marker compounds from Gardeniae fructus by high performance liquid chromatography. Arch. Pharm. Res. 2014, 37, 992–1000. [Google Scholar] [CrossRef]
- Li, J.; Xu, B.; Zhang, Y.; Dai, S.; Sun, F.; Shi, X.; Qiao, Y. Determination of geniposide in Gardenia jasminoides Ellis fruit by near-infrared spectroscopy and chemometrics. Anal. Lett. 2016, 49, 2063–2076. [Google Scholar] [CrossRef]
- Slusarewicz, P.; Zhu, K.; Hedman, T. Kinetic analysis of genipin degradation in aqueous solution. Nat. Prod. Commun. 2010, 5, 1853–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pubchem, Lawsone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6755#sec-%20tion=Top (accessed on 24 June 2020).




| Compound | Chemical Name | Chemical Structure | Purity (%) | CAS No. |
|---|---|---|---|---|
| Genipin | methyl (1R,4aS,7aS)-1-hydroxy-7-(hydroxymethyl)-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-4-carboxylate |  | ≥98% | 6902-77-8 |
| Geniposide | methyl (1S,4aS,7aS)-7-(hydroxymethyl)-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-4-carboxylate |  | 98% | 24512-63-8 |
| Lawsone | 2-Hydroxy-1,4-naphthoquinone |  | >98% | 83-72-7 |
| PPD | p-Phenylenediamine; benzene-1,4-diamine |  | >98% | 106-50-3 |
| Sample Code | Color | Sample Type | Labelled Ingredients | Other Comments on the Label |
|---|---|---|---|---|
| HNT-1 | Black | |||
| HNT-2 | Red | |||
| HNT-3 | White | |||
| HNT-4 | Orange | 100% VEG, India origin, does not contain PPD, clinically tested | ||
| HNT-5 | Pink | Paste | None | |
| HNT-6 | Reddish | |||
| HNT-7 | Green | |||
| HNT-8 | Blue | |||
| HNT-9 | Violet | |||
| HNT-10 | Black | |||
| HNT-11 | Brown | Paste | None | 100% VEG, India origin |
| HNT-12 | Red | |||
| HNT-13 | Black | Paste | None | Pakistan origin |
| HNT-14 | Red | Paste | None | Pakistan origin |
| JNT-1 | Black | Paste | Genipa americana fruit juice, xanthan gum, citric acid, potassium sorbate, Lavandula angustifolia flower oil, limonene, linalool | Non tested on animals, non-toxic, PPD free, latex free |
| JNT-2 | Black | Paste | Water, alcohol denat, Genipa americana, xanthan gum, citric acid, potassium sorbate | 100% Natural, for external use only |
| JNT-3 | Black | Paste | Genipa americana fruit extract, xanthan gum, citric acid, Lavandula angustifolia herb oil, potassium sorbate | Dermatologically tested, vegan |
| JNT-4 | Black | Solid | Genipa Americana, sugar, xanthan gum, citric acid | Safe and non-toxic, not for use by children 12 years and under, adult supervision advised, non-permanent, 100% natural |
| HPT | Black | Liquid-paste | None | Herbaceous plant tattoo fluid. vegetable dye, skin retention time varies between individuals because skin metabolism is slightly different |
| Compound | Linearity | Precision RSD (%) | LODs (μg·mL−1) | LOQs (μg·mL−1) |
|---|---|---|---|---|
| R2 | Intra-Day a | |||
| Genipin | 0.9994 | 0.9 | 0.1 | 0.3 |
| Geniposide | 0.9991 | 1.4 | 0.1 | 0.4 |
| Lawsone | 0.9990 | 1.3 | 0.2 | 0.6 |
| HPLC-DAD Analysis | ||||
|---|---|---|---|---|
| Genipin | Geniposide | |||
| Sample Code | (μg·mL−1) | (μg·g−1) | (μg·mL−1) | (μg·g−1) |
| JNT-1 | 4.44 | 2051.10 | 0.40 | 184.92 |
| JNT-2 | 8.43 | 3060.32 | − | − |
| JNT-3 | 0.32 | 133.61 | − | − |
| JNT-4 | 25.86 | 11,423.48 | 13.09 | 5783.10 |
| HPT | − | − | − | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio, L.; Lores, M.; Garcia-Jares, C. Monitoring of Natural Pigments in Henna and Jagua Tattoos for Fake Detection. Cosmetics 2020, 7, 74. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics7040074
Rubio L, Lores M, Garcia-Jares C. Monitoring of Natural Pigments in Henna and Jagua Tattoos for Fake Detection. Cosmetics. 2020; 7(4):74. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics7040074
Chicago/Turabian StyleRubio, Laura, Marta Lores, and Carmen Garcia-Jares. 2020. "Monitoring of Natural Pigments in Henna and Jagua Tattoos for Fake Detection" Cosmetics 7, no. 4: 74. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics7040074