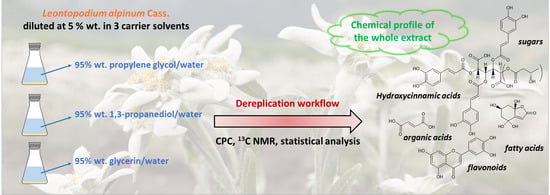
Dereplication of Natural Extracts Diluted in Propylene Glycol, 1,3-Propanediol and Glycerin. Comparison of Leontopodium alpinum Cass. (Edelweiss) Extracts as a Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals and Reagents
2.1.2. Plant Material and Preparation of the Extracts
2.2. Ternary Diagrams
2.3. Evaporation of Propanediols
2.4. Centrifugal Partition Chromatography
2.5. NMR Analysis and Dereplication
2.6. Analyses by LC-MS and HPTLC
3. Results and Discussion
3.1. Development of a Strategy for the Physical Suppression of Propylene Glycol and Propanediol in Natural Extracts
3.2. Workflow for the Dereplication of Natural Extracts Diluted in Glycerin, Propylene Glycol and Propanediol
3.3. Implementation of the Dereplication Workflow on an Extract of L. alpinum Diluted in Propylene Glycol, Propanediol and Glycerin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The European Parliament and the Council of the European Union Regulation. (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union 2009, 342, 59–209.
- Fernandez, X.; Michel, T.; Kerdudo, A. Analyse des principes actifs et substances réglementées en cosmétique. Tech. l’Ingénieur 2015. Available online: https://www.techniques-ingenieur.fr/base-documentaire/procedes-chimie-bio-agro-th2/cosmetiques-produits-42689210/analyse-des-principes-actifs-et-substances-reglementees-en-cosmetique-j3300/ (accessed on 30 November 2020).
- Pellegrini, M.; Marchei, E.; Pacifici, R.; Rotolo, M.C.; Pichini, S. Advances in the analysis of non-allowed pharmacologically active substances in cosmetic products. J. Pharm. Biomed. Anal. 2011, 55, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Coptis Ingredients. Available online: https://www.coptis.com/Coptis-Ingredients-Solution?lang=fr (accessed on 21 January 2021).
- Kerdudo, A.; Fontaine-Vive, F.; Dingas, A.; Faure, C.; Fernandez, X. Optimization of cosmetic preservation: Water activity reduction. Int. J. Cosmet. Sci. 2015, 37, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Herman, A. Antimicrobial Ingredients as Preservative Booster and Components of Self-Preserving Cosmetic Products. Curr. Microbiol. 2019, 76, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Canton, M.; Hubert, J.; Poigny, S.; Roe, R.; Brunel, Y.; Nuzillard, J.-M.; Renault, J.-H. Dereplication of Natural Extracts Diluted in Glycerin: Physical Suppression of Glycerin by Centrifugal Partition Chromatography Combined with Presaturation of Solvent Signals in 13C-Nuclear Magnetic Resonance Spectroscopy. Molecules 2020, 25, 5061. [Google Scholar] [CrossRef] [PubMed]
- Hubert, J.; Nuzillard, J.-M.; Purson, S.; Hamzaoui, M.; Borie, N.; Reynaud, R.; Renault, J.-H. Identification of Natural Metabolites in Mixture: A Pattern Recognition Strategy Based on 13C NMR. Anal. Chem. 2014, 86, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Alabdul Magid, A.; Hubert, J.; Etique, N.; Duca, L.; Voutquenne-Nazabadioko, L. Bio-guided isolation of new phenolic compounds from Hippocrepis emerus flowers and investigation of their antioxidant, tyrosinase and elastase inhibitory activities. Phytochem. Lett. 2020, 35, 28–36. [Google Scholar] [CrossRef]
- Abedini, A.; Colin, M.; Hubert, J.; Charpentier, E.; Angelis, A.; Bounasri, H.; Bertaux, B.; Kotland, A.; Reffuveille, F.; Nuzillard, J.-M.; et al. Abundant Extractable Metabolites from Temperate Tree Barks: The Specific Antimicrobial Activity of Prunus Avium Extracts. Antibiotics 2020, 9, 111. [Google Scholar] [CrossRef] [Green Version]
- Angelis, A.; Hubert, J.; Aligiannis, N.; Michalea, R.; Abedini, A.; Nuzillard, J.-M.; Gangloff, S.; Skaltsounis, A.-L.; Renault, J.-H. Bio-Guided Isolation of Methanol-Soluble Metabolites of Common Spruce (Picea abies) Bark by-Products and Investigation of Their Dermo-Cosmetic Properties. Molecules 2016, 21, 1586. [Google Scholar] [CrossRef] [Green Version]
