Synthesis and Application of a Thermoplastic Plate of Poly(lactide-ε-caprolactone) for Radiation Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Poly(lactide-ε-caprolactone) Copolymer
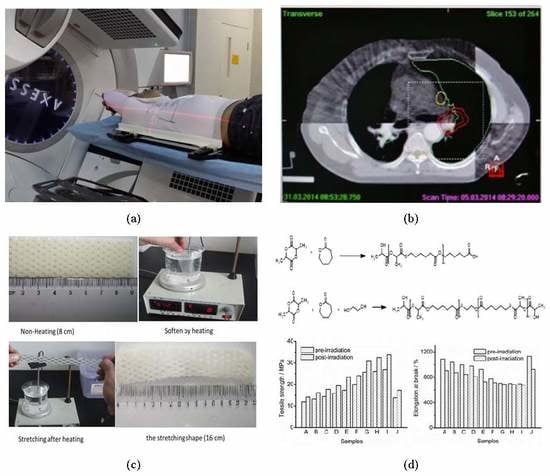
2.2.2. Preparation of Thermoplastic Plates
2.2.3. Measurements
1H-NMR Measurements
GPC Measurements
SEM Measurements
Shape Memory Process Measurements
Shore Hardness Measurements
DSC Measurements
Clinical Trials of P(LA-CL) Low-Temperature Thermoplastic Plates
Statistical Analysis
3. Results and Discussion
3.1. Characterization of Copolymers
3.2. Characterization of Thermoplastic Plates
3.3. Mechanical Property Analysis of Thermoplastic Plates
3.4. DSC Analysis of Thermoplastic Plates
3.5. SEM Analysis of Thermoplastic Plates
3.6. Shape Memory Process Analysis of Thermoplastic Plates
3.7. Clinic Data Analysis of Thermoplastic Plates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fukushima, K.; Feijoo, J.L.; Yang, M.-C. Abiotic degradation of poly (dl-lactide), poly (ɛ-caprolactone) and their blends. Polym. Degrad. Stab. 2012, 97, 2347–2355. [Google Scholar] [CrossRef]
- Diban, N.; Haimi, S.; Bolhuis-Versteeg, L.; Teixeira, S.; Miettinen, S.; Poot, A.; Grijpma, D.; Stamatialis, D. Hollow fibers of poly (lactide-co-glycolide) and poly (ε-caprolactone) blends for vascular tissue engineering applications. Acta Biomater. 2013, 9, 6450–6458. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bai, Y.; Liao, G.; Peng, E.; Wu, B.; Wang, Y.; Zeng, X.; Xie, X. Electrospun poly (L-lactide)/poly (ε-caprolactone) blend nanofibrous scaffold: Characterization and biocompatibility with human adipose-derived stem cells. PLoS ONE 2013, 8, e71265. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, B.; Mohammadi-Rovshandeh, J.; Davachi, S.M.; Zamanian, A. Synthesis and characterization of nanocomposite scaffolds based on triblock copolymer of L-lactide, ε-caprolactone and nano-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C 2014, 42, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tsutsui, A.; Tanaka, K.; Yamamoto, K.; Kadokawa, J.-I. Evaluation of stability of amylose inclusion complexes depending on guest polymers and their application to supramolecular polymeric materials. Biomolecules 2017, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, R.; Wu, L.; Wang, J.; Yang, Z.; Tu, Q.; Zhang, X.; Huang, N. Surface-Degradable Drug-Eluting Stent with Anticoagulation, Antiproliferation, and Endothelialization Functions. Biomolecules 2019, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.-Z.; Duan, D.-R.; Tang, C.-Y.; Tsui, C.-P.; Chen, D.-Z. Flexural properties of poly-L-lactide and polycaprolactone shape memory composites filled with nanometer calcium carbonate. J. Macromol. Sci. Part B 2013, 52, 964–972. [Google Scholar] [CrossRef]
