Small in Size, but Large in Action: microRNAs as Potential Modulators of PTEN in Breast and Lung Cancers
Abstract
:1. Introduction
2. MicroRNAs in Oncology
3. PTEN in Oncology
3.1. Signaling
3.2. Role in Cancer
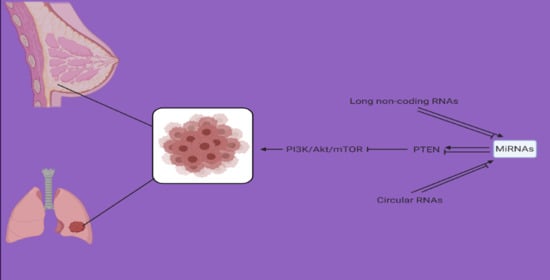
4. MicroRNA and PTEN Relationship
4.1. MicroRNAs and PTEN Inhibition
4.1.1. Breast Cancer
4.1.2. Lung Cancer
4.2. MicroRNAs and PTEN Induction
4.2.1. Breast Cancer
4.2.2. Lung Cancer
4.3. MicroRNAs, PTEN, and Chemotherapy
4.3.1. Breast Cancer
4.3.2. Lung Cancer
4.4. Regulation of microRNA/PTEN Axis
4.4.1. Breast Cancer
4.4.2. Lung Cancer
4.5. MicroRNA/PTEN Axis: A Target of Anti-Tumor Compounds
4.5.1. Breast Cancer
- Phytochemicals can be considered as epigenetic drugs in regulating miRNA expression,
- By targeting miRNAs, natural compounds can modulate PTEN expression,
- PTEN upregulation can impair PI3K/Akt signaling as the important pathway required for cancer progression,
4.5.2. Lung Cancer
5. Conclusions and Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Quintanal-Villalonga, Á.; Molina-Pinelo, S. Epigenetics of lung cancer: A translational perspective. Cellular Oncol. 2019, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, B.; Chong, Q.Y.; Pandey, V.; Guo, Z.; Chen, R.M.; Wang, L.; Wang, Y.; Ma, L.; Kumar, A.P.; et al. A novel small-molecule inhibitor of trefoil factor 3 (TFF3) potentiates MEK1/2 inhibition in lung adenocarcinoma. Oncogenesis 2019, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Mohan, C.D.; Basappa, S.; Rangappa, S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Kumar, A.P.; Sethi, G.; Ahn, K.S.; et al. The IκB Kinase Inhibitor ACHP Targets the STAT3 Signaling Pathway in Human Non-Small Cell Lung Carcinoma Cells. Biomolecules 2019, 9, 875. [Google Scholar] [CrossRef] [Green Version]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Bordoloi, D.; Banik, K.; Padmavathi, G.; Vikkurthi, R.; Harsha, C.; Roy, N.K.; Singh, A.K.; Monisha, J.; Wang, H.; Kumar, A.P.; et al. TIPE2 Induced the Proliferation, Survival, and Migration of Lung Cancer Cells Through Modulation of Akt/mTOR/NF-κB Signaling Cascade. Biomolecules 2019, 9, 836. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.Y.; Shanmugam, M.K.; Narula, A.S.; Kim, C.; Lee, J.H.; Namjoshi, O.A.; Blough, B.E.; Sethi, G.; Ahn, K.S. Oxymatrine Attenuates Tumor Growth and Deactivates STAT5 Signaling in a Lung Cancer Xenograft Model. Cancers 2019, 11, 49. [Google Scholar] [CrossRef] [Green Version]
- Pio, R.; Montuenga, L.M. Alternative splicing in lung cancer. J. Thorac. Oncol. 2009, 4, 674–678. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, C.; Lee, S.G.; Sethi, G.; Ahn, K.S. Ophiopogonin D, a Steroidal Glycoside Abrogates STAT3 Signaling Cascade and Exhibits Anti-Cancer Activity by Causing GSH/GSSG Imbalance in Lung Carcinoma. Cancers 2018, 10, 427. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.H.; Lee, J.H.; Ko, J.H.; Jung, S.H.; Sethi, G.; Ahn, K.S. Brassinin Represses Invasive Potential of Lung Carcinoma Cells through Deactivation of PI3K/Akt/mTOR Signaling Cascade. Molecules 2019, 24, 1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royal College of Physicians. National Lung Cancer Audit Annual Report 2017 (for the Audit Period 2016). Available online: https://www.rcplondon.ac.uk/projects/outputs/nlca-annual-report-2017 (accessed on 18 February 2021).
- Wang, L.; Syn, N.L.; Subhash, V.V.; Any, Y.; Thuya, W.L.; Cheow, E.S.H.; Kong, L.; Yu, F.; Peethala, P.C.; Wong, A.L.; et al. Pan-HDAC inhibition by panobinostat mediates chemosensitization to carboplatin in non-small cell lung cancer via attenuation of EGFR signaling. Cancer Lett. 2018, 417, 152–160. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.M.; Hall, L.J.; Robinson, S.D. The microbiota, antibiotics and breast cancer. Future Med. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Kar, S.; Lai, X.; Cai, W.; Arfuso, F.; Sethi, G.; Lobie, P.E.; Goh, B.C.; Lim, L.H.K.; Hartman, M.; et al. Triple negative breast cancer in Asia: An insider’s view. Cancer Treat. Rev. 2018, 62, 29–38. [Google Scholar] [CrossRef]
- Jia, L.Y.; Shanmugam, M.K.; Sethi, G.; Bishayee, A. Potential role of targeted therapies in the treatment of triple-negative breast cancer. Anti-Cancer Drugs 2016, 27, 147–155. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, Y.; Kar, S.; Kanchi, M.M.; Arora, S.; Kim, J.E.; Koh, P.F.; Yousef, E.; Samy, R.P.; Shanmugam, M.K.; et al. PPARγ Ligand-induced Annexin A1 Expression Determines Chemotherapy Response via Deubiquitination of Death Domain Kinase RIP in Triple-negative Breast Cancers. Mol. Cancer Ther. 2017, 16, 2528–2542. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F.; et al. Thymoquinone Inhibits Bone Metastasis of Breast Cancer Cells Through Abrogation of the CXCR4 Signaling Axis. Front. Pharmacol. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Ong, P.S.; Wang, L.; Chia, D.M.; Seah, J.Y.; Kong, L.R.; Thuya, W.L.; Chinnathambi, A.; Lau, J.Y.; Wong, A.L.; Yong, W.P.; et al. A novel combinatorial strategy using Seliciclib(®) and Belinostat(®) for eradication of non-small cell lung cancer via apoptosis induction and BID activation. Cancer Lett. 2016, 381, 49–57. [Google Scholar] [CrossRef]
- Ong, M.S.; Cai, W.; Yuan, Y.; Leong, H.C.; Tan, T.Z.; Mohammad, A.; You, M.L.; Arfuso, F.; Goh, B.C.; Warrier, S.; et al. ‘Lnc’-ing Wnt in female reproductive cancers: Therapeutic potential of long non-coding RNAs in Wnt signalling. Br. J. Pharmacol. 2017, 174, 4684–4700. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Sun, Y. Lidocaine inhibits proliferation and metastasis of lung cancer cell via regulation of miR-539/EGFR axis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2866–2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Zhang, J.; Chen, F.; Sun, Y. MiR-148b suppressed non-small cell lung cancer progression via inhibiting ALCAM through the NF-κB signaling pathway. Thorac Cancer 2020, 11, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lang, T.Y.; Zou, D.L.; Zhou, L.; Lou, M.; Liu, J.S.; Li, Y.Z.; Ding, D.Y.; Li, Y.C.; Zhang, N.; et al. miR-520b Promotes Breast Cancer Stemness Through Hippo/YAP Signaling Pathway. Onco Targets Ther. 2019, 12, 11691–11700. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Miao, J.; Ding, Y.; Zhang, Y.; Huang, X.; Zhou, X.; Tang, R. MiR-4458 inhibits breast cancer cell growth, migration, and invasiveness by targeting CPSF4. Biochem. Cell Biol. 2019, 97, 722–730. [Google Scholar] [CrossRef]
- Cai, Y.; Hao, Y.; Ren, H.; Dang, Z.; Xu, H.; Xue, X.; Gao, Y. miR-1305 Inhibits The Progression Of Non-Small Cell Lung Cancer By Regulating MDM2. Cancer Manag. Res. 2019, 11, 9529–9540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Pan, J.; Jin, Y.; Li, M.; Chen, M. MiR-195-5p Inhibits Proliferation and Induces Apoptosis of Non-Small Cell Lung Cancer Cells by Targeting CEP55. Onco Targets Ther 2019, 12, 11465–11474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, D.K.; Park, J.; Cho, M.; Ban, E.; Jang, M.; Yoo, Y.S.; Kim, E.E.; Baik, J.H.; Song, E.J. MiR-195 and miR-497 suppress tumorigenesis in lung cancer by inhibiting SMURF2-induced TGF-β receptor I ubiquitination. Mol. Oncol. 2019, 13, 2663–2678. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Deng, Q.; Lv, Z.; Ling, Y.; Hou, X.; Chen, Z.; Dinglin, X.; Ma, S.; Li, D.; Wu, Y.; et al. N6-methyladenosine induced miR-143-3p promotes the brain metastasis of lung cancer via regulation of VASH1. Mol. Cancer 2019, 18, 181. [Google Scholar] [CrossRef] [Green Version]
- Zou, P.; Zhu, M.; Lian, C.; Wang, J.; Chen, Z.; Zhang, X.; Yang, Y.; Chen, X.; Cui, X.; Liu, J.; et al. miR-192-5p suppresses the progression of lung cancer bone metastasis by targeting TRIM44. Sci. Rep. 2019, 9, 19619. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Hua, X.; Qi, N.; Han, G.; Yu, J.; Yu, Y.; Wei, X.; Li, H.; Chen, X.; Leng, C.; et al. MiR-27b suppresses epithelial-mesenchymal transition and chemoresistance in lung cancer by targeting Snail1. Life Sci. 2020, 254, 117238. [Google Scholar] [CrossRef]
- Huang, L.; Tang, X.; Shi, X.; Su, L. miR-532-5p promotes breast cancer proliferation and migration by targeting RERG. Exp. Ther. Med. 2020, 19, 400–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, F.; Chen, L.; Sun, Y.; He, C.; Fu, D.; Tang, J. MiR-539 inhibits the malignant behavior of breast cancer cells by targeting SP1. Biochem. Cell Biol. 2020, 98, 426–433. [Google Scholar] [CrossRef]
- Hong, T.; Ding, J.; Li, W. miR-7 Reverses Breast Cancer Resistance To Chemotherapy By Targeting MRP1 And BCL2. Onco Targets Ther. 2019, 12, 11097–11105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Zhang, W.; Liu, C.; Li, G. miR-200 affects tamoxifen resistance in breast cancer cells through regulation of MYB. Sci. Rep. 2019, 9, 18844. [Google Scholar] [CrossRef]
- Duan, W.J.; Bi, P.D.; Ma, Y.; Liu, N.Q.; Zhen, X. MiR-512-3p regulates malignant tumor behavior and multi-drug resistance in breast cancer cells via targeting Livin. Neoplasma 2020, 67, 102–110. [Google Scholar] [CrossRef]
- Pasculli, B.; Barbano, R.; Rendina, M.; Fontana, A.; Copetti, M.; Mazza, T.; Valori, V.M.; Morritti, M.; Maiello, E.; Graziano, P.; et al. Hsa-miR-210-3p expression in breast cancer and its putative association with worse outcome in patients treated with Docetaxel. Sci. Rep. 2019, 9, 14913. [Google Scholar] [CrossRef]
- Estevão-Pereira, H.; Lobo, J.; Salta, S.; Amorim, M.; Lopes, P.; Cantante, M.; Reis, B.; Antunes, L.; Castro, F.; Palma de Sousa, S.; et al. Overexpression of circulating MiR-30b-5p identifies advanced breast cancer. J. Transl. Med. 2019, 17, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wang, Q.; Rui, Y.; Zhang, C.; Wang, W.; Gu, J.; Tang, J.; Ding, Y. HOXC13-AS promotes breast cancer cell growth through regulating miR-497-5p/PTEN axis. J. Cell Physiol. 2019, 234, 22343–22351. [Google Scholar] [CrossRef]
- Yu, Y.; Cao, X.-C. miR-190-5p in human diseases. Cancer Cell Int. 2019, 19, 257. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ang, H.L.; Moghadam, E.R.; Mohammadi, S.; Zarrin, V.; Hushmandi, K.; Samarghandian, S.; Zarrabi, A.; Najafi, M.; Mohammadinejad, R.; et al. MicroRNAs and Their Influence on the ZEB Family: Mechanistic Aspects and Therapeutic Applications in Cancer Therapy. Biomolecules 2020, 10, 1040. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Hushmandi, K.; Hashemi, M.; Akbari, M.E.; Kubatka, P.; Raei, M.; Koklesova, L.; Shahinozzaman, M.; Mohammadinejad, R.; Najafi, M.; et al. Role of microRNA/Epithelial-to-Mesenchymal Transition Axis in the Metastasis of Bladder Cancer. Biomolecules 2020, 10, 1159. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Hashemi, F.; Moghadam, E.R.; Owrang, M.; Hashemi, F.; Makvandi, P.; Goharrizi, M.A.S.B.; Najafi, M. Lung cancer cells and their sensitivity/resistance to cisplatin chemotherapy: Role of microRNAs and upstream mediators. Cell. Signal. 2020, 109871. [Google Scholar]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2010, 39, 152–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witten, L.; Slack, F.J. miR-155 as a novel clinical target for hematological malignancies. Carcinogenesis 2020, 41, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.P.; Zhang, Y.; Liu, Z.T.; Qi, H.; Zheng, X.M.; Qi, L.H.; Wang, J.Y. MiR-203 regulates proliferation and apoptosis of ovarian cancer cells by targeting SOCS3. Eur. Rev. Med. Pharmacol Sci. 2019, 23, 9286–9294. [Google Scholar]
