Proteinuria-Lowering Effects of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors in Chronic Kidney Disease Patients: A Real-World Multicentric Study
Abstract
:1. Introduction
2. Results
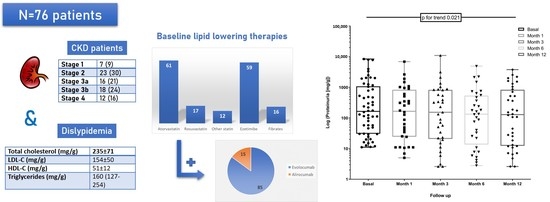
2.1. Baseline Characteristics
2.2. Baseline Kidney Function
2.3. Baseline Lipid Profile
2.4. Lipid and Kidney Outcomes
3. Discussion
4. Materials and Methods
4.1. Study Design and Patient Characteristics
4.2. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anavekar, N.S.; McMurray, J.J.; Velazquez, E.J.; Solomon, S.D.; Kober, L.; Rouleau, J.L.; White, H.D.; Nordlander, R.; Maggioni, A.; Dickstein, K.; et al. Relation Between Renal Dysfunction and Cardiovascular Outcomes after Myocardial Infarction. N. Engl. J. Med. 2004, 351, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Allon, M. Evidence-based cardiology in hemodialysis patients. J. Am. Soc. Nephrol. 2013, 24, 1934–1943. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, M.A.; Wanner, C.; Cass, A.; Garg, A.X.; Holdaas, H.; Jardine, A.G.; Jiang, L.; Kronenberg, F.; Parekh, R.S.; Shoji, T.; et al. Kidney Disease, Improving Global Outcomes (KDIGO) Lipid Work Group. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 259–305. [Google Scholar]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol, a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [PubMed] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; de Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias, lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Massy, Z.A.; Ferrières, J.; Bruckert, E.; Lange, C.; Liabeuf, S.; Velkovski-Rouyer, M.; Stengel, B. CKD-REIN Collaborators. Achievement of Low-Density Lipoprotein Cholesterol Targets in CKD. Achievement of Low-Density Lipoprotein Cholesterol Targets in CKD. Kidney Int. Rep. 2019, 4, 1546–1554. [Google Scholar] [CrossRef] [Green Version]
- Lambert, G.; Krempf, M.; Costet, P. PCSK9, a Promising Therapeutic Target for Dyslipidemias? Trends Endocrinol. Metab. 2006, 17, 79–81. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. FOURIER Steering Committee and Investigators. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Toth, P.P.; Dwyer, J.P.; Cannon, C.P.; Colhoun, H.M.; Rader, D.J.; Upadhyay, A.; Louie, M.J.; Koren, A.; Letierce, A.; Mandel, J.; et al. Efficacy and safety of lipid lowering by alirocumab in chronic kidney disease. Kidney Int. 2018, 93, 1397–1408. [Google Scholar] [CrossRef] [Green Version]
- Charytan, D.M.; Sabatine, M.S.; Pedersen, T.R.; Im, K.; Park, J.G.; Pineda, A.L.; Wasserman, S.M.; Deedwania, P.; Olsson, A.G.; Sever, P.S.; et al. FOURIER Steering Committee and Investigators. Efficacy and Safety of Evolocumab in Chronic Kidney Disease in the FOURIER Trial. J. Am. Coll. Cardiol. 2019, 73, 2961–2970. [Google Scholar] [CrossRef]
- Agrawal, S.; Zaritsky, J.J.; Fornoni, A.; Smoyer, W.E. Dyslipidaemia in nephrotic syndrome, mechanisms and treatment. Nat. Rev. Nephrol. 2018, 14, 57–70. [Google Scholar] [CrossRef]
- Bermúdez-López, M.; Betriu, À.; Valdivielso, J.M.; Bretones del Pino, T.; Arroyo, D.; Fernández, E. Beyond the traditional lipid parameters in chronic kidney disease. Nefrologia 2018, 38, 109–113. [Google Scholar] [CrossRef]
- Bermúdez-López, M.; Arroyo, D.; Betriu, À.; Masana, L.; Fernández, E.; Valdivielso, J.M. New perspectives on CKD-induced dyslipidemia. Expert Opin. Ther. Targets 2017, 21, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Ferro, C.J.; Mark, P.B.; Kanbay, M.; Sarafidis, P.; Heine, G.H.; Rossignol, P.; Massy, Z.A.; Mallamaci, F.; Valdivielso, J.M.; Malyszko, J.; et al. Lipid management in patients with chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 727–749. [Google Scholar] [CrossRef] [Green Version]
- Quiroga, B.; Muñoz Ramos, P.; Álvarez Chiva, V. Efficacy and safety of the PCSK9 inhibitors in the treatment of dyslipidemia in chronic kidney disease. Nefrologia 2020, 40, 499–505. [Google Scholar] [CrossRef]
