Optimization of Ultrasound-Assisted Emulsification of Emollient Nanoemulsions of Seed Oil of Passiflora edulis var. edulis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vegetal Material
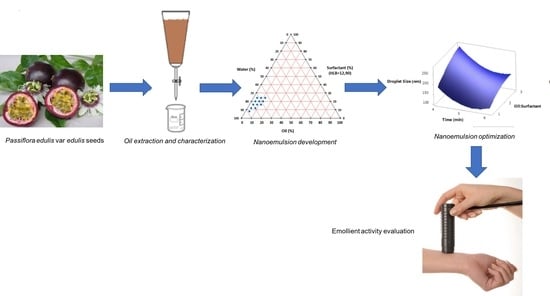
2.2. Extraction of P. edulis var. edulis Seed Oil (PEO)
2.3. Chemical Characterization of Seed Oil of Passiflora edulis var. edulis
2.3.1. Preparation of Methyl Esters of Fatty Acids
2.3.2. Gas Chromatography Analysis Coupled to GC-MS Mass Spectrometry
2.4. Physicochemical Characterization of Fixed Seed Oil of P. edulis var. edulis
Anisidine Index
2.5. Preparation of the Nanoemulsion Containing Soil of Passiflora edulis var. edulis
2.6. Optimization of Nanoemulsions Containing Seed Oil of Passiflora edulis var. edulis
2.7. Characterization of Nanoemulsions of the Optimized Design
2.7.1. Droplet Size Analysis, PDI, Potential ζ and p-Anisidine Value of Nanoemulsions of the Optimized Design
2.7.2. Transmission Electron Microscopy (TEM)
2.7.3. Viscosity, pH and Conductivity
2.8. Stability Evaluation
2.9. Evaluation of Influence of Nanoemulsions of Seed Oil of Passiflora edulis var. edulis on Skin Parameters
2.9.1. Skin Viscoelasticity and Firmness
2.9.2. Transepidermal Water Loss
2.10. Statistical Analysis
3. Results and Discussion
3.1. Chemical and Physicochemical Characterization of Fixed Seed Oil of Passiflora edulis var. edulis
3.2. Preparation of the Nanoemulsion Containing Seed Oil of Passiflora edulis var. edulis
3.3. Optimization of Nanomulsions Containing Seed Oil of Passiflora edulis var. edulis
3.3.1. Response Surface Models
3.3.2. Analysis of the Response Surface of the Effects of the Formulation Variables on the Droplet Size of the Nanoemulsions
3.3.3. Analysis of the Response Surface of the Effects of the Formulation Variables on the PDI of the Nanoemulsions
3.3.4. Analysis of the Response Surface of the Effects of the Formulation Variables on the Potential ζ of the Nanoemulsions
3.3.5. Analysis of the Response Surface of the Effects of the Formulation Variables on the p-Anisidine Value of the Nanoemulsions
3.4. Validation of the Model for Optimized Nanoemulsions Containing Seed Oil of Passiflora edulis var. edulis
3.5. Stability Evaluation of the Optimized Nanoemulsion of Passiflora edulis var. edulis
3.6. Evaluation of the Emollient Effect of the Seed Oil of Passiflora edulis var. edulis and Its Optimized Nanoemulsion
3.6.1. Transepidermal Water Loss
3.6.2. Skin Moisturizing
3.6.3. Skin Viscoelasticity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aburjai, T.; Natsheh, F.M. Plants Used in Cosmetics. Phyther. Res. 2003, 17, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Guglielmini, G. Nanostructured novel carrier for topical application. Clin. Derm. 2008, 26, 341–346. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Xiao, H. Potential biological fate of ingested nanoemulsions: Influence of particle characteristics. Food Funct. 2012, 3, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Coca, C.A.; Nates Parra, G.; Ospina Torres, R.; Melo Ortiz, C.D. Biología floral y reproductiva de la gulupa Passiflora edulis sims F. edulis. Caldasia 2011, 33, 433–451. [Google Scholar]
- Hernandez, Y.; Aragón, M.; Martinez, J.; Rojas, M. Evaluation of Passiflora tripartita var. mollisima seed oil as potential nanoemulsion excipient. J. Excip. Food Chem. 2018, 9, 16–27. [Google Scholar]
- The Commission of the European Communities. Commission Regulation (EC) No. 796/2002 of May 6 of 2002; Amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-pomace oil and on the relevant methods of analysis. Off. J. Eur. Union. 2002, 15, 23–26. [Google Scholar]
- International Organization for Standardization. ISO 5508: 1990: Animal and Vegetable Fats and Oils—Analysis by Gas Chromatography of Methyl Esters of Fatty Acids; International Organization for Standardization: Geneva, Switzerland, 1990; Volume 2, p. 12. [Google Scholar]
- U.S. Pharmacopoeia-National Formulary (USP 40 NF 35); United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2017; Volume 1, Chemical Test (401). Fats and Fixed oils; pp. 332–336.