- Hu, Z.; Cai, M.; Zhang, Y.; Tao, L.; Guo, R. miR-29c-3p inhibits autophagy and cisplatin resistance in ovarian cancer by regulating FOXP1/ATG14 pathway. Cell Cycle 2020, 19, 193–206. [Google Scholar] [CrossRef]
- Han, Z.; Zhan, R.; Chen, S.; Deng, J.; Shi, J.; Wang, W. miR-181b/Oncostatin m axis inhibits prostate cancer bone metastasis via modulating osteoclast differentiation. J. Cell Biochem. 2020, 121, 1664–1674. [Google Scholar] [CrossRef]
- Wu, Q.; Zhong, H.; Jiao, L.; Wen, Y.; Zhou, Y.; Zhou, J.; Lu, X.; Song, X.; Ying, B. MiR-124-3p inhibits the migration and invasion of Gastric cancer by targeting ITGB3. Pathol. Res. Pract. 2020, 216, 152762. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhu, W.; Chen, Q.; Yuan, Y.; Wang, Y.; Wang, J.; Wu, X. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics 2019, 9, 8206–8220. [Google Scholar] [CrossRef]
- Sun, S.L.; Shu, Y.G.; Tao, M.Y. miR-503 Inhibits Proliferation, Migration, And Angiogenesis of Glioma by Acting on VEGFA through Targeting LRIG2. Cancer Manag. Res. 2019, 11, 10599–10608. [Google Scholar] [CrossRef] [Green Version]
- Mu, H.Q.; He, Y.H.; Wang, S.B.; Yang, S.; Wang, Y.J.; Nan, C.J.; Bao, Y.F.; Xie, Q.P.; Chen, Y.H. MiR-130b/TNF-α/NF-κB/VEGFA loop inhibits prostate cancer angiogenesis. Clin. Transl. Oncol. 2020, 22, 111–121. [Google Scholar] [CrossRef]
- Yang, F.; Shao, C.; Wei, K.; Jing, X.; Qin, Z.; Shi, Y.; Shu, Y.; Shen, H. miR-942 promotes tumor migration, invasion, and angiogenesis by regulating EMT via BARX2 in non-small-cell lung cancer. J. Cell Physiol. 2019, 234, 23596–23607. [Google Scholar] [CrossRef]
- Pourdavoud, P.; Pakzad, B.; Mosallaei, M.; Saadatian, Z.; Esmaeilzadeh, E.; Alimolaie, A.; Shaygannejad, A. MiR-196: Emerging of a new potential therapeutic target and biomarker in colorectal cancer. Mol. Biol. Rep. 2020, 47, 9913–9920. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, N.; Zhao, S.; Chen, X.; Li, F.; Tao, X. miR-221-3p and miR-15b-5p promote cell proliferation and invasion by targeting Axin2 in liver cancer. Oncol. Lett. 2019, 18, 6491–6500. [Google Scholar] [CrossRef]
- Wei, J.; Yang, L.; Wu, Y.N.; Xu, J. Serum miR-1290 and miR-1246 as Potential Diagnostic Biomarkers of Human Pancreatic Cancer. J. Cancer 2020, 11, 1325–1333. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, X.; Chen, L.; Zhao, Y.; Huang, Z.; Zhou, H.; Shi, M. MiR-181a Promotes Apoptosis and Reduces Cisplatin Resistance by Inhibiting Osteopontin in Cervical Cancer Cells. Cancer Biother. Radiopharm. 2019, 34, 559–565. [Google Scholar] [CrossRef]
- Tang, H.; Song, C.; Ye, F.; Gao, G.; Ou, X.; Zhang, L.; Xie, X.; Xie, X. miR-200c suppresses stemness and increases cellular sensitivity to trastuzumab in HER2+ breast cancer. J. Cell Mol. Med. 2019, 23, 8114–8127. [Google Scholar] [CrossRef]
- Yan, S.; Wang, H.; Chen, X.; Liang, C.; Shang, W.; Wang, L.; Li, J.; Xu, D. MiR-182-5p inhibits colon cancer tumorigenesis, angiogenesis, and lymphangiogenesis by directly downregulating VEGF-C. Cancer Lett. 2020, 488, 18–26. [Google Scholar] [CrossRef]
- Dai, W.; Zhou, J.; Wang, H.; Zhang, M.; Yang, X.; Song, W. miR-424-5p promotes the proliferation and metastasis of colorectal cancer by directly targeting SCN4B. Pathol. Res. Pract. 2020, 216, 152731. [Google Scholar] [CrossRef]
- Zhou, Y.; He, A.; Zhang, L.; Yi, G. MiR-744 mediates the Oxaliplatin chemoresistance in colorectal cancer through inhibiting BIN1. Neoplasma 2020, 67, 296–303. [Google Scholar] [CrossRef]
- Lv, L.; Li, Q.; Chen, S.; Zhang, X.; Tao, X.; Tang, X.; Wang, S.; Che, G.; Yu, Y.; He, L. miR-133b suppresses colorectal cancer cell stemness and chemoresistance by targeting methyltransferase DOT1L. Exp. Cell Res. 2019, 385, 111597. [Google Scholar] [CrossRef]
- Yang, L.W.; Wu, X.J.; Liang, Y.; Ye, G.Q.; Che, Y.C.; Wu, X.Z.; Zhu, X.J.; Fan, H.L.; Fan, X.P.; Xu, J.F. miR-155 increases stemness and decitabine resistance in triple-negative breast cancer cells by inhibiting TSPAN5. Mol. Carcinog 2020, 59, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ding, Y.; Wang, X.; Wang, X.; Zhao, L.; Bi, H. miR-650 promotes non-small cell lung cancer cell proliferation and invasion by targeting ING4 through Wnt-1/β-catenin pathway. Oncol. Lett. 2019, 18, 4621–4628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, W.; Dong, N.; Wu, S.; Gui, D.; Ye, Z.; Wu, H.; Zhong, X. miR-4500 suppresses cell proliferation and migration in bladder cancer via inhibition of STAT3/CCR7 pathway. J. Cell Biochem. 2019. [Google Scholar] [CrossRef]
- Wu, G.; Zheng, H.; Xu, J.; Guo, Y.; Zheng, G.; Ma, C.; Hao, S.; Liu, X.; Chen, H.; Wei, S.; et al. miR-429 suppresses cell growth and induces apoptosis of human thyroid cancer cell by targeting ZEB1. Artif. Cells Nanomed. Biotechnol. 2019, 47, 548–554. [Google Scholar] [CrossRef]
- Zhang, P.F.; Pei, X.; Li, K.S.; Jin, L.N.; Wang, F.; Wu, J.; Zhang, X.M. Circular RNA circFGFR1 promotes progression and anti-PD-1 resistance by sponging miR-381-3p in non-small cell lung cancer cells. Mol. Cancer 2019, 18, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Z.H.; Xu, X.W.; Fu, X.Y.; Zhou, L.D.; Liu, S.P.; Tan, D.M. Long non-coding RNA MALAT1 promotes angiogenesis and immunosuppressive properties of HCC cells by sponging miR-140. Am. J. Physiol. Cell Physiol. 2020, 318, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Cruise, E.S.; Dowling, R.J.; Stambolic, V. PTEN nuclear functions. Cold Spring Harb. Perspect. Med. 2020, 10. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Najafi, M.; Ang, H.L.; Moghadam, E.R.; Mahabady, M.K.; Zabolian, A.; Jafaripour, L.; Bejandi, A.K.; Hushmandi, K.; Saleki, H.; et al. PTEN, a Barrier for Proliferation and Metastasis of Gastric Cancer Cells: From Molecular Pathways to Targeting and Regulation. Biomedicines 2020, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Braglia, L.; Zavatti, M.; Vinceti, M.; Martelli, A.M.; Marmiroli, S. Deregulated PTEN/PI3K/AKT/mTOR signaling in prostate cancer: Still a potential druggable target? Biochim. Biophys. Acta BBA Mol. Cell Res. 2020, 118731. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, P.S.; Wang, L.Z.; Dai, X.; Tseng, S.H.; Loo, S.J.; Sethi, G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front. Pharmacol. 2016, 7, 395. [Google Scholar] [CrossRef]
- Singh, S.S.; Yap, W.N.; Arfuso, F.; Kar, S.; Wang, C.; Cai, W.; Dharmarajan, A.M.; Sethi, G.; Kumar, A.P. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J. Gastroenterol. 2015, 21, 12261–12273. [Google Scholar] [CrossRef]
- Mohan, C.D.; Srinivasa, V.; Rangappa, S.; Mervin, L.; Mohan, S.; Paricharak, S.; Baday, S.; Li, F.; Shanmugam, M.K.; Chinnathambi, A.; et al. Trisubstituted-Imidazoles Induce Apoptosis in Human Breast Cancer Cells by Targeting the Oncogenic PI3K/Akt/mTOR Signaling Pathway. PLoS ONE 2016, 11, e0153155. [Google Scholar] [CrossRef] [Green Version]
- Stokoe, D.; Stephens, L.R.; Copeland, T.; Gaffney, P.R.; Reese, C.B.; Painter, G.F.; Holmes, A.B.; McCormick, F.; Hawkins, P.T. Dual role of phosphatidylinositol-3, 4, 5-trisphosphate in the activation of protein kinase B. Science 1997, 277, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Calleja, V.; Laguerre, M.; Parker, P.J.; Larijani, B. Role of a novel PH-kinase domain interface in PKB/Akt regulation: Structural mechanism for allosteric inhibition. PLoS Biol. 2009, 7, e1000017. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Um, J.Y.; Sethi, G.; Ahn, K.S. Casticin-Induced Inhibition of Cell Growth and Survival Are Mediated through the Dual Modulation of Akt/mTOR Signaling Cascade. Cancers 2019, 11, 254. [Google Scholar] [CrossRef] [Green Version]
- Si, X.; Xu, F.; Xu, F.; Wei, M.; Ge, Y.; Chenge, S. CADM1 inhibits ovarian cancer cell proliferation and migration by potentially regulating the PI3K/Akt/mTOR pathway. Biomed. Pharmacother. 2020, 123, 109717. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.S.; Seo, K.I. Lupiwighteone induces caspase-dependent and -independent apoptosis on human breast cancer cells via inhibiting PI3K/Akt/mTOR pathway. Food Chem. Toxicol 2020, 135, 110863. [Google Scholar] [CrossRef]
- Bu, T.; Wang, C.; Jin, H.; Meng, Q.; Huo, X.; Sun, H.; Sun, P.; Wu, J.; Ma, X.; Liu, Z.; et al. Organic anion transporters and PI3K-AKT-mTOR pathway mediate the synergistic anticancer effect of pemetrexed and rhein. J. Cell Physiol. 2020, 235, 3309–3319. [Google Scholar] [CrossRef] [PubMed]
- Perez-Juarez, C.E.; Arechavaleta-Velasco, F.; Zeferino-Toquero, M.; Alvarez-Arellano, L.; Estrada-Moscoso, I.; Diaz-Cueto, L. Inhibition of PI3K/AKT/mTOR and MAPK signaling pathways decreases progranulin expression in ovarian clear cell carcinoma (OCCC) cell line: A potential biomarker for therapy response to signaling pathway inhibitors. Med. Oncol. 2019, 37, 4. [Google Scholar] [CrossRef]
- Jiang, C.; Zeng, X.; Shan, R.; Wen, W.; Li, J.; Tan, J.; Li, L.; Wan, R. The Emerging Picture of the Roles of CircRNA-CDR1as in Cancer. Front Cell Dev Biol 2020, 8, 590478. [Google Scholar] [CrossRef]
- Lee, J.H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.; Sethi, G.; Ahn, K.S. Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharmacol. Res. 2019, 150, 104504. [Google Scholar] [CrossRef] [PubMed]
- Vidotto, T.; Melo, C.M.; Castelli, E.; Koti, M.; dos Reis, R.B.; Squire, J.A. Emerging role of PTEN loss in evasion of the immune response to tumours. Br. J. Cancer 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Ho, J.; Srikumar, T.; Dowling, R.; Gorrini, C.; Miller, S.; Mak, T.; Neel, B.; Raught, B.; Stambolic, V. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science 2013, 341, 395–399. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.H.; Balajee, A.S.; Wang, J.; Wu, H.; Eng, C.; Pandolfi, P.P.; Yin, Y. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 2007, 128, 157–170. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.H.; Lee, J.H.; Kim, C.; Ko, J.H.; Ryu, S.H.; Lee, S.G.; Yang, W.M.; Um, J.Y.; Chinnathambi, A.; Alharbi, S.A.; et al. Ginkgolic Acid C 17:1, Derived from Ginkgo biloba Leaves, Suppresses Constitutive and Inducible STAT3 Activation through Induction of PTEN and SHP-1 Tyrosine Phosphatase. Molecules 2017, 22, 276. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Wang, Y.; Ly, A.; Hosono, Y.; Murchie, E.; Walmsley, C.S.; Huynh, T.; Healy, C.; Peterson, R.; Yanase, S.; et al. PTEN Loss Mediates Clinical Cross-Resistance to CDK4/6 and PI3Kα Inhibitors in Breast Cancer. Cancer Discov. 2020, 10, 72–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Wang, X.; Chen, Y.; Han, J.; Kong, D.; Zhu, M.; Fu, X.; Wu, Y. Pten loss in Lgr5(+) hair follicle stem cells promotes SCC development. Theranostics 2019, 9, 8321–8331. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Li, X.; Shi, Z.; Xia, Y.; Cai, Q.; Xu, D.; Tan, L.; Du, L.; Zheng, Y.; Zhao, D.; et al. PTEN Suppresses Glycolysis by Dephosphorylating and Inhibiting Autophosphorylated PGK1. Mol. Cell 2019, 76, 516–527. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, F.; Chen, Y.; Wang, R.; Liu, J.; Jin, Y.; An, R. Cryptotanshinone Inhibites Bladder Cancer Cell Proliferation and Promotes Apoptosis via the PTEN/PI3K/AKT Pathway. J. Cancer 2020, 11, 488–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Zhu, D.; Zhu, L.; Hou, Y.; Hou, L.; Huang, X.; Li, L.; Wang, Y.; Li, L.; Zou, H.; et al. Dichloroacetate Overcomes Oxaliplatin Chemoresistance in Colorectal Cancer through the miR-543/PTEN/Akt/mTOR Pathway. J. Cancer 2019, 10, 6037–6047. [Google Scholar] [CrossRef]