- Le, D.M.; Tycon, M.A.; Fecko, C.J.; Ashby, V.S. Near-infrared activation of semi-crystalline shape memory polymer nanocomposites. J. Appl. Polym. Sci. 2013, 130, 4551–4557. [Google Scholar] [CrossRef]
- Behl, M.; Bellin, I.; Kelch, S.; Wagermaier, W.; Lendlein, A. One-step process for creating triple-shape capability of AB polymer networks. Adv. Funct. Mater. 2009, 19, 102–108. [Google Scholar] [CrossRef]
- Zhang, D.; Giese, M.L.; Prukop, S.L.; Grunlan, M.A. Poly (ε-caprolactone)-based shape memory polymers with variable polydimethylsiloxane soft segment lengths. J. Polym. Sci. Part A: Polym. Chem. 2011, 49, 754–761. [Google Scholar] [CrossRef] [Green Version]
- Defize, T.; Riva, R.; Raquez, J.M.; Dubois, P.; Jérôme, C.; Alexandre, M. Thermoreversibly crosslinked Poly (ε-caprolactone) as recyclable shape-memory polymer network. Macromol. Rapid Commun. 2011, 32, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Paderni, K.; Pandini, S.; Passera, S.; Pilati, F.; Toselli, M.; Messori, M. Shape-memory polymer networks from sol–gel cross-linked alkoxysilane-terminated poly (ε-caprolactone). J. Mater. Sci. 2012, 47, 4354–4362. [Google Scholar] [CrossRef]
- Žukienė, K.; Jankauskaitė, V.; Betingytė, V.; Baltušnikas, A. Properties of recycled polycaprolactone-based thermoplastic polyurethane filled with montmorillonites. J. Appl. Polym. Sci. 2013, 128, 2186–2196. [Google Scholar] [CrossRef]
- Garle, A.; Kong, S.; Ojha, U.; Budhlall, B.M. Thermoresponsive semicrystalline poly (ε-caprolactone) networks: Exploiting cross-linking with cinnamoyl moieties to design polymers with tunable shape memory. Acs Appl. Mater. Interfaces 2012, 4, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Li, X.; Xiong, C.; Deng, X. Polymerization of lactides and lactones 5. Ring-opening polymerization of ε-caprolactone and dl-lactide by rare earth 2-methylphenyl samarium. Eur. Polym. J. 1999, 35, 2131–2138. [Google Scholar] [CrossRef]
- Deng, X.; Yuan, M.; Xiong, C.; Li, X. Polymerization of lactides and lactones. II. Ring-opening polymerization of ε-caprolactone and DL-lactide by organoacid rare earth compounds. J. Appl. Polym. Sci. 1999, 71, 1941–1948. [Google Scholar] [CrossRef]
- Zhu, G.; Liang, G.; Xu, Q.; Yu, Q. Radiation effects on structure and properties of poly (epsilon-caprolactone). Acta Polym. Sin. 2003, 667–672. [Google Scholar]
- DENG, X.; YUAN, M.; CAO, X.; LI, X. Study on the ring-opening polymerization of 3-phenyl-ε-caprolactone and 5-phenyl-ε-caprolactone. Macromol. Chem. Phys. 2001, 202, 2417–2424. [Google Scholar] [CrossRef]
- Deng, X.; Yao, J.; Yuan, M.; Li, X.; Xiong, C. Synthesis of poly [(glycolic acid)-alt-(L-glutamic acid)] and poly [18]. Macromol. Chem. Phys. 2000, 201, 2371–2376. [Google Scholar] [CrossRef]