- Alzorqi, I.; Ketabchi, M.R.; Sudheer, S.; Manickam, S. Optimization of ultrasound induced emulsification on the formulation of palm-olein based nanoemulsions for the incorporation of antioxidant β-d-glucan polysaccharides. Ultrason. Sonochem. 2016, 31, 71–84. [Google Scholar] [CrossRef]
- Rodrigues, R.F.; Costa, I.C.; Almeida, F.B.; Cruz, R.A.S.; Ferreira, A.M.; Vilhena, J.C.E.; Florentino, A.C.; Carvalho, J.C.T.; Fernandes, C.P. Development and characterization of evening primrose (Oenothera biennis) oil nanoemulsions. Rev. Bras. Farm. 2015, 25, 422–425. [Google Scholar] [CrossRef] [Green Version]
- Estanqueiro, M.; Conceição, J.; Amaral, M.H.; Sousa Lobo, J.M. Characterization, sensorial evaluation and moisturizing efficacy of nanolipidgel formulations. Int. J. Cosmet. Sci. 2014, 36, 159–166. [Google Scholar] [CrossRef]
- Kapoor, S.; Saraf, S. Formulation and Evaluation of Moisturizer Containing Herbal Extracts for the Management of Dry Ski. Pharmacogn. J. 2010, 2, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, S.; Saraf, S. Assessment of viscoelasticity and hydration effect of herbal moisturizers using bioengineering techniques. Pharm. Mag. 2010, 6, 298–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahshawat, M.S.; Saraf, S.; Saraf, S. Preparation and characterization of herbal creams for improvement of skin viscoelastic properties. Int. J. Cosmet. Sci. 2008, 30, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Malacrida, C.R.; Jorge, N. Yellow passion fruit seed oil (Passiflora edulis f. flavicarpa): Physical and chemical characteristics. Braz. Arch. Biol. Technol. 2012, 55, 127–134. [Google Scholar]
- Liu, S.; Yang, F.; Li, J.; Zhang, C.; Ji, H.; Hong, P. Physical and chemical analysis of Passiflora seeds and seed oil from China. Int. J. Food Sci. Nutr. 2008, 59, 706–715. [Google Scholar] [CrossRef]
- Nyanzi, S.A.; Carstensen, B.; Schwack, W. A comparative study of fatty acid profiles of Passiflora seed oils from Uganda. J. Am. Oil Chem. Soc. 2005, 82, 41–44. [Google Scholar] [CrossRef]
- de Santana, F.C.; Shinagawa, F.B.; Araujo, E.D.; Costa, A.M.; Mancini-Filho, J. Chemical Composition and Antioxidant Capacity of Brazilian Passiflora Seed Oils. J. Food Sci. 2015, 80, C2647–C2654. [Google Scholar] [CrossRef]
- Pereira, M.G.; Hamerski, F.; Andrade, E.F.; Scheer, A.D.; Corazza, M.L. Assessment of subcritical propane, ultrasound-assisted and Soxhlet extraction of oil from sweet passion fruit (Passiflora alata Curtis) seeds. J. Supercrit. Fluids. 2017, 128, 338–348. [Google Scholar] [CrossRef]