- Lee, J.H.; Mohan, C.D.; Deivasigamani, A.; Jung, Y.Y.; Rangappa, S.; Basappa, S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Garg, M.; et al. Brusatol suppresses STAT3-driven metastasis by downregulating epithelial-mesenchymal transition in hepatocellular carcinoma. J. Adv. Res. 2020. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, J.; Chao, J.; Scheuerman, M.P.; Rahimi, S.A.; Lee, L.Y.; Li, S. PTEN suppresses epithelial-mesenchymal transition and cancer stem cell activity by downregulating Abi1. Sci. Rep. 2020, 10, 12685. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, D.; Weng, W.; Lv, X.; Zheng, L.; Chen, M.; Fan, X.; Mao, J.; Mao, C.; Ye, Y.; et al. CPSF7 regulates liver cancer growth and metastasis by facilitating WWP2-FL and targeting the WWP2/PTEN/AKT signaling pathway. Biochim. Biophys. Acta. Mol. Cell Res. 2020, 1867, 118624. [Google Scholar] [CrossRef]
- Ma, L.; Yao, N.; Chen, P.; Zhuang, Z. TRIM27 promotes the development of esophagus cancer via regulating PTEN/AKT signaling pathway. Cancer Cell Int. 2019, 19, 283. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.P.; Qian, J.; Zhao, Y.J. The loss of PTEN expression and microsatellite stability (MSS) were predictors of unfavorable prognosis in gastric cancer (GC). Neoplasma 2020. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Chen, J.; Mo, X. The association of PTEN hypermethylation and breast cancer: A meta-analysis. Onco. Targets Ther. 2016, 9, 5643–5650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yari, K.; Payandeh, M.; Rahimi, Z. Association of the hypermethylation status of PTEN tumor suppressor gene with the risk of breast cancer among Kurdish population from Western Iran. Tumour Biol. 2016, 37, 8145–8152. [Google Scholar] [CrossRef]
- Buckingham, L.; Penfield Faber, L.; Kim, A.; Liptay, M.; Barger, C.; Basu, S.; Fidler, M.; Walters, K.; Bonomi, P.; Coon, J. PTEN, RASSF1 and DAPK site-specific hypermethylation and outcome in surgically treated stage I and II nonsmall cell lung cancer patients. Int. J. Cancer 2010, 126, 1630–1639. [Google Scholar] [CrossRef]
- Yu, D.; Wang, X.Y.; Jin, Z.L. Linc00702 inhibits cell growth and metastasis through regulating PTEN in colorectal cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3624–3632. [Google Scholar] [CrossRef]
- Yan, J.; Huang, X.; Zhang, X.; Chen, Z.; Ye, C.; Xiang, W.; Huang, Z. LncRNA LINC00470 promotes the degradation of PTEN mRNA to facilitate malignant behavior in gastric cancer cells. Biochem. Biophys. Res. Commun. 2020, 521, 887–893. [Google Scholar] [CrossRef]
- Dai, X.; Guo, X.; Liu, J.; Cheng, A.; Peng, X.; Zha, L.; Wang, Z. Circular RNA circGRAMD1B inhibits gastric cancer progression by sponging miR-130a-3p and regulating PTEN and p21 expression. Aging 2019, 11, 9689–9708. [Google Scholar] [CrossRef]
- Duan, X.M.; Liu, X.N.; Li, Y.X.; Cao, Y.Q.; Silayiding, A.; Zhang, R.K.; Wang, J.P. MicroRNA-498 promotes proliferation, migration, and invasion of prostate cancer cells and decreases radiation sensitivity by targeting PTEN. Kaohsiung J. Med. Sci. 2019, 35, 659–671. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.P.; Liu, G.L.; Li, S.; Liu, X.L. miR-17-5p knockdown inhibits proliferation, autophagy and promotes apoptosis in thyroid cancer via targeting PTEN. Neoplasma 2020, 67, 249–258. [Google Scholar] [CrossRef]
- Li, N.; Miao, Y.; Shan, Y.; Liu, B.; Li, Y.; Zhao, L.; Jia, L. MiR-106b and miR-93 regulate cell progression by suppression of PTEN via PI3K/Akt pathway in breast cancer. Cell Death Dis. 2017, 8, 2796. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, C.; Wang, M.; Su, L.; Qu, Y.; Li, J.; Yu, B.; Yan, M.; Yu, Y.; Liu, B.; et al. Decrease of miR-202-3p expression, a novel tumor suppressor, in gastric cancer. PLoS ONE 2013, 8, e69756. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.G.; He, J.H.; Yu, L.; Hang, Z.P.; Li, W.; Shun, W.H.; Huang, G.X. microRNA-202 suppresses MYCN expression under the control of E2F1 in the neuroblastoma cell line LAN-5. Mol. Med. Rep. 2014, 9, 541–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Guo, J.; Zhang, X. MiR-202-5p/PTEN mediates doxorubicin-resistance of breast cancer cells via PI3K/Akt signaling pathway. Cancer Biol. Ther. 2019, 20, 989–998. [Google Scholar] [CrossRef]
- Kia, V.; Sharif Beigli, M.; Hosseini, V.; Koochaki, A.; Paryan, M.; Mohammadi-Yeganeh, S. Is miR-144 an effective inhibitor of PTEN mRNA: A controversy in breast cancer. In Vitro Cell Dev. Biol. Anim. 2018, 54, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Zhang, J.H. miR-181c promotes proliferation via suppressing PTEN expression in inflammatory breast cancer. Int. J. Oncol. 2015, 46, 2011–2020. [Google Scholar] [CrossRef] [Green Version]
- Xin, Y.; Wang, X.; Meng, K.; Ni, C.; Lv, Z.; Guan, D. Identification of exosomal miR-455-5p and miR-1255a as therapeutic targets for breast cancer. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Li, D.; Wang, J.; Ma, L.J.; Yang, H.B.; Jing, J.F.; Jia, M.M.; Zhang, X.J.; Guo, F.; Gao, J.N. Identification of serum exosomal miR-148a as a novel prognostic biomarker for breast cancer. Eur. Rev. Med. Pharmacol Sci. 2020, 24, 7303–7309. [Google Scholar] [CrossRef] [PubMed]
- Kia, V.; Paryan, M.; Mortazavi, Y.; Biglari, A.; Mohammadi-Yeganeh, S. Evaluation of exosomal miR-9 and miR-155 targeting PTEN and DUSP14 in highly metastatic breast cancer and their effect on low metastatic cells. J. Cell Biochem. 2019, 120, 5666–5676. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Milde-Langosch, K.; Steinbach, B.; Müller, V.; Pantel, K. Diagnostic potential of PTEN-targeting miR-214 in the blood of breast cancer patients. Breast Cancer Res. Treat. 2012, 134, 933–941. [Google Scholar] [CrossRef]
- Walter, B.A.; Gómez-Macias, G.; Valera, V.A.; Sobel, M.; Merino, M.J. miR-21 Expression in Pregnancy-Associated Breast Cancer: A Possible Marker of Poor Prognosis. J. Cancer 2011, 2, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Zhu, H.; Luo, J.; Wu, Z.; Xie, M. miR-425-5p is associated with poor prognosis in patients with breast cancer and promotes cancer cell progression by targeting PTEN. Oncol. Rep. 2019, 42, 2550–2560. [Google Scholar] [CrossRef]
- Clarke, M.F.; Fuller, M. Stem cells and cancer: Two faces of eve. Cell 2006, 124, 1111–1115. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Wang, Y.Y.; Xin, H.W.; Wang, L.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Wang, H.; Tang, F.R.; Warrier, S.; et al. The expanding roles of long non-coding RNAs in the regulation of cancer stem cells. Int. J. Biochem. Cell Biol. 2019, 108, 17–20. [Google Scholar] [CrossRef]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charafe-Jauffret, E.; Ginestier, C.; Iovino, F.; Tarpin, C.; Diebel, M.; Esterni, B.; Houvenaeghel, G.; Extra, J.-M.; Bertucci, F.; Jacquemier, J. Aldehyde dehydrogenase 1–Positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clinl. Cancer Res. 2010, 16, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginestier, C.; Liu, S.; Diebel, M.E.; Korkaya, H.; Luo, M.; Brown, M.; Wicinski, J.; Cabaud, O.; Charafe-Jauffret, E.; Birnbaum, D. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J. Clin. Investig. 2010, 120, 485–497. [Google Scholar] [CrossRef]
- Li, B.; Lu, Y.; Wang, H.; Han, X.; Mao, J.; Li, J.; Yu, L.; Wang, B.; Fan, S.; Yu, X.; et al. miR-221/222 enhance the tumorigenicity of human breast cancer stem cells via modulation of PTEN/Akt pathway. Biomed. Pharmacother. 2016, 79, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Cui, R.; Bahr, J.; Zanesi, N.; Luo, Z.; Meng, W.; Liang, G.; Croce, C.M. miR-130a Deregulates PTEN and Stimulates Tumor Growth. Cancer Res. 2017, 77, 6168–6178. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Zhao, J.; Liang, Y.; Chen, M.; Luo, Y.; Cui, X.; Jiang, B.; Peng, L.; Wang, X. MicroRNA-10b controls the metastasis and proliferation of colorectal cancer cells by regulating Krüppel-like factor 4. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1722–1729. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhang, J.; Fu, Z.; Dong, L.; Tang, Y.; Xu, C.; Wang, H.; Zhang, T.; Wu, Y.; Dong, C.; et al. Hypoxia-induced microRNA-10b-3p promotes esophageal squamous cell carcinoma growth and metastasis by targeting TSGA10. Aging 2019, 11, 10374–10384. [Google Scholar] [CrossRef]
- Xu, C.; Qi, X. MiR-10b inhibits migration and invasion of pancreatic ductal adenocarcinoma via regulating E2F7. J. Clin. Lab. Anal. 2020, e23442. [Google Scholar] [CrossRef]
- Bahena-Ocampo, I.; Espinosa, M.; Ceballos-Cancino, G.; Lizarraga, F.; Campos-Arroyo, D.; Schwarz, A.; Maldonado, V.; Melendez-Zajgla, J.; Garcia-Lopez, P. miR-10b expression in breast cancer stem cells supports self-renewal through negative PTEN regulation and sustained AKT activation. EMBO Rep. 2016, 17, 648–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.S.; Yang, W.C.; Xin, H.W.; Han, J.X.; Ma, S.G. MiR-182-5p Knockdown Targeting PTEN Inhibits Cell Proliferation and Invasion of Breast Cancer Cells. Yonsei Med. J. 2019, 60, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bian, Z.; Wei, D.; Zhang, J.G. miR-29b regulates migration of human breast cancer cells. Mol. Cell Biochem. 2011, 352, 197–207. [Google Scholar] [CrossRef]
- Brody, J.G.; Moysich, K.B.; Humblet, O.; Attfield, K.R.; Beehler, G.P.; Rudel, R.A. Environmental pollutants and breast cancer: Epidemiologic studies. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2007, 109, 2667–2711. [Google Scholar] [CrossRef] [PubMed]
- Brody, J.G.; Rudel, R.A.; Michels, K.B.; Moysich, K.B.; Bernstein, L.; Attfield, K.R.; Gray, S. Environmental pollutants, diet, physical activity, body size, and breast cancer: Where do we stand in research to identify opportunities for prevention? Cancer Interdiscip. Int. J. Am. Cancer Soc. 2007, 109, 2627–2634. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, Y.; Cao, W.; Li, X.; Xie, C.; Geng, S.; Zhu, M.; Liang, Z.; Zhu, J.; Zhu, W.; et al. miR-19 targeting of PTEN mediates butyl benzyl phthalate-induced proliferation in both ER(+) and ER(-) breast cancer cells. Toxicol. Lett. 2018, 295, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, Y.; Qiu, H.; Liu, Y.; Luo, K.; Yi, Y.; Jiang, G.; Lu, M.; Zhang, Z.; Yin, J.; et al. Downregulation of FOXO3a by DNMT1 promotes breast cancer stem cell properties and tumorigenesis. Cell Death Differ. 2020, 27, 966–983. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, M.; Rizza, P.; Donà, A.; Nigro, A.; Ricci, E.; Fiorillo, M.; Perrotta, I.; Lanzino, M.; Giordano, C.; Bonofiglio, D.; et al. FoxO3a as a Positive Prognostic Marker and a Therapeutic Target in Tamoxifen-Resistant Breast Cancer. Cancers 2019, 11, 1858. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Pang, L.; Ke, Y.; Ji, W.; Xiong, J.; Ding, R.; Ding, Y. LncRNA GAS6-AS2 facilitates tumor growth and metastasis of hepatocellular carcinoma by activating the PI3K/AKT/FoxO3a signaling pathway. Int. J. Clin. Exp. Pathol. 2019, 12, 4011–4023. [Google Scholar]
