Silver-Coated Gold Nanorods as Optical Probes for the Sensitive Detection of Ascorbic Acid in Tablets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Materials
2.3. Preparation of Gold Nanorods
2.4. Synthesis of Silver-Coated Gold Nanorods
2.5. The Inhibition of Chemical Etching Reaction by Ascorbic Acid
2.6. Detection of Ascorbic Acid in Tablets
3. Results and Discussion
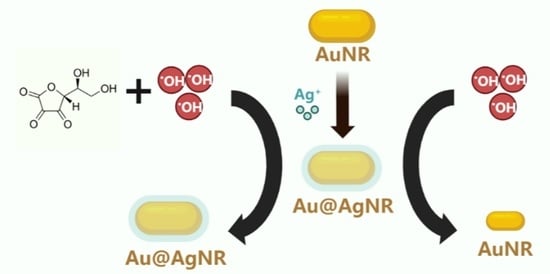
3.1. Working Principles and Spectral Characteristics
3.2. Optimization of Reaction Conditions
3.2.1. Optimization of Silver-Coated Gold nanorod Concentration
3.2.2. Optimization of H2O2 Concentration
3.2.3. Optimization of Fe2+ Concentration
3.2.4. Optimization of Reaction Acidity
3.2.5. Reaction Time Optimization
3.2.6. Reaction Temperature Optimization
3.3. The Sensitivity towards Ascorbic Acid Detection
3.4. The Specificity of the Proposed Method
3.5. The Practicability of the Proposed Method in Detection of Ascorbic Acid in Tablets
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kathiravan, S.; Sundaram, E.; Paulraj, B.A.; Johnson, P.M.; Huang, S.T.; Mani, V.; Vasantha, V.S. Simple and Selective Optical Biosensor Using Ultrasonicator Synthesis of 5-((Anthracen-9-Ylmethylene) Amino)-2,3-Dihydrophthalazine-1,4-Dione for Direct Detection of Ascorbic Acid in Vegetables and Fruits. Food Chem. 2020, 332, 127150. [Google Scholar] [CrossRef] [PubMed]
- Njus, D.; Kelley, P.M.; Tu, Y.J.; Schlegel, H.B. Ascorbic Acid: The Chemistry Underlying Its Antioxidant Properties. Free Radical Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kamoto, R.J.; Kasapila, W.; Ng’ong’ola-Manani, T.A. Use of Fungal Alpha Amylase and Ascorbic Acid in the Optimisation of Grain Amaranth-Wheat Flour Blended Bread. Food Nutr. Res. 2018, 62, 1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdini, R.A.; Lagier, C.M. Voltammetric Iodometric Titration of Ascorbic Acid with Dead-Stop End-Point Detection in Fresh Vegetables and Fruit Samples. J. Agric. Food Chem. 2000, 48, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.N.; Cheng, R.; Kang, K.W.; Chen, M.Y.; Zhan, T.; Wang, J. Size-Dependent Light Scattering of Coooh Nanoflakes for Convenient and Sensitive Detection of Alkaline Phosphatase in Human Serum. Luminescence 2021, 36, 1317–1326. [Google Scholar] [CrossRef]
- Liu, C.; Pang, Q.W.; Wu, T.; Qi, W.J.; Fu, W.S.; Wang, Y. A Rapid Visual Detection of Ascorbic Acid through Morphology Transformation of Silver Triangular Nanoplates. J. Anal. Test. 2021, 5, 210–216. [Google Scholar] [CrossRef]
- Chen, C.X.; Zhang, C.H.; Ni, P.J.; Jiang, Y.Y.; Wang, B.; Lu, Y.Z. “Light-on” Colorimetric Assay for Ascorbic Acid Detection Via Boosting the Peroxidase-Like Activity of Fe-MIL-88. J. Anal. Test. 2022, 6, 67–75. [Google Scholar] [CrossRef]
- Tian, Y.L.; Ji, Y.Y.; Zou, X.; Chen, Q.M.; Zhang, S.L.; Gong, Z.J. N, P Co-Doped Carbon Dots as Multifunctional Fluorescence Nano-Sensor for Sensitive and Selective Detection of Cr(VI) and Ascorbic Acid. J. Anal. Test. 2022, 6, 335–345. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Han, D.F.; Xiao, R.; Wang, T.Q.; Liang, Z.S.; Wu, Z.F.; Han, F.J.; Han, D.X.; Ma, Y.M.; Niu, L. An Enzyme-Free Photoelectrochemical Sensor Platform for Ascorbic Acid Detection in Human Urine. Chemosensors 2022, 10, 247. [Google Scholar] [CrossRef]
- Tortolini, C.; Tasca, F.; Venneri, M.A.; Marchese, C.; Antiochia, R. Gold Nanoparticles/Carbon Nanotubes and Gold Nanoporous as Novel Electrochemical Platforms for L-Ascorbic Acid Detection: Comparative Performance and Application. Chemosensors 2021, 9, 229. [Google Scholar] [CrossRef]
