Functional Groups Dominate Aboveground Net Primary Production under Long-Term Nutrient Additions in a Tibetan Alpine Meadow
Abstract
:1. Introduction
2. Results
2.1. Effects of N and P Additions on ANPP
2.2. Effect of N and P Additions on Species Diversity
2.3. Effect of N and P Additions on Functional Diversity
2.4. Effect of N and P Additions on Functional Groups
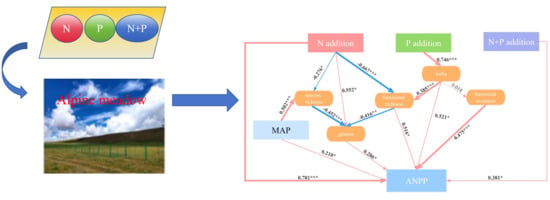
2.5. Direct and Indirect Pathways of N and P Additions Affecting ANPP
3. Discussion
3.1. Effect of N and P Additions on the Relative Abundance of Functional Groups
3.2. Effect of N and P Additions on Species Diversity and Functional Diversity
3.3. Functional Groups Instead of Diversity Drive ANPP
4. Materials and Methods
4.1. Study Area
4.2. Experiment Design and Community Survey
4.3. Soil Analysis
4.4. Calculation of Plant Community Metrics
4.4.1. Community Aboveground Biomass, Relative Abundance, and Response Value
4.4.2. Species Diversity and Functional Diversity
4.4.3. Aridity Index
4.5. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.C.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, Y.Q.; van Groenigen, K.J.; Hungate, B.A.; Cao, J.J.; Zhou, X.H.; Wang, R.W. A keystone microbial enzyme for nitrogen control of soil carbon storage. Sci. Adv. 2018, 4, eaaq1689. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.; Kohli, L.; Rihm, B.; Achermann, B. Nitrogen deposition is negatively related to species richness and species composition of vascular plants and bryophytes in Swiss mountain grassland. Agric. Ecosyst. Environ. 2013, 178, 121–126. [Google Scholar] [CrossRef]
- Borer, E.T.; Stevens, C.J. Nitrogen deposition and climate: An integrated synthesis. Trends Ecol. Evol. 2022, 37, 541–552. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Guo, X.X.; Zuo, X.A.; Medina-Roldán, E.; Guo, A.X.; Yue, P.; Zhao, X.Y.; Qiao, J.J.; Li, X.Y.; Chen, M.; Wei, C.Z.; et al. Effects of multi-resource addition on grassland plant productivity and biodiversity along a resource gradient. Sci. Total Environ. 2023, 857, 159367. [Google Scholar] [CrossRef]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef]
- Li, W.B.; Gan, X.L.; Jiang, Y.; Cao, F.F.; Lu, X.T.; Ceulemans, T.; Zhao, C.Y. Nitrogen effects on grassland biomass production and biodiversity are stronger than those of phosphorus. Environ. Pollut. 2022, 309, 119720. [Google Scholar] [CrossRef]
- Biederman, L.; Mortensen, B.; Fay, P.; Hagenah, N.; Knops, J.; La Pierre, K.; Laungani, R.; Lind, E.; McCulley, R.; Power, S.; et al. Nutrient addition shifts plant community composition towards earlier flowering species in some prairie ecoregions in the U.S. Central Plains. PLoS ONE 2017, 12, e0178440. [Google Scholar] [CrossRef]
- Isbell, F.; Reich, P.B.; Tilman, D.; Hobbie, S.E.; Polasky, S.; Binder, S. Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proc. Natl. Acad. Sci. USA 2013, 110, 11911–11916. [Google Scholar] [CrossRef]
- Tian, Q.Y.; Liu, N.N.; Bai, W.M.; Li, L.H.; Chen, J.Q.; Reich, P.B.; Yu, Q.; Guo, D.L.; Smith, M.D.; Knapp, A.K.; et al. A novel soil manganese mechanism drives plant species loss with increased nitrogen deposition in a temperate steppe. Ecology 2016, 97, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.A.; Yang, B.; Li, M.X.; Xiao, R.Q.; Rao, K.Y.; Wang, J.Q.; Zhang, T.; Guo, J.X. Community composition, structure and productivity in response to nitrogen and phosphorus additions in a temperate meadow. Sci. Total Environ. 2019, 654, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, T.; Merckx, R.; Hens, M.; Honnay, O. Plant species loss from European semi-natural grasslands following nutrient enrichment—Is it nitrogen or is it phosphorus? Glob. Ecol. Biogeogr. 2013, 22, 73–82. [Google Scholar] [CrossRef]