- Yu, Y.; Yang, L.; Han, S.; Wu, Y.; Liu, L.; Chang, Y.; Wang, X.; Chai, J. MIR-190B Alleviates Cell Autophagy and Burn-Induced Skeletal Muscle Wasting via Modulating PHLPP1/Akt/FoxO3A Signaling Pathway. Shock 2019, 52, 513–521. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, R.; Zhu, Q.; Sun, L.; Jian, L.; Wang, X.; Zhao, J.; Li, C.; Liu, X. CXCL16/CXCR6 axis promotes bleomycin-induced fibrotic process in MRC-5 cells via the PI3K/AKT/FOXO3a pathway. Int. Immunopharmacol. 2020, 81, 106035. [Google Scholar] [CrossRef]
- Liu, X.; Feng, J.; Tang, L.; Liao, L.; Xu, Q.; Zhu, S. The regulation and function of miR-21-FOXO3a-miR-34b/c signaling in breast cancer. Int. J. Mol. Sci. 2015, 16, 3148–3162. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Tu, Y.; Xu, Y.; Guo, Y.; Yao, F.; Zhang, X. Endoplasmic reticulum stress-induced exosomal miR-27a-3p promotes immune escape in breast cancer via regulating PD-L1 expression in macrophages. J. Cell. Mol. Med. 2020, 24, 9560–9573. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, H.; Zhang, L.; Hu, M.; Li, F.; Deng, J.; An, M.; Wu, S.; Ma, R.; Lu, J.; et al. Long non-coding RNA LINC00672 contributes to p53 protein-mediated gene suppression and promotes endometrial cancer chemosensitivity. J. Biol. Chem. 2017, 292, 5801–5813. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Lu, Y.; Yu, L.; Han, X.; Wang, H.; Mao, J.; Shen, J.; Wang, B.; Tang, J.; Li, C.; et al. miR-221/222 promote cancer stem-like cell properties and tumor growth of breast cancer via targeting PTEN and sustained Akt/NF-κB/COX-2 activation. Chem. Biol. Interact. 2017, 277, 33–42. [Google Scholar] [CrossRef]
- Han, M.; Liu, M.; Wang, Y.; Chen, X.; Xu, J.; Sun, Y.; Zhao, L.; Qu, H.; Fan, Y.; Wu, C. Antagonism of miR-21 reverses epithelial-mesenchymal transition and cancer stem cell phenotype through AKT/ERK1/2 inactivation by targeting PTEN. PLoS ONE 2012, 7, e39520. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Lee, H.; Bae, H.; Choi, E.H.; Kim, S.J. Epigenetic silencing of miR-19a-3p by cold atmospheric plasma contributes to proliferation inhibition of the MCF-7 breast cancer cell. Sci. Rep. 2016, 6, 30005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Chen, H.; Liao, Y.; Chen, N.; Liu, T.; Zhang, H.; Zhang, H. Expression and clinical evidence of miR-494 and PTEN in non-small cell lung cancer. Tumour Biol. 2015, 36, 6965–6972. [Google Scholar] [CrossRef]
- Xie, X.; Liu, H.T.; Mei, J.; Ding, F.B.; Xiao, H.B.; Hu, F.Q.; Hu, R.; Wang, M.S. miR-106a promotes growth and metastasis of non-small cell lung cancer by targeting PTEN. Int. J. Clin. Exp. Pathol. 2015, 8, 3827–3834. [Google Scholar]
- Ren, W.; Hou, J.; Yang, C.; Wang, H.; Wu, S.; Wu, Y.; Zhao, X.; Lu, C. Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J. Exp. Clin. Cancer Res. 2019, 38, 62. [Google Scholar] [CrossRef]
- Gao, Z.J.; Yuan, W.D.; Yuan, J.Q.; Yuan, K.; Wang, Y. miR-486-5p functions as an oncogene by targeting PTEN in non-small cell lung cancer. Pathol. Res. Pract. 2018, 214, 700–705. [Google Scholar] [CrossRef]
- Yang, W.; Bai, J.; Liu, D.; Wang, S.; Zhao, N.; Che, R.; Zhang, H. MiR-93-5p up-regulation is involved in non-small cell lung cancer cells proliferation and migration and poor prognosis. Gene 2018, 647, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Liu, Y.; Wang, Y.; Meng, M.; Wang, K.; Zang, X.; Zhao, S.; Sun, X.; Cui, L.; Pan, L.; et al. MiR-21 and MiR-155 promote non-small cell lung cancer progression by downregulating SOCS1, SOCS6, and PTEN. Oncotarget 2016, 7, 84508–84519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Q.; Jiang, Z.; Li, Y.; Wu, H.; Yu, J.; Jiang, M. FAM60A promotes cisplatin resistance in lung cancer cells by activating SKP2 expression. Anti-Cancer Drugs 2020, 31, 776–784. [Google Scholar] [CrossRef]
- Bu, W.; Luo, T. miR-1297 Promotes Cell Proliferation of Non-Small Cell Lung Cancer Cells: Involving in PTEN/Akt/Skp2 Signaling Pathway. DNA Cell Biol. 2017, 36, 976–982. [Google Scholar] [CrossRef]
- Ahn, K.J.; Kim, J.; Yun, M.; Park, J.H.; Lee, J.D. Enzymatic properties of the N-and C-terminal halves of human hexokinase II. BMB Rep. 2009. [Google Scholar] [CrossRef] [Green Version]
- Popescu, N.C.; Cheng, S.-y. Chromosomal localization of the gene for a human cytosolic thyroid hormone binding protein homologous to the subunit of pyruvate kinase, subtype M 2. Somatic Cell Mol. Genet. 1990, 16, 593–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Zhang, M.; Jiang, H.; Liu, F.; Liu, H.; Li, Y. Down-regulation of miR-214 inhibits proliferation and glycolysis in non-small-cell lung cancer cells via down-regulating the expression of hexokinase 2 and pyruvate kinase isozyme M2. Biomed. Pharmacother. 2018, 105, 545–552. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Li, X.N.; Qi, Y.; Liu, D.L.; Yang, Y.; Zhao, J.; Zhang, C.Y.; Wu, K.; Zhao, S. MiR-454 promotes the progression of human non-small cell lung cancer and directly targets PTEN. Biomed. Pharmacother 2016, 81, 79–85. [Google Scholar] [CrossRef]
- Aquila, S.; Santoro, M.; Caputo, A.; Panno, M.L.; Pezzi, V.; De Amicis, F. The Tumor Suppressor PTEN as Molecular Switch Node Regulating Cell Metabolism and Autophagy: Implications in Immune System and Tumor Microenvironment. Cells 2020, 9, 1725. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jing, D.; Meng, Z.; Hu, B.; Zhong, B.; Deng, X.; Jin, X.; Shao, Z. FGD1 promotes tumor progression and regulates tumor immune response in osteosarcoma via inhibiting PTEN activity. Theranostics 2020, 10, 2859–2871. [Google Scholar] [CrossRef]
- Wan, J.; Ling, X.; Peng, B.; Ding, G. miR-142-5p regulates CD4+ T cells in human non-small cell lung cancer through PD-L1 expression via the PTEN pathway. Oncol. Rep. 2018, 40, 272–282. [Google Scholar] [CrossRef]
- Hu, P.; Zhou, L.; Wang, C.; Cao, G.; Chang, Y. MiR-181a reduces radiosensitivity of non-small cell lung cancer via inhibiting PTEN. Panminerva Med. 2020. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Hu, L.; He, Y.; Shi, Z.; Tang, S.; Chen, Y. Abnormal Expression of miR-21 and miR-95 in Cancer Stem-Like Cells is Associated with Radioresistance of Lung Cancer. Cancer Investig. 2015, 33, 165–171. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, L.; Chen, C.; Ming, P.; Huang, Q.; Li, C.; Cao, D.; Xu, X.; Ge, W. The extracellular vesicles secreted by lung cancer cells in radiation therapy promote endothelial cell angiogenesis by transferring miR-23a. PeerJ. 2017, 5, e3627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Ma, H.; Chang, H.; Feng, Z.; Zhang, C.; Yang, X. The interaction of interleukin-8 and PTEN inactivation promotes the malignant progression of head and neck squamous cell carcinoma via the STAT3 pathway. Cell Death Dis. 2020, 11, 405. [Google Scholar] [CrossRef]
- Yuan, B.; Sun, R.; Du, Y.; Jia, Z.; Yao, W.; Yang, J. STAT3-Induced Upregulation of lncRNA CASC9 Promotes the Progression of Bladder Cancer by Interacting with EZH2 and Affecting the Expression of PTEN. Onco. Targets Ther. 2020, 13, 9147–9157. [Google Scholar] [CrossRef] [PubMed]
- Parashar, D.; Geethadevi, A.; Aure, M.R.; Mishra, J.; George, J.; Chen, C.; Mishra, M.K.; Tahiri, A.; Zhao, W.; Nair, B.; et al. miRNA551b-3p Activates an Oncostatin Signaling Module for the Progression of Triple-Negative Breast Cancer. Cell Rep. 2019, 29, 4389–4406. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Bai, W.; Zhang, W. MiR-21 suppresses the anticancer activities of curcumin by targeting PTEN gene in human non-small cell lung cancer A549 cells. Clin. Transl. Oncol. 2014, 16, 708–713. [Google Scholar] [CrossRef]
- Guerriero, I.; D’Angelo, D.; Pallante, P.; Santos, M.; Scrima, M.; Malanga, D.; De Marco, C.; Ravo, M.; Weisz, A.; Laudanna, C.; et al. Analysis of miRNA profiles identified miR-196a as a crucial mediator of aberrant PI3K/AKT signaling in lung cancer cells. Oncotarget 2017, 8, 19172–19191. [Google Scholar] [CrossRef]
- Faversani, A.; Amatori, S.; Augello, C.; Colombo, F.; Porretti, L.; Fanelli, M.; Ferrero, S.; Palleschi, A.; Pelicci, P.G.; Belloni, E.; et al. miR-494-3p is a novel tumor driver of lung carcinogenesis. Oncotarget. 2017, 8, 7231–7247. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Zhu, X.; Ma, J.; Wang, W. miR-205 regulates A549 cells proliferation by targeting PTEN. Int. J. Clin. Exp. Pathol. 2015, 8, 1175–1183. [Google Scholar]
- Garofalo, M.; Di Leva, G.; Romano, G.; Nuovo, G.; Suh, S.S.; Ngankeu, A.; Taccioli, C.; Pichiorri, F.; Alder, H.; Secchiero, P.; et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell 2009, 16, 498–509. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Xu, P.; Liu, Z.; Zhen, Y.; Chen, Y.; Liu, Y.; Fu, Q.; Deng, X.; Liang, Z.; Li, Y.; et al. Dual roles of miR-374a by modulated c-Jun respectively targets CCND1-inducing PI3K/AKT signal and PTEN-suppressing Wnt/β-catenin signaling in non-small-cell lung cancer. Cell Death Dis. 2018, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Bach, D.H.; Kim, D.; Bae, S.Y.; Kim, W.K.; Hong, J.Y.; Lee, H.J.; Rajasekaran, N.; Kwon, S.; Fan, Y.; Luu, T.T.; et al. Targeting Nicotinamide N-Methyltransferase and miR-449a in EGFR-TKI-Resistant Non-Small-Cell Lung Cancer Cells. Mol. Ther. Nucleic. Acids 2018, 11, 455–467. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Lyu, J.; Meng, Q.H. MiR-93 Promotes Tumorigenesis and Metastasis of Non-Small Cell Lung Cancer Cells by Activating the PI3K/Akt Pathway via Inhibition of LKB1/PTEN/CDKN1A. J. Cancer 2017, 8, 870–879. [Google Scholar] [CrossRef] [Green Version]
- Marin, I.; Ofek, E.; Bar, J.; Prisant, N.; Perelman, M.; Avivi, C.; Lavy-Shahaf, G.; Onn, A.; Katz, R.; Barshack, I. MiR-21, EGFR and PTEN in non-small cell lung cancer: An in situ hybridisation and immunohistochemistry study. J. Clin. Pathol. 2020, 73, 636–641. [Google Scholar] [CrossRef]
- Dai, L.; Chen, F.; Zheng, Y.; Zhang, D.; Qian, B.; Ji, H.; Long, F.; Cretoiu, D. miR-21 regulates growth and EMT in lung cancer cells via PTEN/Akt/GSK3β signaling. Front. Biosci. (Landmark Ed) 2019, 24, 1426–1439. [Google Scholar]
- Xu, L.F.; Wu, Z.P.; Chen, Y.; Zhu, Q.S.; Hamidi, S.; Navab, R. MicroRNA-21 (miR-21) regulates cellular proliferation, invasion, migration, and apoptosis by targeting PTEN, RECK and Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS ONE 2014, 9, e103698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Wu, X.; Liu, B.; Wang, C.; Liu, Y.; Zhou, Q.; Xu, K. MiR-26a enhances metastasis potential of lung cancer cells via AKT pathway by targeting PTEN. Biochim. Biophys. Acta 2012, 1822, 1692–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.G.; Wang, J.J.; Zhao, F.; Liu, Q.; Jiang, K.; Yang, G.H. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin. Chim. Acta 2010, 411, 846–852. [Google Scholar] [CrossRef]