- Ren, Z.; Li, H.; Sun, X.; Yan, S.; Yang, Y. Fabrication of high toughness poly (lactic acid) by combining plasticization with cross-linking reaction. Ind. Eng. Chem. Res. 2012, 51, 7273–7278. [Google Scholar] [CrossRef]
- Liu, M.; Yin, Y.; Fan, Z.; Zheng, X.; Shen, S.; Deng, P.; Zheng, C.; Teng, H.; Zhang, W. The effects of gamma-irradiation on the structure, thermal resistance and mechanical properties of the PLA/EVOH blends. Nucl. Instrum. Methods Phys. Res. Sect. B: Beam Interact. Mater. At. 2012, 274, 139–144. [Google Scholar] [CrossRef]
- Żenkiewicz, M.; Malinowski, R.; Rytlewski, P.; Richert, A.; Sikorska, W.; Krasowska, K. Some composting and biodegradation effects of physically or chemically crosslinked poly (lactic acid). Polym. Test. 2012, 31, 83–92. [Google Scholar] [CrossRef]
- Rytlewski, P.; Malinowski, R.; Moraczewski, K.; Żenkiewicz, M. Influence of some crosslinking agents on thermal and mechanical properties of electron beam irradiated polylactide. Radiat. Phys. Chem. 2010, 79, 1052–1057. [Google Scholar] [CrossRef]
- Yang, S.L.; Wu, Z.H.; Meng, B.; Yang, W. The effects of dioctyl phthalate plasticization on the morphology and thermal, mechanical, and rheological properties of chemical crosslinked polylactide. J. Polym. Sci. Part B: Polym. Phys. 2009, 47, 1136–1145. [Google Scholar] [CrossRef]
- Quynh, T.M.; Mitomo, H.; Yoneyama, M.; Hien, N.Q. Properties of radiation-induced crosslinking stereocomplexes derived from poly (L-lactide) and different poly (D-lactide). Polym. Eng. Sci. 2009, 49, 970–976. [Google Scholar] [CrossRef]
- Quynh, T.M.; Mitomo, H.; Zhao, L.; Tamada, M. Properties of a poly (L-lactic acid)/poly (D-lactic acid) stereocomplex and the stereocomplex crosslinked with triallyl isocyanurate by irradiation. J. Appl. Polym. Sci. 2008, 110, 2358–2365. [Google Scholar] [CrossRef]
- Quynh, T.M.; Mitomo, H.; Zhao, L.; Asai, S. The radiation crosslinked films based on PLLA/PDLA stereocomplex after TAIC absorption in supercritical carbon dioxide. Carbohydr. Polym. 2008, 72, 673–681. [Google Scholar] [CrossRef]
- Dhakate, S.; Sharma, S.; Borah, M.; Mathur, R.; Dhami, T. Development and characterization of expanded graphite-based nanocomposite as bipolar plate for polymer electrolyte membrane fuel cells (PEMFCs). Energy Fuels 2008, 22, 3329–3334. [Google Scholar] [CrossRef]
- Hamanaka, T.; Hikasa, T.; Ibuki, K. Thermoplastic elastomer composition which has a shore a hardness of less than 45. Patent 5,187,224, 16 February 1993. [Google Scholar]
- Jiang, S.-C.; Dong, D.-W.; Ji, X.-L. Confined Crystallization Behavior of PEO in Organic-Inorganic Hybrid System. Polym. Mater. Sci. Eng. 2001, 17, 141–145. [Google Scholar]














| Sample | mε-caprolactone:mlactide | Total Monomer Weight (g) | Glycol (g) | SnOct (g) |
|---|---|---|---|---|