- DeMalach, N. Toward a mechanistic understanding of the effects of nitrogen and phosphorus additions on grassland diversity. Perspect. Plant Ecol. Evol. Syst. 2018, 32, 65–72. [Google Scholar] [CrossRef]
- Guo, X.X.; Zuo, X.A.; Yue, P.; Li, X.Y.; Hu, Y.; Chen, M.; Yu, Q. Direct and indirect effects of precipitation change and nutrients addition on desert steppe productivity in Inner Mongolia, northern China. Plant Soil 2022, 471, 527–540. [Google Scholar] [CrossRef]
- Hillebrand, H.; Blasius, B.; Borer, E.T.; Chase, J.M.; Downing, J.A.; Eriksson, B.K.; Filstrup, C.T.; Harpole, W.S.; Hodapp, D.; Larsen, S.; et al. Biodiversity change is uncoupled from species richness trends: Consequences for conservation and monitoring. J. Appl. Ecol. 2018, 55, 169–184. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.M.; Díaz-Delgado, R.; de la Riva, E.G.; Villar, R.; Lloret, F.; Marañón, T. Climate variability and community stability in Mediterranean shrublands: The role of functional diversity and soil environment. J. Ecol. 2017, 105, 1335–1346. [Google Scholar] [CrossRef]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Spasojevic, M.J.; Grace, J.B.; Harrison, S.; Damschen, E.I. Functional diversity supports the physiological tolerance hypothesis for plant species richness along climatic gradients. J. Ecol. 2014, 102, 447–455. [Google Scholar] [CrossRef]
- Grime, J.P. Trait convergence and trait divergence in herbaceous plant communities: Mechanisms and consequences. J. Veg. Sci. 2006, 17, 255–260. [Google Scholar] [CrossRef]
- Xu, Z.W.; Li, M.H.; Zimmermann, N.E.; Li, S.P.; Li, H.; Ren, H.Y.; Sun, H.; Han, X.G.; Jiang, Y.; Jiang, L. Plant functional diversity modulates global environmental change effects on grassland productivity. J. Ecol. 2018, 106, 1941–1951. [Google Scholar] [CrossRef]
- Cardinale, B.J. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67, Erratum in Nature 2012, 489, 326. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.F.; Wu, J.G.; Clark, C.M.; Naeem, S.; Pan, Q.M.; Huang, J.H.; Zhang, L.X.; Han, X.G. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: Evidence from inner Mongolia Grasslands. Glob. Change Biol. 2010, 16, 358–372. [Google Scholar] [CrossRef]
- Smith, M.D.; Knapp, A.K.; Collins, S.L. A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology 2009, 90, 3279–3289. [Google Scholar] [CrossRef] [PubMed]
- Avolio, M.L.; Koerner, S.E.; La Pierre, K.J.; Wilcox, K.R.; Wilson, G.W.T.; Smith, M.D.; Collins, S.L. Changes in plant community composition, not diversity, during a decade of nitrogen and phosphorus additions drive above-ground productivity in a tallgrass prairie. J. Ecol. 2014, 102, 1649–1660. [Google Scholar] [CrossRef]
- Ren, Z.; Li, Q.; Chu, C.; Zhao, L.; Zhang, J.; Ai, D.; Yang, Y.; Wang, G. Effects of resource additions on species richness and ANPP in an alpine meadow community. J. Plant Ecol. 2009, 3, 25–31. [Google Scholar] [CrossRef]
- Ren, F.; Song, W.; Chen, L.; Mi, Z.; Zhang, Z.; Zhu, W.; Zhou, H.; Cao, G.; He, J.S. Phosphorus does not alleviate the negative effect of nitrogen enrichment on legume performance in an alpine grassland. J. Plant Ecol. 2016, 10, 822–830. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Q.; Wang, H. The effect of land management on plant community composition, species diversity, and productivity of alpine Kobersia steppe meadow. Ecol. Res. 2006, 21, 181–187. [Google Scholar] [CrossRef]