- Cai, J.; Fang, L.; Huang, Y.; Li, R.; Yuan, J.; Yang, Y.; Zhu, X.; Chen, B.; Wu, J.; Li, M. miR-205 targets PTEN and PHLPP2 to augment AKT signaling and drive malignant phenotypes in non-small cell lung cancer. Cancer Res. 2013, 73, 5402–5415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Ma, Z.; Liu, X.; Zhang, C.; Hu, Y.; Ding, L.; Qi, P.; Wang, J.; Lu, S.; Li, Y. MiR-183-5p is required for non-small cell lung cancer progression by repressing PTEN. Biomed. Pharmacother 2019, 111, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Dastmalchi, N.; Hosseinpourfeizi, M.A.; Khojasteh, S.M.B.; Baradaran, B.; Safaralizadeh, R. Tumor suppressive activity of miR-424-5p in breast cancer cells through targeting PD-L1 and modulating PTEN/PI3K/AKT/mTOR signaling pathway. Life Sci. 2020, 259, 118239. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.B.; Zhang, W.P.; Shi, J.L.; Wang, J.W. MiR-4299 suppresses non-small cell lung cancer cell proliferation, migration and invasion through modulating PTEN/AKT/PI3K pathway. Eur. Rev. Med. Pharmacol Sci. 2018, 22, 3408–3414. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, Y.J.; Yan, F. MicroRNA-130-5p promotes invasion as well as migration of lung adenocarcinoma cells by targeting the EZH2 signaling pathway. Eur. Rev. Med. Pharmacol Sci 2019, 23, 9480–9488. [Google Scholar] [CrossRef]
- Ye, L.; Wang, Y.; Nie, L.; Qian, S.; Xu, M. MiR-130 exerts tumor suppressive function on the tumorigenesis of human non-small cell lung cancer by targeting PTEN. Am. J. Transl. Res. 2017, 9, 1856–1865. [Google Scholar] [PubMed]
- De Angelis, M.L.; Francescangeli, F.; Zeuner, A. Breast Cancer Stem Cells as Drivers of Tumor Chemoresistance, Dormancy and Relapse: New Challenges and Therapeutic Opportunities. Cancers 2019, 11, 1569. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Niu, T.; Xiao, W. Uev1A promotes breast cancer cell survival and chemoresistance through the AKT-FOXO1-BIM pathway. Cancer Cell Int. 2019, 19, 331. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Liu, P.; Li, D.; Yang, Q.; Li, B.; Jiang, X.J. Stat-3 signaling promotes cell proliferation and metastasis of gastric cancer through PDCD4 downregulation. Kaohsiung J. Med. Sci. 2020, 36, 244–249. [Google Scholar] [CrossRef]
- Goh, J.N.; Loo, S.Y.; Datta, A.; Siveen, K.S.; Yap, W.N.; Cai, W.; Shin, E.M.; Wang, C.; Kim, J.E.; Chan, M.; et al. microRNAs in breast cancer: Regulatory roles governing the hallmarks of cancer. Biol. Rev. Camb. Philos. Soc. 2016, 91, 409–428. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.Y.; Hirpara, J.L.; Pandey, V.; Tan, T.Z.; Yap, C.T.; Lobie, P.E.; Thiery, J.P.; Goh, B.C.; Pervaiz, S.; Clément, M.V.; et al. Manganese Superoxide Dismutase Expression Regulates the Switch Between an Epithelial and a Mesenchymal-Like Phenotype in Breast Carcinoma. Antioxid. Redox Signal. 2016, 25, 283–299. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Anbalagan, D.; Lee, L.H.; Samy, R.P.; Shanmugam, M.K.; Kumar, A.P.; Sethi, G.; Lobie, P.E.; Lim, L.H. ANXA1 inhibits miRNA-196a in a negative feedback loop through NF-kB and c-Myc to reduce breast cancer proliferation. Oncotarget 2016, 7, 27007–27020. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Feng, A.; Shim, H. MicroRNA-30c-regulated HDAC9 mediates chemoresistance of breast cancer. Cancer Chemother. Pharmacol. 2020, 85, 413–423. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, F.; Xiang, Z.; Huang, T.; Zhou, W.B. LncRNA XIST promotes chemoresistance of breast cancer cells to doxorubicin by sponging miR-200c-3p to upregulate ANLN. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1464–1472. [Google Scholar] [CrossRef]
- Wang, D.D.; Yang, S.J.; Chen, X.; Shen, H.Y.; Luo, L.J.; Zhang, X.H.; Zhong, S.L.; Zhao, J.H.; Tang, J.H. miR-222 induces Adriamycin resistance in breast cancer through PTEN/Akt/p27(kip1) pathway. Tumour Biol. 2016, 37, 15315–15324. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, J.; Li, Y.-C.; Li, Y.-S.; Shyy, J.Y.-J.; Chien, S. Shear stress and VEGF activate IKK via the Flk-1/Cbl/Akt signaling pathway. Am. J. Physiol. Cell Physiol. 2004, 286, H685–H692. [Google Scholar] [CrossRef] [PubMed]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Siveen, K.S.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Arfuso, F.; Kumar, A.P.; et al. Isorhamnetin augments the anti-tumor effect of capecitabine through the negative regulation of NF-κB signaling cascade in gastric cancer. Cancer Lett. 2015, 363, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Sethi, G.; Jain, A.K.; Jaiswal, A.K.; Aggarwal, B.B. Genetic deletion of NAD(P)H:quinone oxidoreductase 1 abrogates activation of nuclear factor-kappaB, IkappaBalpha kinase, c-Jun N-terminal kinase, Akt, p38, and p44/42 mitogen-activated protein kinases and potentiates apoptosis. J. Biol. Chem. 2006, 281, 19798–19808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugam, M.K.; Ong, T.H.; Kumar, A.P.; Lun, C.K.; Ho, P.C.; Wong, P.T.; Hui, K.M.; Sethi, G. Ursolic acid inhibits the initiation, progression of prostate cancer and prolongs the survival of TRAMP mice by modulating pro-inflammatory pathways. PLoS ONE 2012, 7, e32476. [Google Scholar] [CrossRef]
- Schmedtje, J.F.; Ji, Y.-S.; Liu, W.-L.; DuBois, R.N.; Runge, M.S. Hypoxia induces cyclooxygenase-2 via the NF-κB p65 transcription factor in human vascular endothelial cells. J. Biol. Chem. 1997, 272, 601–608. [Google Scholar] [CrossRef] [Green Version]
- St-Germain, M.-E.; Gagnon, V.; Parent, S.; Asselin, E. Regulation of COX-2 protein expression by Akt in endometrial cancer cells is mediated through NF-κB/IκB pathway. Mol. Cancer 2004, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shin, E.M.; Hay, H.S.; Lee, M.H.; Goh, J.N.; Tan, T.Z.; Sen, Y.P.; Lim, S.W.; Yousef, E.M.; Ong, H.T.; Thike, A.A.; et al. DEAD-box helicase DP103 defines metastatic potential of human breast cancers. J. Clin. Investig. 2014, 124, 3807–3824. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Li, F.; Chen, L.; Siveen, K.S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J. Mol. Med. 2014, 92, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Armoni, M.; Harel, C.; Karni, S.; Chen, H.; Bar-Yoseph, F.; Ver, M.R.; Quon, M.J.; Karnieli, E. FOXO1 represses peroxisome proliferator-activated receptor-γ1 and-γ2 gene promoters in primary adipocytes A NOVEL PARADIGM TO INCREASE INSULIN SENSITIVITY. J Biol. Chem. 2006, 281, 19881–19891. [Google Scholar] [CrossRef] [Green Version]
- Nakae, J.; Oki, M.; Cao, Y. The FoxO transcription factors and metabolic regulation. FEBS Lett. 2008, 582, 54–67. [Google Scholar] [CrossRef] [Green Version]
- Greer, E.L.; Brunet, A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 2005, 24, 7410–7425. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Wang, D.; Li, L.; Yang, S.; Chen, X.; Zhou, S.; Zhong, S.; Zhao, J.; Tang, J. MiR-222 promotes drug-resistance of breast cancer cells to adriamycin via modulation of PTEN/Akt/FOXO1 pathway. Gene 2017, 596, 110–118. [Google Scholar] [CrossRef]
- Zhong, S.; Li, W.; Chen, Z.; Xu, J.; Zhao, J. MiR-222 and miR-29a contribute to the drug-resistance of breast cancer cells. Gene 2013, 531, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Li, Y.; Huang, K.; Wagar, N.; Shim, H. Regulation of miR-19 to breast cancer chemoresistance through targeting PTEN. Pharm. Res. 2011, 28, 3091–3100. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Miller, Z.; Musich, P.R.; Thomas, A.E.; Yao, Z.Q.; Xie, Q.; Howe, P.H.; Jiang, Y. DSTYK Promotes Metastasis and Chemoresistance via EMT in Colorectal Cancer. Front. Pharmacol. 2020, 11, 1250. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Kalantari, M.; Mohammadinejad, R.; Javaheri, T.; Sethi, G. Association of the Epithelial-Mesenchymal Transition (EMT) with Cisplatin Resistance. Int. J. Mol. Sci. 2020, 21, 4002. [Google Scholar] [CrossRef]
- Cheng, J.T.; Wang, L.; Wang, H.; Tang, F.R.; Cai, W.Q.; Sethi, G.; Xin, H.W.; Ma, Z. Insights into Biological Role of LncRNAs in Epithelial-Mesenchymal Transition. Cells 2019, 8, 1178. [Google Scholar] [CrossRef] [Green Version]
- Chu, S.; Liu, G.; Xia, P.; Chen, G.; Shi, F.; Yi, T.; Zhou, H. miR-93 and PTEN: Key regulators of doxorubicin-resistance and EMT in breast cancer. Oncol. Rep. 2017, 38, 2401–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, T.N.; Lin, C.S.; Li, G.D.; Kuo, C.Y.; Kao, S.H. Sesamin inhibits cervical cancer cell proliferation by promoting p53/PTEN-mediated apoptosis. Int. J. Med. Sci. 2020, 17, 2292–2298. [Google Scholar] [CrossRef]
- Li, S.; Li, Q.; Lü, J.; Zhao, Q.; Li, D.; Shen, L.; Wang, Z.; Liu, J.; Xie, D.; Cho, W.C.; et al. Targeted Inhibition of miR-221/222 Promotes Cell Sensitivity to Cisplatin in Triple-Negative Breast Cancer MDA-MB-231 Cells. Front. Genet. 2019, 10, 1278. [Google Scholar] [CrossRef] [Green Version]
- Geng, W.; Song, H.; Zhao, Q.; Dong, K.; Pu, Q.; Gao, H.; Lv, Y. miR-520h Stimulates Drug Resistance to Paclitaxel by Targeting the OTUD3-PTEN Axis in Breast Cancer. Biomed. Res. Int. 2020, 2020, 9512793. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Chen, C.; Li, Z.Y.; Zou, Z.M.; Gao, D.C.; Zhang, X.; Kuang, X.W.; Sun, Z.H.; Zheng, W.J.; Zhou, P.; et al. The MyD88 inhibitor TJ-M2010-2 suppresses proliferation, migration and invasion of breast cancer cells by regulating MyD88/GSK-3β and MyD88/NF-κB signalling pathways. Exp. Cell Res. 2020, 394, 112157. [Google Scholar] [CrossRef]
- Xiong, R.; Sun, X.X.; Wu, H.R.; Xu, G.W.; Wang, G.X.; Sun, X.H.; Xu, M.Q.; Xie, M.R. Mechanism research of miR-34a regulates Axl in non-small-cell lung cancer with gefitinib-acquired resistance. Thorac. Cancer 2020, 11, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wang, Z.; Tong, F.; Dong, X.; Wu, G.; Zhang, R. lncRNA UCA1 Promotes Gefitinib Resistance as a ceRNA to Target FOSL2 by Sponging miR-143 in Non-small Cell Lung Cancer. Mol. Ther. Nucleic. Acids 2020, 19, 643–653. [Google Scholar] [CrossRef]
- Cui, F.; Zhou, Q.; Xiao, K.; Qian, H. MicroRNA-28 promotes the proliferation of non-small-cell lung cancer cells by targeting PTEN. Mol. Med. Rep. 2020, 21, 2589–2596. [Google Scholar] [CrossRef]
- Zhou, J.S.; Yang, Z.S.; Cheng, S.Y.; Yu, J.H.; Huang, C.J.; Feng, Q. miRNA-425-5p enhances lung cancer growth via the PTEN/PI3K/AKT signaling axis. BMC Pulm. Med. 2020, 20, 223. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.M.; Hong, S.H.; Kim, H.I.; Kim, M.J.; Kim, S.K.; Kim, M.; Choi, S.Y.; Park, J.; Kim, H.K.; Kim, J.H.; et al. Synergistic anticancer effect of combined use of Trichosanthes kirilowii with cisplatin and pemetrexed enhances apoptosis of H1299 non-small-cell lung cancer cells via modulation of ErbB3. Phytomedicine 2020, 66, 153109. [Google Scholar] [CrossRef]
- Medina-Reyes, E.I.; Mancera-Rodríguez, M.A.; Delgado-Buenrostro, N.L.; Moreno-Rodríguez, A.; Bautista-Martínez, J.L.; Díaz-Velásquez, C.E.; Martínez-Alarcón, S.A.; Torrens, H.; de Los Ángeles Godínez-Rodríguez, M.; Terrazas-Valdés, L.I.; et al. Novel thiosemicarbazones induce high toxicity in estrogen-receptor-positive breast cancer cells (MCF7) and exacerbate cisplatin effectiveness in triple-negative breast (MDA-MB231) and lung adenocarcinoma (A549) cells. Invest. New Drugs 2020, 38, 558–573. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; You, X.; Xu, L.; Wang, L.; Chen, Y. PAQR3 suppresses the growth of non-small cell lung cancer cells via modulation of EGFR-mediated autophagy. Autophagy 2020, 16, 1236–1247. [Google Scholar] [CrossRef]