- Hubert, J.; Chollet, S.; Purson, S.; Reynaud, R.; Harakat, D.; Martinez, A.; Nuzillard, J.-M.; Renault, J.-H. Exploiting the Complementarity between Dereplication and Computer-Assisted Structure Elucidation for the Chemical Profiling of Natural Cosmetic Ingredients: Tephrosia purpurea as a Case Study. J. Nat. Prod. 2015, 78, 1609–1617. [Google Scholar] [CrossRef]
- Lehbili, M.; Alabdul Magid, A.; Hubert, J.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Renault, J.-H.; Nuzillard, J.-M.; Morjani, H.; Abedini, A.; Gangloff, S.C.; et al. Two new bis-iridoids isolated from Scabiosa stellata and their antibacterial, antioxidant, anti-tyrosinase and cytotoxic activities. Fitoterapia 2018, 125, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Canton, M.; Roe, R.; Poigny, S.; Renault, J.-H.; Nuzillard, J.-M. Multiple solvent signal presaturation and decoupling artifact removal in 13C{1H} nuclear magnetic resonance. Magn. Reson. 2020, 1, 155–164. [Google Scholar] [CrossRef]
- Tauchen, J.; Kokoska, L. The chemistry and pharmacology of Edelweiss: A review. Phytochem. Rev. 2016, 16, 295–308. [Google Scholar] [CrossRef]
- Schwaiger, S.; Seger, C.; Wiesbauer, B.; Schneider, P.; Ellmerer, E.P.; Sturm, S.; Stuppner, H. Development of an HPLC-PAD-MS assay for the identification and quantification of major phenolic edelweiss (Leontopodium alpium Cass.) constituents. Phytochem. Anal. 2006, 17, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Dobner, M.J.; Ellmerer, E.P.; Schwaiger, S.; Batsugkh, O.; Narantuya, S.; Stütz, M.; Stuppner, H. New Lignan, Benzofuran, and Sesquiterpene Derivatives from the Roots of Leontopodium alpinum and L. leontopodioides. Helv. Chim. Acta 2003, 86, 733–738. [Google Scholar] [CrossRef]
- Wang, L.; Ladurner, A.; Latkolik, S.; Schwaiger, S.; Linder, T.; Hošek, J.; Palme, V.; Schilcher, N.; Polanský, O.; Heiss, E.H.; et al. Leoligin, the Major Lignan from Edelweiss (Leontopodium nivale subsp. alpinum), Promotes Cholesterol Efflux from THP-1 Macrophages. J. Nat. Prod. 2016, 79, 1651–1657. [Google Scholar] [CrossRef] [Green Version]
- Marchal, L.; Intes, O.; Foucault, A.; Legrand, J.; Nuzillard, J.-M.; Renault, J.-H. Rational improvement of centrifugal partition chromatographic settings for the production of 5-n-alkylresorcinols from wheat bran lipid extract I. Flooding conditions—optimizing the injection step. J. Chromatogr. A 2003, 51–62. [Google Scholar] [CrossRef]
- Ganzera, M.; Greifeneder, V.; Schwaiger, S.; Stuppner, H. Chemical profiling of Edelweiss (Leontopodium alpinum Cass.) extracts by micellar electrokinetic capillary chromatography. Fitoterapia 2012, 83, 1680–1686. [Google Scholar] [CrossRef]
- Scharinger, B.; Messner, B.; Türkcan, A.; Schuster, D.; Vuorinen, A.; Pitterl, F.; Heinz, K.; Arnhard, K.; Laufer, G.; Grimm, M.; et al. Leoligin, the major lignan from Edelweiss, inhibits 3-hydroxy-3-methyl-glutaryl-CoA reductase and reduces cholesterol levels in ApoE −/− mice. J. Mol. Cell. Cardiol. 2016, 99, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, Y.; Ruan, J.; Chen, Q.; Li, J.; Guo, Y.; Han, L.; Wang, T. Isobenzofuranones from the aerial parts of Leontopodium leontopodioides (Wild.) Beauv. Fitoterapia 2018, 124, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Safer, S.; Cicek, S.S.; Pieri, V.; Schwaiger, S.; Schneider, P.; Wissemann, V.; Stuppner, H. Metabolic fingerprinting of Leontopodium species (Asteraceae) by means of 1H NMR and HPLC-ESI-MS. Phytochemistry 2011, 72, 1379–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renault, J.-H.; Nuzillard, J.-M.; Intes, O.; Maciuk, A. Chapter 3: Solvent systems. In Comprehensive Analytical Chromatography: Countercurrent Chromatography; Elsevier: Amsterdam, The Netherlands, 2002; Volume 38, pp. 49–83. [Google Scholar]
- Faure, K. Chromatographie de partage centrifuge—principes et applications. Tech. l’Ingénieur 2016. Available online: https://www.techniques-ingenieur.fr/base-documentaire/mesures-analyses-th1/chromatographie-et-techniques-separatives-42385210/chromatographie-de-partage-centrifuge-p1496/ (accessed on 30 November 2020).