- Wang, Y.F.; Xue, K.; Hu, R.H.; Ding, B.Y.; Zeng, H.; Li, R.J.; Xu, B.; Pang, Z.; Song, X.N.; Li, C.J.; et al. Vegetation structural shift tells environmental changes on the Tibetan Plateau over 40 years. Sci. Bull. 2023, 68, 1928–1937. [Google Scholar] [CrossRef]
- Zong, N.; Hou, G.; Shi, P.L.; Zhou, T.C.; Yu, J.L.; Tian, J. Different responses of community temporal stability to nitrogen and phosphorus addition in a non-degraded alpine grassland. Ecol. Indic. 2022, 143, 109310. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Q.; He, N.; Smith, M.D.; Elser, J.J.; Du, J.; Yuan, G.; Yu, G.; Yu, Q. Imbalanced atmospheric nitrogen and phosphorus depositions in China: Implications for nutrient limitation. J. Geophys. Res. Biogeosci. 2016, 121, 1605–1616. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Ma, Q.; Zhang, X.; Chen, Y.; Isbell, F.; Wang, D. Nitrogen addition reduced ecosystem stability regardless of its impacts on plant diversity. J. Ecol. 2019, 107, 2427–2435. [Google Scholar] [CrossRef]
- Zhang, Y.; Loreau, M.; Lü, X.; He, N.; Zhang, G.; Han, X. Nitrogen enrichment weakens ecosystem stability through decreased species asynchrony and population stability in a temperate grassland. Glob. Change Biol. 2016, 22, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Duan, J.; Xu, G.; Wang, Y.; Zhang, Z.; Rui, Y.; Luo, C.; Xu, B.; Zhu, X.; Chang, X.; et al. Effects of warming and grazing on soil N availability, species composition, and ANPP in an alpine meadow. Ecology 2012, 93, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Ouyang, H.; Cao, G.M.; Richter, A.; Wanek, W.; Kuzyakov, Y. Dominant plant species shift their nitrogen uptake patterns in response to nutrient enrichment caused by a fungal fairy in an alpine meadow. Plant Soil 2011, 341, 495–504. [Google Scholar] [CrossRef]
- Hautier, Y.; Niklaus, P.A.; Hector, A. Competition for Light Causes Plant Biodiversity Loss After Eutrophication. Science 2009, 324, 636–638. [Google Scholar] [CrossRef]
- Galloway, J.N. The global nitrogen cycle: Changes and consequences. Environ. Pollut. 1998, 102, 15–24. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Cassman, K.G.; Specht, J.E.; Walters, D.T.; Weiss, A.; Dobermann, A. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crops Res. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Png, G.K.; Turner, B.L.; Albornoz, F.E.; Hayes, P.E.; Lambers, H.; Laliberté, E. Greater root phosphatase activity in nitrogen-fixing rhizobial but not actinorhizal plants with declining phosphorus availability. J. Ecol. 2017, 105, 1246–1255. [Google Scholar] [CrossRef]
- Sulieman, S.; Ha, C.V.; Schulze, J.; Tran, L.-S.P. Growth and nodulation of symbiotic Medicago truncatula at different levels of phosphorus availability. J. Exp. Bot. 2013, 64, 2701–2712. [Google Scholar] [CrossRef]
- Divito, G.A.; Sadras, V.O. How do phosphorus, potassium and sulphur affect plant growth and biological nitrogen fixation in crop and pasture legumes? A meta-analysis. Field Crops Res. 2014, 156, 161–171. [Google Scholar] [CrossRef]
- Zong, N.; Shi, P.; Zhao, G.; Zheng, L.; Niu, B.; Zhou, T.; Hou, G. Variations of nitrogen and phosphorus limitation along the environmental gradient in alpine grasslands on the Northern Xizang Plateau. Chin. J. Plant Ecol. 2021, 45, 444–455. (In Chinese) [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 2012, 104, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.M.; Lan, Z.C.; Bai, X.; Grace, J.B.; Bai, Y.F. Evidence that acidification-induced declines in plant diversity and productivity are mediated by changes in below-ground communities and soil properties in a semi-arid steppe. J. Ecol. 2013, 101, 1322–1334. [Google Scholar] [CrossRef]