- Liu, J.; Deng, X.; Sun, X.; Dong, J.; Huang, J. Inhibition of autophagy enhances timosaponin AIII-induced lung cancer cell apoptosis and anti-tumor effect in vitro and in vivo. Life Sci. 2020, 257, 118040. [Google Scholar] [CrossRef]
- Singh, S.S.; Vats, S.; Chia, A.Y.; Tan, T.Z.; Deng, S.; Ong, M.S.; Arfuso, F.; Yap, C.T.; Goh, B.C.; Sethi, G.; et al. Dual role of autophagy in hallmarks of cancer. Oncogene 2018, 37, 1142–1158. [Google Scholar] [CrossRef]
- Deng, S.; Shanmugam, M.K.; Kumar, A.P.; Yap, C.T.; Sethi, G.; Bishayee, A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer 2019, 125, 1228–1246. [Google Scholar] [CrossRef]
- Bordoloi, D.; Banik, K.; Shabnam, B.; Padmavathi, G.; Monisha, J.; Arfuso, F.; Dharmarajan, A.; Mao, X.; Lim, L.H.K.; Wang, L.; et al. TIPE Family of Proteins and Its Implications in Different Chronic Diseases. Int. J. Mol. Sci. 2018, 19, 2974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Xing, Y.; Rong, L. miR-181 regulates cisplatin-resistant non-small cell lung cancer via downregulation of autophagy through the PTEN/PI3K/AKT pathway. Oncol. Rep. 2018, 39, 1631–1639. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-M.; Song, T.-J.; Xiao, J.-H.; Huang, Z.-H.; Li, Y.; Lin, T.-Y. Tripchlorolide induces autophagy in lung cancer cells by inhibiting the PI3K/AKT/mTOR pathway and improves cisplatin sensitivity in A549/DDP cells. Oncotarget 2017, 8, 63911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Shao, Q.H.; Zhou, H.; Wu, J.L.; Quan, W.Q.; Ji, P.; Yao, Y.W.; Li, D.; Sun, Z.J. Ginkgolide B inhibits lung cancer cells promotion via beclin-1-dependent autophagy. BMC Complement Med. Ther. 2020, 20, 194. [Google Scholar] [CrossRef]
- Jia, R.; Wang, C. MiR-29b-3p Reverses Cisplatin Resistance by Targeting COL1A1 in Non-Small-Cell Lung Cancer A549/DDP Cells. Cancer Manag. Res. 2020, 12, 2559–2566. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Zhou, X.; Li, S.; Qin, Y.; Chen, Y.; Liu, H. Inhibition of miR-23a increases the sensitivity of lung cancer stem cells to erlotinib through PTEN/PI3K/Akt pathway. Oncol. Rep. 2017, 38, 3064–3070. [Google Scholar] [CrossRef] [Green Version]
- Shen, K.H.; Hung, J.H.; Liao, Y.C.; Tsai, S.T.; Wu, M.J.; Chen, P.S. Sinomenine Inhibits Migration and Invasion of Human Lung Cancer Cell through Downregulating Expression of miR-21 and MMPs. Int. J. Mol. Sci. 2020, 21, 3080. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, C.; Li, W.; He, Y.; Bao, Y. Matrine Inhibits Proliferation, Invasion, and Migration and Induces Apoptosis of Colorectal Cancer Cells Via miR-10b/PTEN Pathway. Cancer Biother. Radiopharm. 2020. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhu, F.; Liu, J.; Xu, T.; Pei, D.; Wang, R.; Qian, Y.; Li, Q.; Wang, L.; Shi, Z.; et al. Alteration in Mir-21/PTEN expression modulates gefitinib resistance in non-small cell lung cancer. PLoS ONE 2014, 9, e103305. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Liu, X.; Wang, H.; Li, J.; Dai, L.; Li, J.; Xu, Z. Hypoxic non-small-cell lung cancer cell-derived exosomal miR-21 promotes resistance of normoxic cell to cisplatin. Onco. Targets Ther. 2019, 12, 1947–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Ren, S.; Li, X.; Wang, Y.; Garfield, D.; Zhou, S.; Chen, X.; Su, C.; Chen, M.; Kuang, P.; et al. MiR-21 overexpression is associated with acquired resistance of EGFR-TKI in non-small cell lung cancer. Lung Cancer 2014, 83, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Wang, H.; Liu, J.; Wang, Z.X. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol. Cell Biochem. 2013, 372, 35–45. [Google Scholar] [CrossRef]
- Chen, L.; Liu, D.; Yi, X.; Qi, L.; Tian, X.; Sun, B.; Dong, Q.; Han, Z.; Li, Q.; Song, T.; et al. The novel miR-1269b-regulated protein SVEP1 induces hepatocellular carcinoma proliferation and metastasis likely through the PI3K/Akt pathway. Cell Death Dis. 2020, 11, 320. [Google Scholar] [CrossRef]
- Yang, W.; Xiao, W.; Cai, Z.; Jin, S.; Li, T. miR-1269b Drives Cisplatin Resistance of Human Non-Small Cell Lung Cancer via Modulating the PTEN/PI3K/AKT Signaling Pathway. Onco. Targets Ther. 2020, 13, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Huang, Y.; Gong, W. miR-205 promotes the growth, metastasis and chemoresistance of NSCLC cells by targeting PTEN. Oncol. Rep. 2013, 30, 2897–2902. [Google Scholar] [CrossRef] [Green Version]
- Sailo, B.L.; Banik, K.; Girisa, S.; Bordoloi, D.; Fan, L.; Halim, C.E.; Wang, H.; Kumar, A.P.; Zheng, D.; Mao, X.; et al. FBXW7 in Cancer: What Has Been Unraveled Thus Far? Cancers 2019, 11, 246. [Google Scholar] [CrossRef] [Green Version]
- Monisha, J.; Roy, N.K.; Padmavathi, G.; Banik, K.; Bordoloi, D.; Khwairakpam, A.D.; Arfuso, F.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; et al. NGAL is Downregulated in Oral Squamous Cell Carcinoma and Leads to Increased Survival, Proliferation, Migration and Chemoresistance. Cancers 2018, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- Raghunath, A.; Sundarraj, K.; Arfuso, F.; Sethi, G.; Perumal, E. Dysregulation of Nrf2 in Hepatocellular Carcinoma: Role in Cancer Progression and Chemoresistance. Cancers 2018, 10, 481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Jiang, B.; Yan, D.; Wang, X.; Ge, H.; Sun, Y. Knockdown of TMEM45A overcomes multidrug resistance and epithelial-mesenchymal transition in human colorectal cancer cells through inhibition of TGF-β signalling pathway. Clin. Exp. Pharmacol. Physiol. 2020, 47, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Wang, Z.; Tan, F.; Chen, J.; Lin, J.; Yang, J.; Li, H.; Wang, X.; Sali, A.; Zhang, L.; et al. Enhancer Reprogramming within Pre-existing Topologically Associated Domains Promotes TGF-β-Induced EMT and Cancer Metastasis. Mol. Ther. 2020, 28, 2083–2095. [Google Scholar] [CrossRef]
- Ko, J.H.; Nam, D.; Um, J.Y.; Jung, S.H.; Sethi, G.; Ahn, K.S. Bergamottin Suppresses Metastasis of Lung Cancer Cells through Abrogation of Diverse Oncogenic Signaling Cascades and Epithelial-to-Mesenchymal Transition. Molecules 2018, 23, 1601. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.T.; Yang, M.H.; Kumar, A.P.; Sethi, G.; Ahn, K.S. Corilagin Represses Epithelial to Mesenchymal Transition Process Through Modulating Wnt/β-Catenin Signaling Cascade. Biomolecules 2020, 10, 1406. [Google Scholar] [CrossRef]
- Kitamura, K.; Seike, M.; Okano, T.; Matsuda, K.; Miyanaga, A.; Mizutani, H.; Noro, R.; Minegishi, Y.; Kubota, K.; Gemma, A. MiR-134/487b/655 cluster regulates TGF-β-induced epithelial-mesenchymal transition and drug resistance to gefitinib by targeting MAGI2 in lung adenocarcinoma cells. Mol. Cancer Ther. 2014, 13, 444–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Fang, S.; Di, Y.; Ying, W.; Tan, Y.; Gu, W. Modulation of NF-κB/miR-21/PTEN pathway sensitizes non-small cell lung cancer to cisplatin. PLoS ONE 2015, 10, e0121547. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Guan, Y.; Liu, X.; Meng, Q.; Guo, Q. MiR-92b regulates the cell growth, cisplatin chemosensitivity of A549 non small cell lung cancer cell line and target PTEN. Biochem. Biophys. Res. Commun. 2013, 440, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Liu, Y.; Liang, P.; Wang, B.; Tan, H.; Zhang, Y.; Gao, X.; Gao, J. TP53TG1 enhances cisplatin sensitivity of non-small cell lung cancer cells through regulating miR-18a/PTEN axis. Cell Biosci. 2018, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Li, X.; Luan, Y.; Zhao, R.; Li, Y.; Liu, L.; Hao, Y.; Oleg Vladimir, B.; Jia, L. Circulating lncRNA UCA1 Promotes Malignancy of Colorectal Cancer via the miR-143/MYO6 Axis. Mol. Ther. Nucleic. Acids. 2020, 19, 790–803. [Google Scholar] [CrossRef]
- Liu, G.; Liu, S.; Xing, G.; Wang, F. lncRNA PVT1/MicroRNA-17-5p/PTEN Axis Regulates Secretion of E2 and P4, Proliferation, and Apoptosis of Ovarian Granulosa Cells in PCOS. Mol. Ther. Nucleic. Acids. 2020, 20, 205–216. [Google Scholar] [CrossRef]
- Zhu, X.P.; Pan, S.A.; Chu, Z.; Zhou, Y.X.; Huang, Y.K.; Han, D.Q. LncRNA GAS5 regulates epithelial-mesenchymal transition and viability of glioma cells by targeting microRNA-106b and regulating PTEN expression. Neurosci Res. 2020. [Google Scholar] [CrossRef]
- Gao, X.; Qin, T.; Mao, J.; Zhang, J.; Fan, S.; Lu, Y.; Sun, Z.; Zhang, Q.; Song, B.; Li, L. PTENP1/miR-20a/PTEN axis contributes to breast cancer progression by regulating PTEN via PI3K/AKT pathway. J. Exp. Clin. Cancer Res 2019, 38, 256. [Google Scholar] [CrossRef]
- Shi, X.; Tang, X.; Su, L. Overexpression of Long Noncoding RNA PTENP1 Inhibits Cell Proliferation and Migration via Suppression of miR-19b in Breast Cancer Cells. Oncol. Res. 2018, 26, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wang, Y.; Wang, X.; Zhou, D.; Shao, C.; Zhou, M.; He, Z. Downregulation of lncRNA GAS5 confers tamoxifen resistance by activating miR-222 in breast cancer. Cancer lett. 2018, 434, 1–10. [Google Scholar] [CrossRef]
- Shao, G.; Zhao, Z.; Zhao, W.; Hu, G.; Zhang, L.; Li, W.; Xing, C.; Zhang, X. Long non-coding RNA MALAT1 activates autophagy and promotes cell proliferation by downregulating microRNA-204 expression in gastric cancer. Oncol. Lett. 2020, 19, 805–812. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, T.; Feng, H.; Yang, R.; Zhao, X.; Chen, W.; Jiang, B.; Qin, H.; Guo, X.; Liu, M.; et al. Circular RNA circSLC8A1 acts as a sponge of miR-130b/miR-494 in suppressing bladder cancer progression via regulating PTEN. Mol. Cancer 2019, 18, 111. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Zhang, X.; Zai, H.Y.; Jiang, W.; Zhang, K.J.; He, Y.Q.; Hu, Y. circSLC8A1 sponges miR-671 to regulate breast cancer tumorigenesis via PTEN/PI3k/Akt pathway. Genomics 2020. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Mao, L. Circular RNA Circ_0000442 acts as a sponge of MiR-148b-3p to suppress breast cancer via PTEN/PI3K/Akt signaling pathway. Gene 2021, 766, 145113. [Google Scholar] [CrossRef] [PubMed]
- Griffith, T.S.; Lynch, D.H. TRAIL: A molecule with multiple receptors and control mechanisms. Curr. Opin. Immunol. 1998, 10, 559–563. [Google Scholar] [CrossRef]
- Rahman, M.; Davis, S.R.; Pumphrey, J.G.; Bao, J.; Nau, M.M.; Meltzer, P.S.; Lipkowitz, S. TRAIL induces apoptosis in triple-negative breast cancer cells with a mesenchymal phenotype. Breast Cancer Res. Treat. 2009, 113, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xu, C.; Kong, X.; Li, X.; Kong, X.; Wang, Y.; Ding, X.; Yang, Q. Trail resistance induces epithelial-mesenchymal transition and enhances invasiveness by suppressing PTEN via miR-221 in breast cancer. PLoS ONE 2014, 9, e99067. [Google Scholar] [CrossRef] [Green Version]
- Su, S.; Chen, J.; Yao, H.; Liu, J.; Yu, S.; Lao, L.; Wang, M.; Luo, M.; Xing, Y.; Chen, F.; et al. CD10(+)GPR77(+) Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell 2018, 172, 841–856. [Google Scholar] [CrossRef]
