Evaluation of Tear Evaporation Rate in Patients with Diabetes Using a Hand-Held Evaporimeter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. The OSDI
2.3. The TER Test
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, S.Y.; Wong, J.L.M.; Sim, Y.J.; Wong, S.S.; Elhassan, S.A.M.; Tan, S.H.; Ling, G.P.L.; Tay, N.W.R.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Syndr. 2019, 13, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Gharravi, A.M.; Jafar, A.; Ebrahimi, M.; Mahmodi, A.; Pourhashemi, E.; Haseli, N.; Talaie, N.; Hajiasgarli, P. Current status of stem cell therapy, scaffolds for the treatment of diabetes. Diabetes Metab. Syndr. 2018, 12, 1133–1139. [Google Scholar] [CrossRef]
- Gale, E.A.M.; Gillespie, K.M. Diabetes and gender. Diabetologia 2001, 44, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, K.; Sahoo, B.; Choudhury, A.K.; Sofi, N.Y.; Kumar, R.; Bhadoria, A.S. Childhood obesity: Causes and consequences. J. Fam. Med. Prim. Care 2015, 4, 187–192. [Google Scholar] [CrossRef]
- Huo, L.; Harding, J.L.; Peeters, A.; Shaw, J.E.; Magliano, D.J. Life expectancy of type 1 diabetic patients during 1997–2010: A national Australian registry-based cohort study. Diabetologia 2016, 59, 1177–1185. [Google Scholar] [CrossRef] [Green Version]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- English, E.; Lenters-Westra, E. HbA1c method performance: The great success story of global standardization. Crit. Rev. Clin. Lab. Sci. 2018, 55, 408–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, A.A.; Al-Dawish, A.; Mujammami, M.; Dawish, M.A.A. Type 1 diabetes mellitus in Saudi Arabia: A soaring epidemic. Int. J. Pediatr. 2018, 2018, 9408370. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Evangelou, E. Risk factors for type 2 diabetes mellitus: An exposure-wide umbrella review of meta-analyses. PLoS ONE 2018, 13, e0194127. [Google Scholar] [CrossRef] [PubMed]
- Ismail, L.; Materwala, H.; Al Kaabi, J. Association of risk factors with type 2 diabetes: A systematic review. Comput. Struct. Biotechnol. J. 2021, 19, 1759–1785. [Google Scholar] [CrossRef]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.R.; Ann, S.H.; Won, K.B.; Park, G.M.; Kim, Y.G.; Yang, D.H.; Kang, J.W.; Lim, T.H.; Kim, H.K.; Choe, J.; et al. Association between insulin resistance, hyperglycemia, and coronary artery disease according to the presence of diabetes. Sci. Rep. 2019, 9, 6129. [Google Scholar] [CrossRef] [Green Version]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivares, D.E.; Chambi, F.R.; Chañi, E.M.; Craig, W.J.; Pacheco, S.O.; Pacheco, F.J. Risk factors for chronic diseases and multimorbidity in a primary care context of central Argentina: A web-based interactive and cross-sectional study. Int. J. Environ. Res. Public Health 2017, 14, 251. [Google Scholar] [CrossRef] [PubMed]
- Forbang, N.I.; McDermott, M.M.; Liao, Y.; Ix, J.H.; Allison, M.A.; Liu, K.; Tian, L.; Evans, N.; Criqui, M.H. Associations of diabetes mellitus and other cardiovascular disease risk factors with decline in the ankle-brachial index. Vasc.Med. 2014, 19, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Dokken, B.B. The pathophysiology of cardiovascular disease and diabetes: Beyond blood pressure and lipids. Diabetes Spectr. 2008, 21, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Chen, X.; Qin, G.; Xie, H.; Lv, P. Tear film in type 2 diabetic patients with retinopathy. Ophthalmologica 2008, 222, 284–291. [Google Scholar] [CrossRef]
- Cousen, P.; Cackett, P.; Bennett, H.; Swa, K.; Dhillon, B. Tear production and corneal sensitivity in diabetes. J. Diabetes Complications 2007, 21, 371–373. [Google Scholar] [CrossRef]
- Inoue, K.; Okugawa, K.; Amano, S.; Oshika, T.; Takamura, E.; Egami, F.; Umizu, G.; Aikawa, K.; Kato, S. Blinking and superficial punctate keratopathy in patients with diabetes mellitus. Eye 2005, 19, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.J.; Patel, S.; Kong, N.; Ryder, R.E.; Marshall, J. Non-invasive assessment of corneal sensitivity in young and elderly diabetics and non-diabetics. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1737–1742. [Google Scholar] [CrossRef] [Green Version]