- Labrinea, E.P.; Thomaidis, N.S.; Georgiou, C.A. Direct olive oil anisidine value determination by flow injection. Anal. Chim. Acta. 2001, 448, 201–206. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Ha, H.-K.; Lee, M.-R.; Kim, J.W.; Kim, H.-J.; Lee, W.-J. Physicochemical Property and Oxidative Stability of Whey Protein Concentrate Multiple Nanoemulsion Containing Fish Oil. J. Food Sci. 2017, 82, 437–444. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.A.; Vicente, A.A. Influence of surfactant and processing conditions in the stability of oil-in-water nanoemulsions. J. Food Eng. 2015, 167, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Xu, J. Thermophysical Properties and SANS Studies of Nanoemulsion Heat Transfer Fluids. In Neutron Scattering; InTech: London, UK, 2016. [Google Scholar]
- Woitiski, C.B.; Veiga, F.; Ribeiro, A.; Neufeld, R. Design for optimization of nanoparticles integrating biomaterials for orally dosed insulin. Eur. J. Pharm. Biopharm. 2009, 73, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kentish, S.; Wooster, T.J.; Ashokkumar, M.; Balachandran, S.; Mawson, R.; Simons, L. The use of ultrasonics for nanoemulsion preparation. Innov. Food Sci. Emerg. Technol. 2008, 9, 170–175. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Li, P.H.; Chiang, B.H. Process optimization and stability of D-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface methodology. Ultrason. Sonochem. 2012, 19, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Chanana, G.D.; Sheth, B.B. Particle Size Reduction of Emulsions by Formulation Design-II: Effect of Oil and Surfactant Concentration. PDA J. Pharm Sci. Technol. 1995, 49, 71–76. [Google Scholar]
- Leong, T.S.H.; Wooster, T.J.; Kentish, S.E.; Ashokkumar, M. Minimising oil droplet size using ultrasonic emulsification. Ultrason. Sonochem. 2009, 16, 721–727. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Gao, Y.; Zhao, J.; Mao, L. Characterization and stability evaluation of β-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditions. Food Res. Int. 2008, 41, 61–68. [Google Scholar] [CrossRef]
- Meor Mohd, A.; Bustami Effendi, T.J.; Abdul Majeed, A.B. Development and stability evaluation of Astaxanthin nanoemulsion. Asian J. Pharm. Clin. Res. 2011, 4, 143–148. [Google Scholar]
- Wang, L.; Dong, J.; Chen, J.; Eastoe, J.; Li, X. Design and optimization of a new self-nanoemulsifying drug delivery system. J. Colloid Interface Sci. 2009, 330, 443–448. [Google Scholar] [CrossRef]
- Maruno, M.; da Rocha-Filho, P.A. O/W Nanoemulsion after 15 Years of Preparation: A Suitable Vehicle for Pharmaceutical and Cosmetic Applications. J. Dispers. Sci. Technol. 2009, 31, 17–22. [Google Scholar] [CrossRef]
- Canselier, J.P.; Delmas, H.; Wilhelm, A.M.; Abismaïl, B. Ultrasound Emulsification—An Overview. J. Dispers. Sci. Technol. 2002, 23, 333–349. [Google Scholar] [CrossRef]