- Ershaid, N.; Sharon, Y.; Doron, H.; Raz, Y.; Shani, O.; Cohen, N.; Monteran, L.; Leider-Trejo, L.; Ben-Shmuel, A.; Yassin, M.; et al. NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat. Commun. 2019, 10, 4375. [Google Scholar] [CrossRef] [Green Version]
- Vennin, C.; Mélénec, P.; Rouet, R.; Nobis, M.; Cazet, A.S.; Murphy, K.J.; Herrmann, D.; Reed, D.A.; Lucas, M.C.; Warren, S.C.; et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat. Commun. 2019, 10, 3637. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Li, X.; Zeng, C.; Liu, C.; Hao, Q.; Li, W.; Zhang, K.; Zhang, W.; Wang, S.; Zhao, H.; et al. CD63(+) Cancer-Associated Fibroblasts Confer Tamoxifen Resistance to Breast Cancer Cells through Exosomal miR-22. Adv. Sci. (Weinh) 2020, 7, 2002518. [Google Scholar] [CrossRef] [PubMed]
- Muppala, S.; Mudduluru, G.; Leupold, J.H.; Buergy, D.; Sleeman, J.P.; Allgayer, H. CD24 induces expression of the oncomir miR-21 via Src, and CD24 and Src are both post-transcriptionally downregulated by the tumor suppressor miR-34a. PLoS ONE 2013, 8, e59563. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, J.; Qian, X.; Han, L.; Zhang, K.; Chen, L.; Liu, J.; Ren, Y.; Yang, M.; Zhang, A.; et al. AC1MMYR2, an inhibitor of dicer-mediated biogenesis of Oncomir miR-21, reverses epithelial-mesenchymal transition and suppresses tumor growth and progression. Cancer Res. 2013, 73, 5519–5531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.; Li, G.; Li, Y.; Zhu, Z. Upregulation of Long Noncoding RNA GAS5 Inhibits Lung Cancer Cell Proliferation and Metastasis via miR-205/PTEN Axis. Med. Sci. Monit. 2019, 25, 2311–2319. [Google Scholar] [CrossRef]
- Cao, L.; Chen, J.; Ou, B.; Liu, C.; Zou, Y.; Chen, Q. GAS5 knockdown reduces the chemo-sensitivity of non-small cell lung cancer (NSCLC) cell to cisplatin (DDP) through regulating miR-21/PTEN axis. Biomed. Pharmacother. 2017, 93, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ren, P.; Zhang, Y.; Gong, B.; Yu, D.; Sun, X. Long non-coding RNA GAS5 increases the radiosensitivity of A549 cells through interaction with the miR-21/PTEN/Akt axis. Oncol. Rep. 2020, 43, 897–907. [Google Scholar] [CrossRef] [Green Version]
- Xiang, C.; Zhang, Y.; Zhang, Y.; Liu, C.; Hou, Y.; Zhang, Y. lncRNA LEF1-AS1 Promotes Proliferation and Induces Apoptosis of Non-Small-Cell Lung Cancer Cells by Regulating miR-221/PTEN Signaling. Cancer Manag. Res. 2020, 12, 3845–3850. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Buhrmann, C.; Varghese, E.; Abotaleb, M.; Qaradakhi, T.; Zulli, A.; et al. Flavonoids against the Warburg phenotype—Concepts of predictive, preventive and personalised medicine to cut the Gordian knot of cancer cell metabolism. EPMA J. 2020, 11, 377–398. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Guo, Q.; Mao, G.; Zhu, J.; Li, F. CircLARP4 Suppresses Cell Proliferation, Invasion and Glycolysis and Promotes Apoptosis in Non-Small Cell Lung Cancer by Targeting miR-135b. Onco. Targets Ther. 2020, 13, 3717–3728. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shanmugam, M.K.; Chen, L.; Chatterjee, S.; Basha, J.; Kumar, A.P.; Kundu, T.K.; Sethi, G. Garcinol, a polyisoprenylated benzophenone modulates multiple proinflammatory signaling cascades leading to the suppression of growth and survival of head and neck carcinoma. Cancer Prev. Res. (Philadelphia, Pa.) 2013, 6, 843–854. [Google Scholar] [CrossRef] [Green Version]
- Puar, Y.R.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Sethi, G.; Tergaonkar, V. Evidence for the Involvement of the Master Transcription Factor NF-κB in Cancer Initiation and Progression. Biomedicines 2018, 6, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.Y.; Shanmugam, M.K.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.M.; Um, J.Y.; Sethi, G.; Ahn, K.S. Fangchinoline, a Bisbenzylisoquinoline Alkaloid can Modulate Cytokine-Impelled Apoptosis via the Dual Regulation of NF-κB and AP-1 Pathways. Molecules 2019, 24, 3127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attafi, I.M.; Bakheet, S.A.; Korashy, H.M. The role of NF-κB and AhR transcription factors in lead-induced lung toxicity in human lung cancer A549 cells. Toxicol. Mech. Methods 2020, 30, 197–207. [Google Scholar] [CrossRef]
- He, H.; Xu, C.; Zheng, L.; Wang, K.; Jin, M.; Sun, Y.; Yue, Z. Polyphyllin VII induces apoptotic cell death via inhibition of the PI3K/Akt and NF-κB pathways in A549 human lung cancer cells. Mol. Med. Rep. 2020, 21, 597–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Shanmugam, M.K.; Siveen, K.S.; Wang, F.; Ong, T.H.; Loo, S.Y.; Swamy, M.M.; Mandal, S.; Kumar, A.P.; Goh, B.C.; et al. Garcinol sensitizes human head and neck carcinoma to cisplatin in a xenograft mouse model despite downregulation of proliferative biomarkers. Oncotarget 2015, 6, 5147–5163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Cho, S.K.; Kim, K.D.; Nam, D.; Chung, W.S.; Jang, H.J.; Lee, S.G.; Shim, B.S.; Sethi, G.; Ahn, K.S. β-Caryophyllene oxide potentiates TNFα-induced apoptosis and inhibits invasion through down-modulation of NF-κB-regulated gene products. Apoptosis 2014, 19, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.; Garg, M.; Tergaonkar, V.; Tan, S.Y.; Sethi, G. Pharmacological significance of the non-canonical NF-κB pathway in tumorigenesis. BBA 2020, 188449. [Google Scholar] [CrossRef]
- Akgun, S.; Kucuksayan, H.; Ozes, O.N.; Can, O.; Alikanoglu, A.S.; Yildiz, M.; Akca, H. NF-κB-Induced Upregulation of miR-548as-3p Increases Invasion of NSCLC by Targeting PTEN. Anticancer Agents Med. Chem. 2019, 19, 1058–1068. [Google Scholar] [CrossRef]
- Tong, L.; Luo, Y.; Wei, T.; Guo, L.; Wang, H.; Zhu, W.; Zhang, J. KH-type splicing regulatory protein (KHSRP) contributes to tumorigenesis by promoting miR-26a maturation in small cell lung cancer. Mol. Cell Biochem. 2016, 422, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.; Pillai, S.C.; Rochani, A.K.; Palaninathan, V.; Nakajima, Y.; Maekawa, T.; Kumar, D.S. GANT61 and curcumin-loaded PLGA nanoparticles for GLI1 and PI3K/Akt-mediated inhibition in breast adenocarcinoma. Nanotechnology 2020, 31, 185102. [Google Scholar] [CrossRef]
- Guney Eskiler, G.; Sahin, E.; Deveci Ozkan, A.; Cilingir Kaya, O.T.; Kaleli, S. Curcumin induces DNA damage by mediating homologous recombination mechanism in triple negative breast cancer. Nutr. Cancer 2020, 72, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Bagherian, M.; Azami, N.; Bejandi, A.K.; Hushmandi, K.; Ang, H.L.; et al. Polychemotherapy with Curcumin and Doxorubicin via Biological Nanoplatforms: Enhancing Antitumor Activity. Pharmaceutics 2020, 12, 1084. [Google Scholar] [CrossRef]
- Mishra, S.; Verma, S.S.; Rai, V.; Awasthee, N.; Chava, S.; Hui, K.M.; Kumar, A.P.; Challagundla, K.B.; Sethi, G.; Gupta, S.C. Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. CMLS 2019, 76, 1947–1966. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Warrier, S.; Kumar, A.P.; Sethi, G.; Arfuso, F. Potential Role of Natural Compounds as Anti-Angiogenic Agents in Cancer. Curr. Vasc. Pharmacol. 2017, 15, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef] [Green Version]
- Borges, G.A.; Elias, S.T.; Amorim, B.; de Lima, C.L.; Coletta, R.D.; Castilho, R.M.; Squarize, C.H.; Guerra, E.N.S. Curcumin downregulates the PI3K-AKT-mTOR pathway and inhibits growth and progression in head and neck cancer cells. Phytother. Res. 2020, 34, 3311–3324. [Google Scholar] [CrossRef]
- Liu, B.; Shen, Y.; Huang, H.; Croce, K.D.; Wu, M.; Fan, Y.; Liu, Y.; Xu, J.; Yao, G. Curcumin derivative C212 inhibits Hsp90 and eliminates both growing and quiescent leukemia cells in deep dormancy. Cell Commun. Signal 2020, 18, 159. [Google Scholar] [CrossRef]
- Mohammed, F.; Rashid-Doubell, F.; Taha, S.; Cassidy, S.; Fredericks, S. Effects of curcumin complexes on MDA-MB-231 breast cancer cell proliferation. Int. J. Oncol. 2020, 57, 445–455. [Google Scholar] [CrossRef]
- Wang, N.; Feng, T.; Liu, X.; Liu, Q. Curcumin inhibits migration and invasion of non-small cell lung cancer cells through up-regulation of miR-206 and suppression of PI3K/AKT/mTOR signaling pathway. Acta. Pharm. 2020, 70, 399–409. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Hu, C.; Wang, B.; Fan, S.; Jin, W. Curcumin Suppresses Cell Proliferation, Migration, and Invasion Through Modulating miR-21-5p/SOX6 Axis in Hepatocellular Carcinoma. Cancer Biother. Radiopharm. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Hashemipour, M.; Vosough, M.; Najafi, M.; Shahinozzaman, M.; Hushmandi, K.; Khan, H.; Mirzaei, H. Sensing the scent of death: Modulation of microRNAs by Curcumin in gastrointestinal cancers. Pharmacol. Res. 2020, 160, 105199. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Mittal, S.; Sak, K.; Tuli, H.S. Molecular mechanisms underlying chemopreventive potential of curcumin: Current challenges and future perspectives. Life Sci. 2016, 148, 313–328. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Liao, W.; Yu, L.; Hu, Z.; Li, M.; Xia, H. Curcumin suppresses glioblastoma cell proliferation by p-AKT/mTOR pathway and increases the PTEN expression. Arch. Biochem. Biophys. 2020, 689, 108412. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Shao, T.; Zheng, W.; Ding, J. Curcumin suppresses tumor growth of gemcitabine-resistant non-small cell lung cancer by regulating lncRNA-MEG3 and PTEN signaling. Clin. Transl. Oncol. 2021. [Google Scholar] [CrossRef]
- Li, X.; Xie, W.; Xie, C.; Huang, C.; Zhu, J.; Liang, Z.; Deng, F.; Zhu, M.; Zhu, W.; Wu, R.; et al. Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phytother. Res. 2014, 28, 1553–1560. [Google Scholar] [CrossRef]
- Wang, X.; Hang, Y.; Liu, J.; Hou, Y.; Wang, N.; Wang, M. Anticancer effect of curcumin inhibits cell growth through miR-21/PTEN/Akt pathway in breast cancer cell. Oncol. Lett. 2017, 13, 4825–4831. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, H.I.; Ismail, M.B.; Ammar, R.B.; Ahmed, E. Thidiazuron suppresses breast cancer progression via targeting miR-132 and miR-202-5p/PTEN axis mediated dysregulation of PI3K/AKT signaling pathway. Biochem. Cell Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Rauh, R.; Kahl, S.; Tomicic, M.; Böchzelt, H.; Tome, M.E.; Briehl, M.M.; Bauer, R.; Kaina, B. Molecular modes of action of cantharidin in tumor cells. Biochem. Pharmacol. 2005, 69, 811–818. [Google Scholar] [CrossRef]
- Zheng, L.H.; Bao, Y.L.; Wu, Y.; Yu, C.L.; Meng, X.; Li, Y.X. Cantharidin reverses multidrug resistance of human hepatoma HepG2/ADM cells via down-regulation of P-glycoprotein expression. Cancer Lett. 2008, 272, 102–109. [Google Scholar] [CrossRef]
- Sagawa, M.; Nakazato, T.; Uchida, H.; Ikeda, Y.; Kizaki, M. Cantharidin induces apoptosis of human multiple myeloma cells via inhibition of the JAK/STAT pathway. Cancer Sci. 2008, 99, 1820–1826. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, X. Cantharidin modulates the E2F1/MCM7-miR-106b-93/p21-PTEN signaling axis in MCF-7 breast cancer cells. Oncol. Lett. 2015, 10, 2849–2855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Shen, H.; Zhu, X.; Liu, Y.; Yang, H.; Chen, H.; Xiong, S.; Chi, H.; Xu, W. A nuclear lncRNA Linc00839 as a Myc target to promote breast cancer chemoresistance via PI3K/AKT signaling pathway. Cancer Sci. 2020, 111, 3279–3291. [Google Scholar] [CrossRef]