- Saito, J.; Enoki, M.; Hara, M.; Morishige, N.; Chikama, T.; Nishida, T. Correlation of corneal sensation, but not of basal or reflex tear secretion with the stage of diabetic retinopathy. Cornea 2003, 22, 15–18. [Google Scholar] [CrossRef]
- Zagon, I.S.; Sassani, J.W.; Immonen, J.A.; McLaughlin, P.J. Ocular surface abnormalities related to type 2 diabetes are reversed by the opioid antagonist naltrexone. Clin. Exp. Ophthalmol. 2014, 42, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.; Elagin, R.B.; Jume, J.C. Diabetes-associated dry eye syndrome in a new humanized transgenic model of type I diabetes. Mol. Vis. 2013, 19, 1259–1267. [Google Scholar]
- Alves, M.C.; Carvalheira, J.B.; Módulo, C.M.; Rocha, E.M. Tear film and ocular surface changes in diabetes mellitus. Arq. Bras. Oftalmol. 2008, 71, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhao, L.; Deng, S.; Sun, X.; Wang, N. Dry eye syndrome in patients with diabetes mellitus: Prevalence, etiology, and clinical characteristics. J. Ophthalmol. 2016, 2016, 8201053. [Google Scholar] [CrossRef] [Green Version]
- Kaiserman, I.; Kaiserman, N.; Nakar, S.; Vinker, S. Dry eye in diabetic patients. Am. J. Ophthalmol. 2005, 139, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Katakami, C.; Inoue, M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology 2001, 108, 586–592. [Google Scholar] [CrossRef]
- Goebbels, M. Tear secretion and tear film function in insulin dependent diabetics. Br. J. Ophthalmol. 2000, 84, 19–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 75–92. [CrossRef]
- Johnson, M.E.; Murphy, P.J. Changes in the tear film and ocular surface from dry eye syndrome. Prog. Retin. Eye Res. 2004, 23, 449–474. [Google Scholar] [CrossRef] [PubMed]
- Masmali, A.; Alqahtani, T.A.; Alharbi, A.; El-Hiti, G.A. Comparative study of repeatability of phenol red thread test versus Schirmer’s test in normal adults in Saudi Arabia. Eye Contact Lens 2014, 40, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Masmali, A.; Alrabiah, S.; Alharbi, A.; El-Hiti, G.A.; Almubrad, T. Investigation of tear osmolarity using the TearLabTM osmolarity system in normal adults in Saudi Arabia. Eye Contact Lens 2014, 40, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Masmali, M.A.; Al-Shehri, A.; Alanazi, S.A.; Abusharha, A.; Fagehi, R.; El-Hiti, G.A. Assessment of tear film quality among smokers using tear ferning patterns. J. Ophthalmol. 2016, 2016, 8154315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masmali, A.M.; Al-Bahlal, J.M.; El-Hiti, G.A.; Akhtar, S.; Purslow, C.; Murphy, P.J.; Almubrad, T. Repeatability and diurnal variation of tear ferning test. Eye Contact Lens 2015, 41, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masmali, A.M.; Alanazi, S.A.; Almagren, B.; El-Hiti, G.A. Assessment of the tear film in normal eye subjects after consumption of a single dose of hot peppermint drink. Clin. Optom. 2019, 11, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Abusharaha, A.; Alturki, A.A.; Alanazi, S.A.; Fagehi, R.; Al-Johani, N.; El-Hiti, G.A.; Masmali, A.M. An assessment of the tear evaporation rate in thyroid gland patients. Clin. Ophthalmol. 2019, 13, 131–135. [Google Scholar] [CrossRef] [Green Version]
- Alanazi, S.A.; Abusharha, A.; Fagehi, R.; Alsaqr, A.M.; El-Hiti, G.A.; Alahmari, R.A.; Alenazi, F.A.; Alnassar, K.M.; Masmali, A.M. Assessment of the tear evaporation rate in chronic smokers using Delfin VapoMeter. Int. J. Ophthalmol. Vis. Sci. 2019, 4, 37–41. [Google Scholar] [CrossRef]
- Mathers, W.D.; Binarao, G.; Petroll, M. Ocular water evaporation and the dry eye: A new measuring device. Cornea 1993, 12, 335–340. [Google Scholar] [CrossRef]
- Tsubota, K.; Yamada, M. Tear evaporation from the ocular surface. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2942–2950. [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef] [Green Version]