- Humbert, J.-Y.; Dwyer, J.M.; Andrey, A.; Arlettaz, R. Impacts of nitrogen addition on plant biodiversity in mountain grasslands depend on dose, application duration and climate: A systematic review. Glob. Change Biol. 2016, 22, 110–120. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, F.; Quan, Q.; Song, B.; Wang, J.; Zhou, Q.; Niu, S. Common Species Stability and Species Asynchrony Rather than Richness Determine Ecosystem Stability Under Nitrogen Enrichment. Ecosystems 2021, 24, 686–698. [Google Scholar] [CrossRef]
- Song, M.-H.; Yu, F.-H.; Ouyang, H.; Cao, G.-M.; Xu, X.-L.; Cornelissen, J.H.C. Different inter-annual responses to availability and form of nitrogen explain species coexistence in an alpine meadow community after release from grazing. Glob. Change Biol. 2012, 18, 3100–3111. [Google Scholar] [CrossRef]
- Cleland, E.E.; Harpole, W.S. Nitrogen enrichment and plant communities. Ann. N. Y. Acad. Sci. 2010, 1195, 46–61. [Google Scholar] [CrossRef]
- Band, N.; Kadmon, R.; Mandel, M.; DeMalach, N. Assessing the roles of nitrogen, biomass, and niche dimensionality as drivers of species loss in grassland communities. Proc. Natl. Acad. Sci. USA 2022, 119, e2112010119. [Google Scholar] [CrossRef]
- Harpole, W.S.; Sullivan, L.L.; Lind, E.M.; Firn, J.; Adler, P.B.; Borer, E.T.; Chase, J.; Fay, P.A.; Hautier, Y.; Hillebrand, H.; et al. Addition of multiple limiting resources reduces grassland diversity. Nature 2016, 537, 93–96. [Google Scholar] [CrossRef]
- Chen, Q.; Hooper, D.U.; Lin, S. Shifts in Species Composition Constrain Restoration of Overgrazed Grassland Using Nitrogen Fertilization in Inner Mongolian Steppe, China. PLoS ONE 2011, 6, e16909. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.C.; Bai, Y.F. Testing mechanisms of N-enrichment-induced species loss in a semiarid Inner Mongolia grassland: Critical thresholds and implications for long-term ecosystem responses. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3125–3134. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.S.; Niu, S.L. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Johnson, N.C. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 2010, 185, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Petchey, O.L.; Gaston, K.J. Functional diversity: Back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Peng, Y.F.; Li, F.; Yang, G.B.; Wang, J.; Yu, J.C.; Zhou, G.Y.; Yang, Y.H. Changes in above-/below-ground biodiversity and plant functional composition mediate soil respiration response to nitrogen input. Funct. Ecol. 2021, 35, 1171–1182. [Google Scholar] [CrossRef]
- Niu, K.C.; Choler, P.; de Bello, F.; Mirotchnick, N.; Du, G.Z.; Sun, S.C. Fertilization decreases species diversity but increases functional diversity: A three-year experiment in a Tibetan alpine meadow. Agric. Ecosyst. Environ. 2014, 182, 106–112. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef]
- Mason, N.W.H.; de Bello, F.; Dolezal, J.; Leps, J. Niche overlap reveals the effects of competition, disturbance and contrasting assembly processes in experimental grassland communities. J. Ecol. 2011, 99, 788–796. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Luo, R.Y.; Fan, J.L.; Wang, W.J.; Luo, J.F.; Kuzyakov, Y.; He, J.S.; Chu, H.Y.; Ding, W.X. Nitrogen and phosphorus enrichment accelerates soil organic carbon loss in alpine grassland on the Qinghai-Tibetan Plateau. Sci. Total Environ. 2019, 650, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Zhang, X.Z.; Wu, J.S.; Shen, Z.X.; Zhang, Y.J.; Xu, X.L.; Fan, Y.Z.; Zhao, Y.P.; Yan, W. Root biomass distribution in alpine ecosystems of the northern Tibetan Plateau. Environ. Earth Sci. 2011, 64, 1911–1919. [Google Scholar] [CrossRef]