- Li, L.Q.; Li, X.L.; Wang, L.; Du, W.J.; Guo, R.; Liang, H.H.; Liu, X.; Liang, D.S.; Lu, Y.J.; Shan, H.L.; et al. Matrine inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells. Cell Physiol. Biochem. 2012, 30, 631–641. [Google Scholar] [CrossRef]
- Cabello, P.; Pineda, B.; Tormo, E.; Lluch, A.; Eroles, P. The Antitumor Effect of Metformin Is Mediated by miR-26a in Breast Cancer. Int. J. Mol. Sci. 2016, 17, 1298. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.Y.; Wu, A.T.; Yuan, K.S.; Liu, S.H. Brown Seaweed Fucoidan Inhibits Cancer Progression by Dual Regulation of mir-29c/ADAM12 and miR-17-5p/PTEN Axes in Human Breast Cancer Cells. J. Cancer 2016, 7, 2408–2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, F.; Tang, H.; Liu, P.; Shen, J.; Guan, X.; Xie, X.; Gao, J.; Xiong, L.; Jia, L.; Chen, J.; et al. Isoliquiritigenin modulates miR-374a/PTEN/Akt axis to suppress breast cancer tumorigenesis and metastasis. Sci. Rep. 2017, 7, 9022. [Google Scholar] [CrossRef] [Green Version]
- de la Parra, C.; Castillo-Pichardo, L.; Cruz-Collazo, A.; Cubano, L.; Redis, R.; Calin, G.A.; Dharmawardhane, S. Soy Isoflavone Genistein-Mediated Downregulation of miR-155 Contributes to the Anticancer Effects of Genistein. Nutr. Cancer 2016, 68, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Jahanafrooz, Z.; Motamed, N.; Bakhshandeh, B. Effects of miR-21 downregulation and silibinin treatment in breast cancer cell lines. Cytotechnology 2017, 69, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cui, H.; Jin, X.; Gong, X.; Wang, W.; Wang, J. Triptolide inhibits epithelial-mesenchymal transition and induces apoptosis in gefitinib-resistant lung cancer cells. Oncol Rep. 2020, 43, 1569–1579. [Google Scholar] [CrossRef]
- Philips, B.J.; Kumar, A.; Burki, S.; Ryan, J.P.; Noda, K.; D’Cunha, J. Triptolide-induced apoptosis in non-small cell lung cancer via a novel miR204-5p/Caveolin-1/Akt-mediated pathway. Oncotarget 2020, 11, 2793–2806. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zang, A.; Jia, Y.; Zhang, J.; Fan, W.; Feng, J.; Duan, M.; Zhang, L.; Huo, R.; Jiao, J.; et al. Triptolide reduces proliferation and enhances apoptosis of human non-small cell lung cancer cells through PTEN by targeting miR-21. Mol. Med. Rep. 2016, 13, 2763–2768. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, H.; Chen, S.; Yang, R.; Li, H.; Zhang, G. Baicalein inhibits cell growth and increases cisplatin sensitivity of A549 and H460 cells via miR-424-3p and targeting PTEN/PI3K/Akt pathway. J. Cell Mol. Med. 2018, 22, 2478–2487. [Google Scholar] [CrossRef] [Green Version]
- Spear, J.M.; Lu, Z.; Russu, W.A. Pharmacological Inhibition of CDK8 in Triple-Negative Breast Cancer Cell Line MDA-MB-468 Increases E2F1 Protein, Induces Phosphorylation of STAT3 and Apoptosis. Molecules 2020, 25, 5728. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Fang, L.; Yang, Q.; Hibberd, S.; Du, W.W.; Wu, N.; Yang, B.B. Posttranscriptional regulation of AKT by circular RNA angiomotin- like 1 mediates chemoresistance against paclitaxel in breast cancer cells. Aging 2019, 11, 11369–11381. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Chen, S.; Feng, S.; Li, Y.; Zou, J.; Ling, H.; Zeng, Y.; Zeng, X. Function and mechanisms of microRNA-20a in colorectal cancer. Exp. Ther. Med. 2020, 19, 1605–1616. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Qiu, F.; Fu, M.; Chen, H.; Wang, H. Propofol Reduces Epithelial to Mesenchymal Transition, Invasion and Migration of Gastric Cancer Cells through the MicroRNA-195-5p/Snail Axis. Med. Sci. Monit. 2020, 26, 920981. [Google Scholar] [CrossRef]
- Xu, Y.; Pan, S.; Jiang, W.; Xue, F.; Zhu, X. Effects of propofol on the development of cancer in humans. Cell Proliferation 2020, 53, 12867. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Dong, L.; Zhao, S.; Li, Q.; Liu, D.; Zhu, X.; Ge, X.; Li, R.; Wang, G. Propofol Affects Non-Small-Cell Lung Cancer Cell Biology By Regulating the miR-21/PTEN/AKT Pathway In Vitro and In Vivo. Anesth. Analg. 2020, 131, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, P.; Cui, J.; Wang, W.; Chen, Y.; Zhang, T. Panax notoginseng saponins attenuate lung cancer growth in part through modulating the level of Met/miR-222 axis. J. Ethnopharmacol. 2016, 193, 255–265. [Google Scholar] [CrossRef] [PubMed]





| miRNA | Signaling Network | Outcomes | Refs. |
|---|---|---|---|
| miRNA-106b miRNA-93 | PTEN/PI3K/Akt | Increasing cancer cell proliferation and metastasis PTEN downregulation Inducing PI3K/Akt signaling | [139] |
| miRNA-182-5p | - | Silencing miRNA-182-5p impairs cancer malignancy via PTEN upregulation | [127] |
| miRNA-221/222 | PTEN/Akt | Promoting colony formation capacity Inducing Akt signaling via PTEN inhibition | [121] |
| miRNA-19a/b | PTEN/Akt/p21 | Inhibiting cell cycle arrest at G1/S phase Binding to 3′-UTR of PTEN in reducing its expression P21 inhibition Upregulating PCNA and cyclin D1 | [131] |
| miRNA-9 miRNA-155 | - | Involvement of these exosomal miRNAs in metastasis of breast cancer cells via PTEN downregulation | [112] |
| miRNA-10b | PTEN/Akt | Maintaining self-renewal capacity of breast cancer cells Akt hyperactivation via PTEN downregulation | [126] |
| miRNA-221/222 | PTEN/Akt/NF-kB/COX-2 | Enhancing stem cell-like features of breast cancer cells Increasing colony formation capacity Promoting stemness via ALDH1 upregulation PTEN inhibition Activating Akt/NF-kB/COX-2 axis | [140] |
| miRNA-181c | - | Increasing cancer growth by binding to 3′-UTR of PTEN | [109] |
| miRNA-425-5p | - | Association with poor prognosis of breast cancer patients Dually promoting cancer cell proliferation and metastasis PTEN inhibition | [115] |
| miRNA-30a | PTEN/Akt | Downregulating PTEN expression Providing condition for Akt phosphorylation Promoting cancer cell survival and growth | [122] |
| miRNA-21 | PTEN/Akt/ERK1/2 | Silencing miRNA-21 disrupts cancer metastasis (EMT) via PTEN upregulation and subsequent inhibition of Akt/ERK1/2 | [141] |
| miRNA-19a-3p | - | miRNA downregulation by cold atmospheric plasma leads to breast cancer suppression via PTEN upregulation | [142] |
| miRNA | Signaling Network | Outcomes | Refs |
|---|---|---|---|
| miRNA-93 | LKB1/PTEN/CDKN1A/PI3K/Akt | Upregulation of miRNA-93 in lung cancer cells Association with proliferation and metastasis of cancer cells Inhibiting LKB1/PTEN/p21 axis Inducing PI3K/Akt | [171] |
| miRNA-21 | PTEN/EMT | Reverse relationship with PTEN Promoting metastasis via EMT induction | [172] |
| miRNA-21 | PTEN/Akt/GSK-3b | Increasing cyclin D1 and cyclin E1 expressions Enhancing cancer cell proliferation Promoting metastasis via EMT induction Activating Akt/GSK-3b signaling via PTEN downregulation | [173] |
| miRNA-21 | - | Enhancing cell proliferation and invasion Apoptosis inhibition PTEN inhibition | [174] |
| miRNA-26a | PTEN/Akt | Enhancing metastasis via PTEN downregulation and the subsequent induction of Akt signaling | [175] |
| miRNA-21 | - | Binding to 3′-UTR of PTEN Reducing the mRNA level of PTEN Promoting growth and metastatic features | [176] |
| miRNA-205 | PTEN/Akt/mTOR | PTEN inhibition Activating Akt/mTOR signaling Increasing malignancy of lung cancer cells | [177] |
| miRNA-183-5p | PTEN/Akt/p53 | Exerting oncogenic role Promoting Akt phosphorylation via PTEN downregulation Activating p53 | [178] |
| miRNA | Chemotherapeutic Agent | Impact on Chemotherapy | Remarks | Refs. |
|---|---|---|---|---|
| miRNA-93 | Doxorubicin | Resistance | PTEN downregulation EMT induction Increasing cancer metastasis and malignancy | [209] |
| miRNA-202-5p | Doxorubicin | Resistance | Enhancing tumor volume and progression Downregulating PTEN and subsequent induction of PI3K/Akt signaling | [107] |
| miRNA-222 | Adriamycin | Resistance | Activation of PI3K/Akt signaling via PTEN downregulation Association with poor prognosis | [203] |
| miRNA-222 | Adriamycin | Resistance | PTEN downregulation Inducing Akt signaling P27 inhibition Triggering the resistance of cancer cells to apoptosis | [191] |
| miRNA-222 miRNA-29a | Adriamycin Doxorubicin | Resistance | Overexpression in drug-resistant cancer cells Association with PTEN downregulation | [204] |
| miRNA-520h | Paclitaxel | Resistance | Binding to OTUD3 and reducing its expression PTEN inhibition Paving the way for Akt induction | [212] |
| miRNA-221/222 | Cisplatin | Resistance | Downregulation of miRNA-221/222 enhances cisplatin sensitivity Activation of p53/PTEN signaling following miRNA inhibition | [211] |
| miRNA | Chemotherapeutic Agent | Effect on Chemotherapy | Remarks | Refs. |
|---|---|---|---|---|
| miRNA-181 | Cisplatin | Sensitivity | PTEN upregulation Inhibition of PI3K/Akt/mTOR signaling Apoptosis induction Disrupting cancer metastasis | [249] |
| miRNA-29b-3p | Cisplatin | Sensitivity | Disrupting cell viability Reducing proliferation Inducing apoptosis via Bax upregulation Triggering PTEN signaling | [228] |
| miRNA-23a | Erlotinib | Resistance | Silencing miRNA-23a restores PTEN expression to suppress PI3K/Akt signaling, leading to erlotinib sensitivity | [229] |
| miRNA-134/487b/655 | Gefitinib | Resistance | Inducing TGF-b1 signaling in reducing PTEN expression Enhancing cancer metastasis via EMT induction Providing chemoresistance | [246] |
| miRNA-21 | Gefitinib | Resistance | Reverse relationship between miRNA-21 and PTEN Activation of Akt and ERK signaling pathways Association with poor prognosis | [232] |
| miRNA-21 | Cisplatin | Resistance | Hypoxia induces exsoaomal transfer of miRNA-21 Exerting PTEN inhibition | [233] |
| miRNA-92b | Cisplatin | Resistance | Establishing cancer proliferation Reducing sensitivity to chemotherapy PTEN inhibition | [248] |
| miRNA-1269b | Cisplatin | Resistance | Enhancing cancer cell growth Apoptosis inhibition Inducing PI3K/Akt signaling via PTEN downregulation | [237] |
| miRNA-21 | EGFR-TKI | Resistance | Negative association with PTEN expression Triggering PI3K/Akt signaling Reducing chemosensitivity | [234] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abadi, A.J.; Zarrabi, A.; Gholami, M.H.; Mirzaei, S.; Hashemi, F.; Zabolian, A.; Entezari, M.; Hushmandi, K.; Ashrafizadeh, M.; Khan, H.; et al. Small in Size, but Large in Action: microRNAs as Potential Modulators of PTEN in Breast and Lung Cancers. Biomolecules 2021, 11, 304. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11020304
Abadi AJ, Zarrabi A, Gholami MH, Mirzaei S, Hashemi F, Zabolian A, Entezari M, Hushmandi K, Ashrafizadeh M, Khan H, et al. Small in Size, but Large in Action: microRNAs as Potential Modulators of PTEN in Breast and Lung Cancers. Biomolecules. 2021; 11(2):304. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11020304
Chicago/Turabian StyleAbadi, Asal Jalal, Ali Zarrabi, Mohammad Hossein Gholami, Sepideh Mirzaei, Farid Hashemi, Amirhossein Zabolian, Maliheh Entezari, Kiavash Hushmandi, Milad Ashrafizadeh, Haroon Khan, and et al. 2021. "Small in Size, but Large in Action: microRNAs as Potential Modulators of PTEN in Breast and Lung Cancers" Biomolecules 11, no. 2: 304. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11020304