- Mejía, A.; Cartes, M.; Chaparro, G. Isobaric vapor—liquid—liquid equilibrium for water + MTBE + alcohol (ethanol or 1-butanol) mixtures. Fluid Phase Equilibria 2020, 523, 112768. [Google Scholar] [CrossRef]
- Berthod, A.; Friesen, J.B.; Inui, T.; Pauli, G.F. Elution−Extrusion Countercurrent Chromatography: Theory and Concepts in Metabolic Analysis. Anal. Chem. 2007, 79, 3371–3382. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Propanediols Extracts with Sample Preparation (a,b) | Propanediols Extracts Without Sample Preparation (c,d) | Glycerin Extract (e) | |
|---|---|---|---|
| Biphasic solvent system | EtOAc/CH3CN/water 3:3:4 (v/v/v) | MtBE/n-BuOH/water from Σi 45:5:50 to Σf 5:45:50 (v/v/v) | EtOAc/CH3CN/water 3:3:4 (v/v/v) |
| Mode | Ascending | Ascending | Ascending |
| Elution mode | Isocratic | Gradient | Isocratic |
| Stationary phase retention | 73% | 72% | 73% |
| Mass of sample injected (dilution in stationary/mobile phases) | 6.6 g (14/4 mL) - 21.7 g (10/2 mL) | 34.989 - 30.195 g | 39.8 g |
| Elution | 0 to 20 mL/min in 5 min; 20 mL/min for 35 min | Upi 0 to 20 mL/min in 5 min; 100% Upi for 10 min; from 100% Upi to 100% Upf in 1 h; 100% Upf for 45 min | 0 to 20 mL/min in 5 min; 20 mL/min for 65 min |
| Extrusion time | 20 min | 20 min | 20 min |
| Number of pooled fractions | 9 | 12 | 13 |
| Recovery | 89–89% | 93–95% | 110% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canton, M.; Poigny, S.; Roe, R.; Nuzillard, J.-M.; Renault, J.-H. Dereplication of Natural Extracts Diluted in Propylene Glycol, 1,3-Propanediol and Glycerin. Comparison of Leontopodium alpinum Cass. (Edelweiss) Extracts as a Case Study. Cosmetics 2021, 8, 10. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics8010010
Canton M, Poigny S, Roe R, Nuzillard J-M, Renault J-H. Dereplication of Natural Extracts Diluted in Propylene Glycol, 1,3-Propanediol and Glycerin. Comparison of Leontopodium alpinum Cass. (Edelweiss) Extracts as a Case Study. Cosmetics. 2021; 8(1):10. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics8010010
Chicago/Turabian StyleCanton, Marine, Stéphane Poigny, Richard Roe, Jean-Marc Nuzillard, and Jean-Hugues Renault. 2021. "Dereplication of Natural Extracts Diluted in Propylene Glycol, 1,3-Propanediol and Glycerin. Comparison of Leontopodium alpinum Cass. (Edelweiss) Extracts as a Case Study" Cosmetics 8, no. 1: 10. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics8010010