- Ngan, C.L.; Basri, M.; Lye, F.F.; Fard Masoumi, H.R.; Tripathy, M.; Abedi Karjiban, R.; Abdul-Malek, E. Comparison of process parameter optimization using different designs in nanoemulsion-based formulation for transdermal delivery of fullerene. Int. J. Nanomed. 2014, 9, 4375–4386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, D.D.; Colombo, M.; Kelmann, R.G.; Kaiser, S.; Lucca, L.G.; Teixeira, H.F.; Limberger, R.P.; Veiga, V.F., Jr.; Koester, L.S. Optimization of Copaiba oil-based nanoemulsions obtained by different preparation methods. Ind. Crops Prod. 2014, 59, 154–162. [Google Scholar] [CrossRef]
- Manev, E.D.; Pugh, R.J. Diffuse layer electrostatic potential and stability of thin aqueous films containing a nonionic surfactant. Langmuir 1991, 7, 2253–2260. [Google Scholar] [CrossRef]
- Ribeiro, R.; Barreto, S.; Ostrosky, E.; Rocha-Filho, P.; Veríssimo, L.; Ferrari, M. Production and Characterization of Cosmetic Nanoemulsions Containing Opuntia ficus-indica (L.) Mill Extract as Moisturizing Agent. Molecules 2015, 20, 2492–2509. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, T.; Ahmad, A.; Ahmed, A.; Ahmed, Z. Optimization of olive oil based O/W nanoemulsions prepared through ultrasonic homogenization: A response surface methodology approach. Food Chem. 2017, 229, 790–796. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmed, A.; Ahmad, A.; Ahmad, M.S.; Sandhu, M.A. Optimization of mixed surfactants-based β-carotene nanoemulsions using response surface methodology: An ultrasonic homogenization approach. Food Chem. 2018, 253, 179–184. [Google Scholar] [CrossRef]
- Bernardi, D.S.; Pereira, T.A.; Maciel, N.R.; Bortoloto, J.; Viera, G.S.; Oliveira, G.C.; Rocha-Filho, P.A. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: In vitro and in vivo assessments. J. Nanobiotechnol. 2011, 9, 44–47. [Google Scholar] [CrossRef]
- De Morais, J.M.; Dos Santos, O.D.H.; Delicato, T.; Da Rocha-Filho, P.A. Characterization and Evaluation of Electrolyte Influence on Canola Oil/Water Nano-Emulsion. J. Dispers. Sci. Technol. 2006, 27, 1009–1014. [Google Scholar] [CrossRef]
- Ahmad Mustafa, M.E.; Nagib Ali, E.; Hesham Ali, E. Preparation and Evaluation of Olive Oil Nanoemulsion Using Sucrose. Int. J. Pharm. Pharm. Sci. 2013, 5, 434–440. [Google Scholar]
- Eid, A.M.M.; Baie, S.H.; Arafat, O. Development and stability evaluation of olive oil nanoemulsion using sucrose monoester laurate. AIP Conf. Proc. 2012, 1502, 486–494. [Google Scholar]
- Rao, J.; McClements, D.J. Food-grade microemulsions, nanoemulsions and emulsions: Fabrication from sucrose monopalmitate & lemon oil. Food Hydrocoll. 2011, 25, 1413–1423. [Google Scholar]