- Zhao, G.S.; Shi, P.L.; Wu, J.S.; Xiong, D.P.; Zong, N.; Zhang, X.Z. Foliar nutrient resorption patterns of four functional plants along a precipitation gradient on the Tibetan Changtang Plateau. Ecol. Evol. 2017, 7, 7201–7212. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Zhou, T.C.; Shi, P.L.; Sun, J.; Zong, N.; Yu, J.L.; Song, M.H. Multi-year nitrogen accumulation weakens the stabilizing effect of species asynchrony on drought resistance in a Tibetan alpine meadow. Agric. For. Meteorol. 2023, 340, 109617. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis; Wiley: Hoboken, NJ, USA, 1983; pp. 595–624. [Google Scholar]
- Parkinson, J.A.; Allen, S.E. A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Commun. Soil Sci. Plant Anal. 1975, 6, 1–11. [Google Scholar] [CrossRef]
- Song, M.-H.; Zong, N.; Jiang, J.; Shi, P.-L.; Zhang, X.-Z.; Gao, J.-Q.; Zhou, H.-K.; Li, Y.-K.; Loreau, M. Nutrient-induced shifts of dominant species reduce ecosystem stability via increases in species synchrony and population variability. Sci. Total Environ. 2019, 692, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Zhang, D.; Wang, G.; Liu, Y.; Li, Q.; Zheng, Z.; Yang, G.; Peng, Y.; Niu, K.; Yang, Y. Experimental warming altered plant functional traits and their coordination in a permafrost ecosystem. New Phytol. 2023, 240, 1802–1816. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Springer: Berlin/Heidelberg, Germany, 1988; pp. 81–99. [Google Scholar]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- De Martonne, E. L’indice d’aridité. Bull. De L’association De Géographes Français 1926, 9, 3–5. [Google Scholar] [CrossRef]






| Variation | ANPP | Species Richness | Shannon–Wiener | Simpson | Evenness |
|---|---|---|---|---|---|
| Year | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** |
| N | <0.001 *** | 0.022 * | 0.240 | 0.158 | 0.051 |
| Year × N | 0.669 | 0.557 | 0.065 | 0.009 ** | 0.047 * |
| P | 0.031 * | 0.161 | 0.185 | 0.417 | 0.545 |
| Year × P | 0.185 | 0.130 | 0.029 * | 0.155 | 0.466 |
| N + P | <0.001 *** | 0.768 | 0.633 | 0.654 | 0.630 |
| Year × N + P | 0.604 | 0.415 | 0.019 * | 0.046 * | 0.093 |
| Variation | Functional Richness | Functional Evenness | Functional Dispersion |
|---|---|---|---|
| Year | 0.828 | 0.093 | 0.048 * |
| N | 0.024 * | <0.001 *** | 0.271 |
| Year × N | 0.937 | 0.074 | 0.229 |
| P | 0.007 ** | 0.642 | 0.264 |
| Year × P | 0.934 | 0.490 | 0.403 |
| N + P | 0.015 * | 0.001 *** | 0.142 |
| Year × N + P | 0.578 | 0.013 * | 0.334 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Hou, G.; Shi, P.; Zong, N.; Yu, J. Functional Groups Dominate Aboveground Net Primary Production under Long-Term Nutrient Additions in a Tibetan Alpine Meadow. Plants 2024, 13, 344. https://0-doi-org.brum.beds.ac.uk/10.3390/plants13030344
Chen X, Hou G, Shi P, Zong N, Yu J. Functional Groups Dominate Aboveground Net Primary Production under Long-Term Nutrient Additions in a Tibetan Alpine Meadow. Plants. 2024; 13(3):344. https://0-doi-org.brum.beds.ac.uk/10.3390/plants13030344
Chicago/Turabian StyleChen, Xueying, Ge Hou, Peili Shi, Ning Zong, and Jialuo Yu. 2024. "Functional Groups Dominate Aboveground Net Primary Production under Long-Term Nutrient Additions in a Tibetan Alpine Meadow" Plants 13, no. 3: 344. https://0-doi-org.brum.beds.ac.uk/10.3390/plants13030344