| A | 9:1 | 400 | 0.5 | 0.6 |
| B | 8:2 | 400 | 0.5 | 0.6 |
| C | 7:3 | 400 | 0.5 | 0.6 |
| D | 9:1 | 400 | 0.25 | 0.6 |
| E | 8:2 | 400 | 0.25 | 0.6 |
| F | 7:3 | 400 | 0.25 | 0.6 |
| G | 9:1 | 400 | 0 | 0.6 |
| H | 8:2 | 400 | 0 | 0.6 |
| I | 7:3 | 400 | 0 | 0.6 |
| J | 10:0 | 400 | 0 | 0.6 |
| Sample | mε-caprolactone:mlactide | Total Monomer Weight (g) | Conversion (%) | Yield (%) |
|---|---|---|---|---|
| A | 9:1 | 400 | 97.1 | 90.7 |
| B | 8:2 | 400 | 96.8 | 94.2 |
| C | 7:3 | 400 | 96.4 | 96.3 |
| D | 9:1 | 400 | 96.7 | 94.9 |
| E | 8:2 | 400 | 97.7 | 93.7 |
| F | 7:3 | 400 | 97.4 | 92.4 |
| G | 9:1 | 400 | 95.8 | 91.9 |
| H | 8:2 | 400 | 96.2 | 94.6 |
| I | 7:3 | 400 | 95.6 | 95.8 |
| Sample | Mn (kDa) | Mw (kDa) | PDI |
|---|---|---|---|
| A | 4.2 | 6.3 | 1.498 |
| B | 4.4 | 6.5 | 1.475 |
| C | 4.0 | 6.0 | 1.490 |
| D | 6.5 | 10.5 | 1.611 |
| E | 7.49 | 11.1 | 1.475 |
| F | 7.09 | 11.0 | 1.505 |
| G | 11.05 | 19.0 | 1.721 |
| H | 12.44 | 21.0 | 1.687 |
| I | 10.94 | 17.3 | 1.581 |
| Sample | Tm (°C) | Remark |
|---|---|---|
| B | 47.4 | pre-irradiation |
| B1 | 50.1 | post-irradiation |
| E | 52.2 | pre-irradiation |
| E1 | 55.2 | post-irradiation |
| H | 50.9 | pre-irradiation |
| H1 | 56.3 | post-irradiation |
| Project | Group | Setup Error | Median | Maximum | Minimum | p Value |
|---|---|---|---|---|---|---|
| X (mm) | 1 | 0.81 ± 3.78 | 1.33 | 8.70 | −8.70 | 0.02 |
| 2 | −1.35 ± 0.45 | −0.30 | 3.20 | −1.90 | ||
| Y (mm) | 1 | 1.75 ± 4.55 | 2.15 | 10.00 | −8.75 | 0.01 |
| 2 | 1.15 ± 2.55 | 1.60 | 7.55 | −5.00 | ||
| Z (mm) | 1 | 2.45 ± 2.20 | 2.15 | 7.35 | −3.30 | 0.005 |
| 2 | 2.35 ± 0.35 | 1.25 | 5.00 | −2.75 | ||
| U (mm) | 1 | 0.60 ± 0.65 | 0.65 | 2.10 | −1.80 | 0.01 |
| 2 | 0.08 ± 0.32 | 0.00 | 0.65 | 0.70 | ||
| V (mm) | 1 | 0.35 ± 1.40 | 0.45 | 2.55 | −3.85 | 0.03 |
| 2 | −0.10 ± 0.50 | 0.00 | 1.30 | −2.00 | ||
| W (mm) | 1 | 0.45 ± 0.90 | 0.40 | 3.45 | −1.35 | 0.01 |
| 2 | 0.15 ± 0.32 | 0.08 | 1.25 | −1.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Li, W.; Wu, H.; Jiang, D.; Yuan, M.; Yuan, M. Synthesis and Application of a Thermoplastic Plate of Poly(lactide-ε-caprolactone) for Radiation Therapy. Biomolecules 2020, 10, 27. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10010027
Li H, Li W, Wu H, Jiang D, Yuan M, Yuan M. Synthesis and Application of a Thermoplastic Plate of Poly(lactide-ε-caprolactone) for Radiation Therapy. Biomolecules. 2020; 10(1):27. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10010027
Chicago/Turabian StyleLi, Hongli, Wenzhi Li, Hongtao Wu, Dengbang Jiang, Mingwei Yuan, and Minglong Yuan. 2020. "Synthesis and Application of a Thermoplastic Plate of Poly(lactide-ε-caprolactone) for Radiation Therapy" Biomolecules 10, no. 1: 27. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10010027