- Zhang, X.; Sun, X.; Li, J.; Zhang, X.; Gong, T.; Zhang, Z. Lipid nanoemulsions loaded with doxorubicin-oleic acid ionic complex: Characterization, in vitro and in vivo studies. Die Pharm. 2011, 66, 496–505. [Google Scholar]
- Ahmad, K.; Ho, C.C.; Fong, W.K.; Toji, D. Properties of Palm Oil-in-Water Emulsions Stabilized by Nonionic Emulsifiers. J. Colloid Interface Sci. 1996, 181, 595–604. [Google Scholar] [CrossRef]
- Tan, C.; Nakajima, M. β-Carotene nanodispersions: Preparation, characterization and stability evaluation. Food Chem. 2005, 92, 661–671. [Google Scholar] [CrossRef]
- Fluhr, J.W. Practical Aspects of Cosmetic Testing; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Dal’Belo, S.E.; Rigo Gaspar, L. Berardo Gonçalves, P.M.; Moisturizing effect of cosmetic formulations containing Aloe vera extract in different concentrations assessed by skin bioengineering techniques. Ski. Res. Technol. 2006, 12, 241–246. [Google Scholar] [CrossRef]
- Bonté, F. Skin moisturization mechanisms: New data. Ann. Pharm. Fr. 2011, 69, 135–141. [Google Scholar] [CrossRef]
- Bolzinger, M.A.; Briançon, S.; Chevalier, Y. Nanoparticles through the skin: Managing conflicting results of inorganic and organic particles in cosmetics and pharmaceutics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 463–478. [Google Scholar] [CrossRef]
- Kowalska, M.; Wozniak, M.; Pazdzior, M. Assessment of the sensory and moisturizing properties of emulsions with hemp oil. Acta Polytech. Hung. 2017, 14, 183–195. [Google Scholar]











| Injection Volume | 1.0 µL |
|---|---|
| Injection split | 10:1 |
| Capillary Column | Thermo Scientific ™TRACE™ TR1 30 m × 0.25 mm |
| Film thickness | 0.25 µm |
| Injection port temperature | 220 ° C |
| Carrier gas | Helium (≥99.9% purity) |
| Flow rate | 1.0 mL/min |
| Initial oven temperature | 180 °C (15 min) |
| Rate of increase of temperature of the furnace | 0.5 °C/min |
| Final temperature of the oven | 193 °C (43 min) |
| Independent Variables | Codes | Levels | ||
|---|---|---|---|---|
| −1 (−α) | 0 | 1 (+α) | ||
| Power (W) | A | 70 | 80 | 90 |
| Time (min) | B | 4 | 5 | 6 |
| Water (%) | C | 70 | 80 | 90 |
| Oil: Surfactant ratio | D | 1 | 2 | 3 |
| DAY 1 | DAY 7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | % PEO | % S | % W | PEO:S | Droplet Size (nm) | PDI | ζ Potential (mV) | Droplet Size (nm) | PDI | ζ Potential (mV) |
| 1 | 4 | 8 | 88 | 1:02 | 56.72 ± 0.6988 | 0.357 ± 0.0017 | −29.10 ± 0.6798 | 60.87 ± 0.5719 | 0.471 ± 0.0033 | −21.8 ± 0.3091 |
| 2 | 4 | 12 | 84 | 1:03 | 41.59 ± 0.3229 | 0.366 ± 0.0024 | −17.40 ± 1.2657 | 42.01 ± 0.7450 | 0.532 ± 0.0266 | −20.6 ± 0.3741 |