- Artunc, F. Kidney-derived PCSK9-a new driver of hyperlipidemia in nephrotic syndrome? Kidney Int. 2020, 98, 1393–1395. [Google Scholar] [CrossRef]
- Molina-Jijon, E.; Gambut, S.; Macé, C.; Avila-Casado, C.; Clement, L.C. Secretion of the epithelial sodium channel chaperone PCSK9 from the cortical collecting duct links sodium retention with hypercholesterolemia in nephrotic syndrome. Kidney Int. 2020, 98, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Yazdani, S.; Vivès, R.R.; El Masri, R.; Dam, W.; van de Sluis, B.; van den Born, J. Proteinuria converts hepatic heparan sulfate to an effective proprotein convertase subtilisin kexin type 9 enzyme binding partner. Kidney Int. 2021, 99, 1369–1381. [Google Scholar] [CrossRef]
- Ge, M.; Merscher, S.; Fornoni, A. Use of Lipid-Modifying Agents for the Treatment of Glomerular Diseases. J. Pers Med. 2021, 11, 820. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.J.; Huber, T.B.; Gödel, M.; Jarad, G.; Hartleben, B.; Kwoh, C.; Keil, A.; Karpitskiy, A.; Hu, J.; Huh, C.J.; et al. Albumin-associated free fatty acids induce macropinocytosis in podocytes. J. Clin. Investig. 2015, 125, 2307–2316. [Google Scholar] [CrossRef] [Green Version]
- Gyebi, L.; Soltani, Z.; Reisin, E. Lipid nephrotoxicity, new concept for an old disease. Curr. Hypertens Rep. 2012, 14, 177–181. [Google Scholar] [CrossRef]
- Palmer, S.C.; Craig, J.C.; Navaneethan, S.D.; Tonelli, M.; Pellegrini, F.; Strippoli, G.F. Benefits and harms of statin therapy for persons with chronic kidney disease, a systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 263–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, K.; O’Malley, P.G.; Jackson, J.L. Meta-analysis, the effect of statins on albuminuria. Ann. Intern. Med. 2006, 145, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Nikolic, D.; Banach, M.; Nikfar, S.; Salari, P.; Mikhailidis, D.P.; Toth, P.P.; Abdollahi, M.; Ray, K.K.; Pencina, M.J.; Malyszko, J.; et al. Lipid and Blood Pressure Meta-Analysis Collaboration Group. A meta-analysis of the role of statins on renal outcomes in patients with chronic kidney disease. Is the duration of therapy important? Int. J. Cardiol. 2013, 168, 5437–5447. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Verdon, C.; Ninomiya, T.; Barzi, F.; Cass, A.; Patel, A.; Jardine, M.; Gallagher, M.; Turnbull, F.; Chalmers, J.; et al. The relationship between proteinuria and coronary risk, a systematic review and meta-analysis. PLoS Med. 2008, 5, 207. [Google Scholar] [CrossRef] [Green Version]
- Gæde, P.; Oellgaard, J.; Carstensen, B.; Rossing, P.; Lund-Andersen, H.; Parving, H.H.; Pedersen, O. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria, 21 years follow-up on the Steno-2 randomised trial. Diabetologia 2016, 59, 2298–2307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, T.G.; Tam-Tham, H.; Hemmelgarn, B.R.; Elliott, M.; James, M.T.; Ronksley, P.E.; Jun, M. Change in Proteinuria or Albuminuria as a Surrogate for Cardiovascular and Other Major Clinical Outcomes, a Systematic Review and Meta-Analysis. Can. J. Cardiol 2019, 35, 77–91. [Google Scholar] [CrossRef]
- Jatem, E.; Lima, J.; Montoro, B.; Torres-Bondia, F.; Segarra, A. Efficacy and Safety of PCSK9 Inhibitors in Hypercholesterolemia Associated With Refractory Nephrotic Syndrome. Kidney Int. Rep. 2020, 6, 101–109. [Google Scholar] [CrossRef]
- Bai, J.; Gong, L.L.; Li, Q.F.; Wang, Z.H. Long-term efficacy and safety of proprotein convertase subtilisin/kexin 9 monoclonal antibodies, A meta-analysis of 11 randomized controlled trials. J. Clin. Lipidol. 2018, 12, 277–291. [Google Scholar] [CrossRef]