- Rohit, A.; Ehrmann, K.; Naduvilath, T.; Willcox, M.; Stapleton, F. Validating a new device for measuring tear evaporation rates. Ophthalmic Physiol. Opt. 2014, 34, 53–62. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Swamy, N.; Shashikala, P.; Parakash, K.H. Dry eye in type 2 diabetes. J. Eval. Med. Dental Sci. 2013, 2, 3122–3126. [Google Scholar]
- Manaviat, M.R.; Rashidi, M.; Afkhami-Ardekani, M.; Shoja, M.R. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. 2008, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Wee, S.W.; Chun, Y.S.; Moon, N.J.; Kim, J.C. Clinical usefulness of the phenol red thread test as diagnostic tool in dry eye patient. J. Korean Ophthalmol. Soc. 2012, 53, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Masmali, A.M.; Maeni, Y.A.; El-Hiti, G.A.; Murphy, P.J.; Almubrad, T. Investigation of ocular tear ferning in controlled and uncontrolled diabetic subjects. Eye Contact Lens 2018, 44, S70–S75. [Google Scholar] [CrossRef]
- Fuerst, N.; Langelier, N.; Massaro-Giordano, M.; Pistilli, M.; Stasi, K.; Burns, C.; Cardillo, S.; Bunya, V.Y. Tear osmolarity and dry eye symptoms in diabetics. Clin. Ophthalmol. 2014, 8, 507–715. [Google Scholar] [CrossRef] [Green Version]
- Aljarousha, M.; Badarudin, N.E.; Che Azemin, M.Z. Comparison of dry eye parameters between diabetics and non-diabetics in district of Kuantan, Pahang. Malays. J. Med. Sci. 2016, 23, 72–77. [Google Scholar]
- Derakhshan, A.; Abrishami, M.; Khajedaluee, M.; Omidtabrizi, A.; Moghaddam, S.G. Comparison between tear film osmolar cocentration and other tear film function parameters in patients with diabetes mellitus. Korean J. Ophthalmol. 2019, 33, 326–332. [Google Scholar] [CrossRef]
- Kesarwani, D.; Rizvi, S.W.A.; Khan, A.A.; Amitava, A.K.; Vasenwala, S.M.; Siddiqui, Z. Tear film and ocular surface dysfunction in diabetes mellitus in an Indian population. Indian J. Ophthalmol. 2017, 65, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.L.; Patel, D.V.; McGhee, C.N.J.; Pradhan, M.; Kilfoyle, D.; Braatvedt, G.D.; Crai, J.P. Peripheral neuropathy and tear film dysfunction in type 1 diabetes mellitus. J. Diabetes Res. 2014, 2014, 848659. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, S.; Zhou, J.; Liu, C.; Xu, M. Relationship between lipid layer thickness, incomplete blinking rate and tear film instability in patients with different myopia degrees after small-incision lenticule extraction. PLoS ONE 2020, 15, e0230119. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.M.; Tien, L.; Han, A.; Lee, J.M.; Kim, D.; Markoulli, M.; P Craig, J.P. Impact of blinking on ocular surface and tear film parameters. Ocul. Surf. 2018, 16, 424–429. [Google Scholar] [CrossRef] [PubMed]


| Score | Diabetes Group (n = 30) | Control Group (n = 30) |
|---|---|---|
| Age (year) | 33.1 ± 7.9 | 32.2 ± 6.5 |
| OSDI * | 12.0 (8.3) | 5.6 (7.0) |
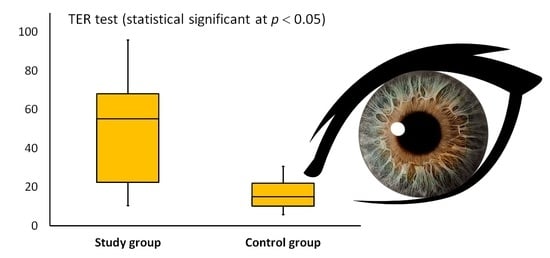
| TER (g/m2h) * | 46.4 (36.7) | 15.1 (11.9) |
| Score | Controlled Diabetes (n = 17) | Uncontrolled Diabetes (n = 13) |
|---|---|---|
| Age (year) | 28.8 ± 7.8 | 37.3 ± 5.3 |
| OSDI * | 11.0 (8.0) | 13.0 (11.5) |
| TER (g/m2h) * | 27.3 (32.6) | 53.4 (14.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abusharha, A.; El-Hiti, G.A.; Alsubaie, M.H.; Munshi, A.F.; Alnasif, A.R.; Fagehi, R.; Alanazi, M.A.; Masmali, A.M. Evaluation of Tear Evaporation Rate in Patients with Diabetes Using a Hand-Held Evaporimeter. Healthcare 2022, 10, 104. https://0-doi-org.brum.beds.ac.uk/10.3390/healthcare10010104
Abusharha A, El-Hiti GA, Alsubaie MH, Munshi AF, Alnasif AR, Fagehi R, Alanazi MA, Masmali AM. Evaluation of Tear Evaporation Rate in Patients with Diabetes Using a Hand-Held Evaporimeter. Healthcare. 2022; 10(1):104. https://0-doi-org.brum.beds.ac.uk/10.3390/healthcare10010104
Chicago/Turabian StyleAbusharha, Ali, Gamal A. El-Hiti, Mushawwat H. Alsubaie, Abdulaziz F. Munshi, Ahmed R. Alnasif, Raied Fagehi, Mana A. Alanazi, and Ali M. Masmali. 2022. "Evaluation of Tear Evaporation Rate in Patients with Diabetes Using a Hand-Held Evaporimeter" Healthcare 10, no. 1: 104. https://0-doi-org.brum.beds.ac.uk/10.3390/healthcare10010104