| 3 | 4 | 16 | 80 | 1:04 | 46.60 ± 1.5248 | 0.592 ± 0.0925 | −38.00 ± 2.2691 | 37.81 ± 0.9338 | 0.581 ± 0.0252 | −16.2 ± 0.6847 |
| 4 | 4 | 20 | 76 | 1:05 | 85.66 ± 0.6907 | 0.087 ± 0.0128 | −32.60 ± 1.1085 | 86.04 ± 1.1880 | 0.092 ± 0.0176 | −17.1 ± 0.1577 |
| 5 | 4 | 24 | 72 | 1:06 | 88.79 ± 0.6150 | 0.103 ± 0.0164 | −39.00 ± 0.0471 | 89.31 ± 0.5897 | 0.081 ± 0.0107 | −19.9 ± 06128 |
| 6 | 8 | 4 | 88 | 2:01 | 171.10 ± 1.7795 | 0.219 ± 0.0046 | −31.40 ± 1.1430 | 166.00 ± 1.7795 | 0.222 ± 0.0046 | −36.8 ± 0.2494 |
| 7 | 8 | 8 | 84 | 1:01 | 106.10 ± 1.0625 | 0.224 ± 0.0237 | −37.80 ± 0.5734 | 106.00 ± 1.0625 | 0.228 ± 0.0237 | −42.1 ± 0.5734 |
| 8 | 8 | 12 | 80 | 2:03 | 83.26 ± 1.3258 | 0.292 ± 0.0024 | −33.10 ± 1.5195 | 90.80 ± 1.0581 | 0.322 ± 0.0005 | −28.9 ± 0.3500 |
| 9 | 8 | 16 | 76 | 1:02 | 59.72 ± 1.8150 | 0.354 ± 0.0305 | −29.60 ± 0.3681 | 61.19 ± 0.4648 | 0.486 ± 0.0065 | −26.2 ± 1.1953 |
| 10 | 8 | 20 | 72 | 2:05 | 64.34 ± 1.7478 | 0.688 ± 0.0384 | −20.70 ± 1.4884 | 56.66 ± 1.1153 | 0.432 ± 0.1970 | −22.5 ± 2.1275 |
| 11 | 8 | 24 | 68 | 1:03 | 34.47 ± 0.1078 | 0.256 ± 0.0034 | −21.20 ± 1.3735 | 33.98 ± 1.7598 | 0.447 ± 0.0400 | −20.7 ± 1.5412 |
| 12 | 12 | 4 | 84 | 3:01 | 195.50 ± 2.2106 | 0.232 ± 0.0094 | −42.90 ± 0.4320 | 189.50 ± 2.5850 | 0.223 ± 0.0041 | −37.4 ± 0.6480 |
| 13 | 12 | 8 | 80 | 3:02 | 133.50 ± 0.1886 | 0.178 ± 0.0078 | −33.20 ± 0.7788 | 139.00 ± 1.4055 | 0.186 ± 0.0025 | −32.8 ± 0.8060 |
| 14 | 12 | 12 | 76 | 1:01 | 136.90 ± 1.7745 | 0.201 ± 0.0092 | −29.80 ± 0.8259 | 135.30 ± 1.8673 | 0.176 ± 0.0096 | −36.3 ± 0.8055 |
| 15 | 12 | 16 | 72 | 3:04 | 81.97 ± 0.5107 | 0.218 ± 0.0045 | −28.80 ± 1.4522 | 84.00 ± 1.0138 | 0.252 ± 0.0016 | −26.6 ± 1.3021 |
| 16 | 12 | 20 | 68 | 3:05 | 72.95 ± 1.6562 | 0.275 ± 0.0059 | −23.90 ± 1.2027 | 73.84 ± 0.9060 | 0.303 ± 0.0031 | −26.9 ± 0.4921 |
| 17 | 12 | 24 | 64 | 1:02 | 52.68 ± 0.2423 | 0.26 ± 0.0014 | −18.40 ± 0.6847 | 55.27 ± 1.1875 | 0.458 ± 0.0290 | −22.4 ± 1.0873 |
| 18 | 16 | 4 | 80 | 4:01 | 219.00 ± 2.0532 | 0.239 ± 0.0071 | −41.90 ± 0.6164 | 305.00 ± 1.7259 | 0.447 ± 0.0076 | −44.4 ± 0.6480 |
| 19 | 16 | 8 | 76 | 2:01 | 165.40 ± 2.0072 | 0.208 ± 0.0198 | −38.00 ± 1.4337 | 162.30 ± 1.8457 | 0.215 ± 0.0041 | −43.9 ± 0.6128 |
| 20 | 16 | 12 | 72 | 4:03 | 169.40 ± 1.4166 | 0.251 ± 0.0082 | −35.00 ± 1.2027 | 168.90 ± 0.9978 | 0.258 ± 0.0107 | −40.3 ± 0.0816 |
| 21 | 16 | 16 | 68 | 1:01 | 131.00 ± 1.9754 | 0.239 ± 0.0128 | −30.50 ± 0.6531 | 127.0 ± 1.1614 | 0.211 ± 0.0118 | −32.5 ± 0.9285 |
| 22 | 20 | 4 | 76 | 5:01 | 281.70 ± 1.8642 | 0.377 ± 0.0052 | −47.60 ± 0.7845 | 272.6 ± 0.4643 | 0.375 ± 0.0135 | −46.2 ± 1.3767 |