- Busuioc, R.M.; Covic, A.; Kanbay, M.; Banach, M.; Burlacu, A.; Mircescu, G. Protein convertase subtilisin/kexin type 9 biology in nephrotic syndrome, implications for use as therapy. Nephrol. Dial. Transplant. 2020, 35, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Park, S.; Lee, S.; Kim, Y.; Kang, M.W.; Cho, S.; Park, S.; Han, K.; Kim, Y.C.; Han, S.S.; et al. Lipid profiles and risk of major adverse cardiovascular events in CKD and diabetes, A nationwide population-based study. PLoS ONE 2020, 15, e0231328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Pickett, J.K.; Shah, M.; Gillette, M.; Jones, P.; Virani, S.; Ballantyne, C.; Nambi, V. Acute Tubular Injury in a Patient on a Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitor. JACC Case Rep. 2020, 2, 1042–1045. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; van Lente, F.; Greene, T.; et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]



| Epidemiological Variables and Comorbidities | |
|---|---|
| Age (years) | 66 ± 10 |
| Sex (male, %) | 52 (68) |
| Hypertension (n, %) | 64 (84) |
| Diabetes mellitus (n, %) | 52 (68) |
| Ischemic heart disease (n, %) | 38 (50) |
| Heart failure (n, %) | 17 (22) |
| Cerebrovascular disease (n, %) | 12 (16) |
| Peripheral vascular disease (n, %) | 14 (18) |
| Treatment | |
| Statins (n, %) | 68 (90) |
| - Atorvastatin | 46 (61) |
| - Rosuvastatin | 13 (17) |
| - Others | 9 (12) |
| Ezetimibe (n, %) | 45 (59) |
| Fibrates (n, %) | 12 (16) |
| RAAS inhibitors (n, %) | 49 (65) |
| SGLT2i (n, %) | 2 (3) |
| Immunosuppression (n, %) | 0 (0) |
| Kidney Function | |
| Creatinine (mg/dL) | 1.55 ± 0.77 |
| eGFR (mL/min/1.73 m2) | 52 ± 22 |
| UPCR (mg/g) | 57 (9–481) |
| CKD stages (n, %) | |
| - Stage 1 | 7 (9) |
| - Stage 2 | 23 (30) |
| - Stage 3a | 16 (21) |
| - Stage 3b | 18 (24) |
| - Stage 4 | 12 (16) |
| CKD etiology (n, %) | |
| - Hypertension | 32 (42) |
| - Diabetic renal disease | 16 (21) |
| - Unknown | 15 (20) |
| - Glomerulonephritis | 7 (9) |
| - Others | 3 (4) |
| - Interstitial | 2 (3) |
| - Hereditary | 1(1) |
| Lipid Profile | |
| - Total cholesterol (mg/g) | 235 ± 71 |
| - LDL-C (mg/g) | 154 ± 50 |
| - HDL-C (mg/g) | 51 ± 12 |
| - Triglycerides (mg/g) | 160 (127–254) |
| Baseline | 1 Month | 3 Months | 6 Months | 12 Months | p for Trend | |
|---|---|---|---|---|---|---|
| Total cholesterol (mg/dL) | 235 ± 71 | 156 ± 71 | 145 ± 58 | 139 ± 47 | 147 ± 41 | <0.001 |
| LDL-C (mg/dL) | 154 ± 50 | 72 ± 43 | 64 ± 58 | 63 ± 39 | 62 ± 38 | <0.001 |
| HDL-C (mg/dL) | 51 ± 12 | 50 ± 13 | 50 ± 13 | 50 ± 11 | 48 ± 11 | 0.583 |
| Triglycerides (mg/dL) † | 160 (127–254) | 138 (95–191) | 130 (90–193) | 130 (102–199) | 146 (111–188) | 0.002 |
| Creatinine (mg/dL) | 1.55 ± 0.77 | 1.58 ± 0.66 | 1.59 ± 0.61 | 1.55 ± 0.84 | 1.66 ± 0.91 | 0.737 |
| eGFR (mL/min/1.73 m2) | 52 ± 22 | 47 ± 20 | 46 ± 18 | 51 ± 21 | 48 ± 22 | 0.287 |
| UPCR (mg/g) † | 57 (9–481) | 34 (5–353) | 22 (4–376) | 25 (4–248) | 30 (7–520) | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz Ramos, P.; Gil Giraldo, Y.; Álvarez-Chiva, V.; Arroyo, D.; Sango Merino, C.; Moncho Francés, F.; Ocaña, J.; Reque, J.; Sánchez-Álvarez, E.; Górriz, J.L.; et al. Proteinuria-Lowering Effects of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors in Chronic Kidney Disease Patients: A Real-World Multicentric Study. Metabolites 2021, 11, 760. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11110760
Muñoz Ramos P, Gil Giraldo Y, Álvarez-Chiva V, Arroyo D, Sango Merino C, Moncho Francés F, Ocaña J, Reque J, Sánchez-Álvarez E, Górriz JL, et al. Proteinuria-Lowering Effects of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors in Chronic Kidney Disease Patients: A Real-World Multicentric Study. Metabolites. 2021; 11(11):760. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11110760
Chicago/Turabian StyleMuñoz Ramos, Patricia, Yohana Gil Giraldo, Vicente Álvarez-Chiva, David Arroyo, Cristina Sango Merino, Francesc Moncho Francés, Javier Ocaña, Javier Reque, Emilio Sánchez-Álvarez, José Luis Górriz, and et al. 2021. "Proteinuria-Lowering Effects of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors in Chronic Kidney Disease Patients: A Real-World Multicentric Study" Metabolites 11, no. 11: 760. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11110760