| 23 | 20 | 8 | 72 | 5:02 | 202.50 ± 1.0652 | 0.254 ± 0.0043 | −29.70 ± 0.1885 | 184.1 ± 2.5223 | 0.21 ± 0.0133 | −36.8 ± 0.0816 |
| 24 | 20 | 12 | 68 | 5:03 | 166.90 ± 1.9431 | 0.236 ± 0.0054 | −30.70 ± 1.4429 | 156.4 ± 1.6438 | 0.202 ± 0.0048 | −32.5 ± 0.4109 |
| Independent Variables. | Response Variables | |||||||
|---|---|---|---|---|---|---|---|---|
| No | W (%) | PEO:S | Power (W) | Time (m) | Droplet Size (nm) | PDI | Potential ζ (mV) | p-Anisidine Value |
| 1 | 70 | 3:01 | 80 | 5 | 197.6 ± 2.6281 | 0.212 ± 0.0135 | 39.90 ± 0.2625 | 5.2187 ± 0.2730 |
| 2 | 80 | 3:01 | 90 | 5 | 195.5 ± 1.4384 | 0.224 ± 0.0111 | 37.20 ± 0.3266 | 5.3447 ± 0.1274 |
| 3 | 90 | 2:01 | 90 | 5 | 167.1 ± 1.9816 | 0.207 ± 0.0065 | 39.10 ± 0.4190 | 4.7094 ± 1.1095 |
| 4 | 90 | 2:01 | 70 | 5 | 175.0 ± 1.1431 | 0.238 ± 0.0034 | 39.50 ± 0.5793 | 5.7191 ± 0.0152 |
| 5 | 90 | 1:01 | 80 | 5 | 108.5 ± 1.1441 | 0.236 ± 0.0019 | 38.20 ± 0.7483 | 5.9547 ± 0.4382 |
| 6 | 70 | 1:01 | 80 | 5 | 122.9 ± 1.3474 | 0.184 ± 0.0123 | 32.80 ± 1.2832 | 6.4179 ± 0.2582 |
| 7 | 70 | 2:02 | 80 | 4 | 168.2 ± 2.7797 | 0.198 ± 0.0061 | 37.70 ± 0.2055 | 6.0636 ± 0.7051 |
| 8 | 90 | 2:02 | 80 | 4 | 183.7 ± 2.5747 | 0.224 ± 0.0043 | 39.80 ± 0.1414 | 6.6534 ± 0.5350 |
| 9 | 80 | 2:02 | 80 | 5 | 161.4 ± 0.5907 | 0.198 ± 0.0082 | 38.20 ± 0.4320 | 5.8233 ± 0.0495 |
| 10 | 80 | 1:01 | 70 | 5 | 132.9 ± 1.6990 | 0.233 ± 0.0033 | 37.70 ± 0.6481 | 6.2062 ± 0.1885 |
| 11 | 70 | 2:02 | 70 | 5 | 205.1 ± 2.2485 | 0.265 ± 0.0090 | 39.60 ± 0.5354 | 7.9133 ± 0.0339 |
| 12 | 80 | 2:02 | 70 | 6 | 170.1 ± 1.8779 | 0.225 ± 0.0123 | 38.30 ± 1.0614 | 7.5773 ± 0.0131 |
| 13 | 80 | 2:02 | 80 | 5 | 167.3 ± 1.2257 | 0.210 ± 0.0012 | 36.00 ± 1.0965 | 7.5622 ± 0.5468 |
| 14 | 80 | 2:02 | 80 | 5 | 166.1 ± 1.5297 | 0.203 ± 0.0140 | 37.80 ± 0.6377 | 6.0865 ± 0.3119 |
| 15 | 80 | 1:01 | 90 | 5 | 107.2 ± 1.2356 | 0.218 ± 0.0050 | 36.70 ± 0.5437 | 5.1968 ± 0.2999 |
| 16 | 80 | 3:01 | 80 | 6 | 193.3 ± 0.9463 | 0.212 ± 0.0074 | 36.90 ± 0.0943 | 5.7673 ± 0.3119 |
| 17 | 90 | 2:02 | 80 | 6 | 168.9 ± 1.3199 | 0.215 ± 0.0037 | 36.50 ± 0.5558 | 6.8725 ± 0.2999 |
| 18 | 80 | 3:03 | 80 | 4 | 285.3 ± 2.0237 | 0.431 ± 0.0062 | 46.60 ± 0.3300 | 4.5363 ± 1.5573 |
| 19 | 80 | 2:01 | 90 | 6 | 178.3 ± 2.4536 | 0.241 ± 0.0070 | 35.90 ± 0.4922 | 4.9296 ± 0.2981 |
| 20 | 90 | 3:01 | 80 | 5 | 170.8 ± 0.8042 | 0.224 ± 0.043 | 47.40 ± 1.0403 | 5.5833 ± 0.1280 |
| 21 | 70 | 2:01 | 80 | 6 | 164.3 ± 0.8731 | 0.180 ± 0.0142 | 35.00 ± 0.6848 | 6.0818 ± 1.0589 |
| 22 | 80 | 1:01 | 80 | 4 | 177.0 ± 3.0137 | 0.369 ± 0.0347 | 40.90 ± 2.2196 | 6.4257 ± 0.6207 |
| 23 | 80 | 1:01 | 80 | 6 | 117.4 ± 0.4320 | 0.227 ± 0.0127 | 38.40 ± 1.8625 | 6.3627 ± 0.5862 |
| 24 | 70 | 2:01 | 90 | 5 | 166.4 ± 1.9224 | 0.188 ± 0.0053 | 35.50 ± 0.2625 | 6.9292 ± 0.7144 |
| 25 | 80 | 3:01 | 70 | 5 | 197.5 ± 0.4082 | 0.245 ± 0.0070 | 38.50 ± 0.6481 | 6.3500 ± 0.5181 |
| 26 | 80 | 2:01 | 70 | 4 | 177.0 ± 2.3156 | 0.251 ± 0.0029 | 41.90 ± 1.1086 | 5.3320 ± 0.8545 |
| 27 | 80 | 2:01 | 90 | 4 | 279.1 ± 2.1417 | 0.351 ± 0.0151 | 38.40 ± 1.7250 | 4.5409 ± 0.8396 |
| Variable | Drop Size | PDI | Potential ζ | p-Anisidine | |||||
|---|---|---|---|---|---|---|---|---|---|
| F Value | p Value | F Value | p Value | F Value | p Value | F Value | p Value | ||
| Linear terms | A | 12.28 | 0.008 | 8.84 | 0.025 | 12.63 | 0.007 | 6.08 | 0.03 |
| B | 147.23 | 0.000 | 0.06 | 0.819 | 25.77 | 0.001 | 1.79 | 0.206 | |
| C | 11.17 | 0.010 | 35.24 | 0.001 | 3.55 | 0.096 | 1.07 | 0.32 | |
| D | 335.93 | 0.000 | 2.58 | 0.159 | 1.42 | 0.267 | 1.55 | 0.237 | |
| Quadratic terms | A2 | 7.92 | 0.023 | 15.35 | 0.008 | 5.28 | 0.051 | 0.84 | 0.376 |
| B2 | 104.77 | 0.000 | 1.12 | 0.331 | 0.01 | 0.916 | 0.62 | 0.446 | |
| C2 | 0.23 | 0.644 | 0.75 | 0.420 | 0.07 | 0.799 | 0.04 | 0.848 | |
| D2 | 20.02 | 0.002 | 7.54 | 0.033 | 1.96 | 0.199 | 1.90 | 0.194 | |
| Terms of interactions | AB | 5.14 | 0.053 | 8.79 | 0.025 | 0.28 | 0.608 | 1.13 | 0.308 |
| AC | 4.25 | 0.073 | 0.29 | 0.608 | 3.21 | 0.111 | 0.00 | 0.989 | |
| AD | 2.52 | 0.151 | 0.16 | 0.702 | 0.02 | 0.888 | 0.00 | 0.998 | |
| BC | 3.55 | 0.096 | 0.00 | 0.973 | 0.08 | 0.779 | 0.01 | 0.910 | |
| BD | 4.71 | 0.062 | 3.95 | 0.094 | 4.89 | 0.058 | 0.55 | 0.472 | |
| CD | 0.69 | 0.430 | 7.15 | 0.037 | 6.32 | 0.036 | 0.23 | 0.644 | |
| Response Variable | Predicted Value | Experimental Value | Error (%) |
|---|---|---|---|
| Drop Size (nm) | 130.00 | 130.33 ± 0.858 | 0.25 |
| PDI | 0.200 | 0.202 ± 0.015 | 0.80 |
| Potential ζ | * 30.00 | −30.40 ± 0.829 | 1.33 |
| p-anisidine | 6.00 | 5.44 ± 0.235 | 9.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán, C.; Rojas, M.A.; Aragón, M. Optimization of Ultrasound-Assisted Emulsification of Emollient Nanoemulsions of Seed Oil of Passiflora edulis var. edulis. Cosmetics 2021, 8, 1. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics8010001
Guzmán C, Rojas MA, Aragón M. Optimization of Ultrasound-Assisted Emulsification of Emollient Nanoemulsions of Seed Oil of Passiflora edulis var. edulis. Cosmetics. 2021; 8(1):1. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics8010001
Chicago/Turabian StyleGuzmán, Caterine, Maritza Adelina Rojas, and Marcela Aragón. 2021. "Optimization of Ultrasound-Assisted Emulsification of Emollient Nanoemulsions of Seed Oil of Passiflora edulis var. edulis" Cosmetics 8, no. 1: 1. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics8010001