- Chen, H.J.; Wang, Q.; Shen, Q.P.; Liu, X.; Li, W.; Nie, Z.; Yao, S.Z. Nitrogen Doped Graphene Quantum Dots Based Long-Persistent Chemiluminescence System for Ascorbic Acid Imaging. Biosens. Bioelectron. 2017, 91, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Samper, I.C.; Mcmahon, C.J.; Schenkel, M.S.; Clark, K.M.; Khamcharoen, W.; Anderson, L.; Terry, J.S.; Gallichotte, E.N.; Ebel, G.D.; Geiss, B.J. Electrochemical Immunoassay for the Detection of SARS-CoV-2 Nucleocapsid Protein in Nasopharyngeal Samples. Anal. Chem. 2022, 94, 15155–15161. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, L.; Peng, G.; Wang, G.; Liang, X. Chemical Redox-Modulated Etching of Plasmonic Nanoparticles for Nitrite Detection: Comparison among Gold Nanosphere, Nanorod, and Nanotriangle. J. Anal. Test. 2021, 5, 350–359. [Google Scholar] [CrossRef]
- Liu, J.J.; Yan, H.H.; Zhang, Q.; Gao, P.F.; Li, C.M.; Liang, G.L.; Huang, C.Z.; Wang, J. High-Resolution Vertical Polarization Excited Dark-Field Microscopic Imaging of Anisotropic Gold Nanorods for the Sensitive Detection and Spatial Imaging of Intracellular MicroRNA-21. Anal. Chem. 2020, 92, 13118–13125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Z.; Li, R.; Gao, P.F.; Wang, N.; Lei, G.; Huang, C.Z.; Wang, J. Real-Time Dark-Field Light Scattering Imaging to Monitor the Coupling Reaction with Gold Nanorods as Optical Probe. Nanoscale 2017, 9, 3568–3575. [Google Scholar] [CrossRef]
- Gao, Z.; Deng, K.; Wang, X.D.; Miro, M.; Tang, D. High-Resolution Colorimetric Assay for Rapid Visual Readout of Phosphatase Activity Based on Gold/Silver Core/Shell Nanorod. ACS Appl. Mater. Interfaces 2014, 6, 18243–18250. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid Synthesis of Au, Ag, and Bimetallic Au Core-Ag Shell Nanoparticles Using Neem (Azadirachta Indica) Leaf Broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef]
- Gu, Y.; Song, J.; Li, M.-X.; Zhang, T.-T.; Zhao, W.; Xu, J.-J.; Liu, M.; Chen, H.-Y. Ultrasensitive MicroRNA Assay via Surface Plasmon Resonance Responses of Au@Ag Nanorods Etching. Anal. Chem. 2017, 89, 10585–10591. [Google Scholar] [CrossRef]
- Cai, Z.X.; Chen, Y.Z.; Meteku, B.E.; Zheng, Q.W.; Li, F.M.; Zhang, M.S.; Zeng, J.B.; Chen, X. Cu2+-Assisted Synthesis of Au@Agl Core/Shell Nanorods via In Situ Oxidation of Iodide: A Strategy for Colorimetric Iodide Sensing. J. Anal. Test. 2022, 2022, 6, 374–381. [Google Scholar]
- Zhao, Y.; Gao, X.-Y.; Wang, H.; Wang, J.; Zhou, J.; Zhao, W.; Xu, J.-J.; Chen, H.-Y. Ultrasensitive Detection of MicroRNA via a Au@Ag Nanosnowman. Anal. Chem. 2019, 91, 15988–15992. [Google Scholar] [CrossRef]
- Cheng, R.; Zhu, F.; Huang, M.; Zhang, Q.; Yan, H.H.; Zhao, X.H.; Luo, F.K.; Li, C.M.; Liu, H.; Liang, G.L.; et al. “Hepatitis Virus Indicator”--The Simultaneous Detection of Hepatitis B and Hepatitis C Viruses Based on the Automatic Particle Enumeration. Biosens. Bioelectron. 2022, 202, 114001. [Google Scholar] [CrossRef] [PubMed]
- Gole, A.; Murphy, C.J. Seed-Mediated Synthesis of Gold Nanorods: Role of the Size and Nature of the Seed. Chem. Mater. 2004, 16, 3633–3640. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, H.H.; Ru, C.; Zhu, F.; Zou, H.Y.; Gao, P.F.; Huang, C.Z.; Wang, J. Plasmonic Biosensor for the Highly Sensitive Detection of MicroRNA-21 Via the Chemical Etching of Gold Nanorods under a Dark-Field Microscope. Biosens. Bioelectron. 2022, 201, 113942. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, S.; Tan, P.; Zhou, J.; Huang, Y.; Nie, Z.; Yao, S. A Plasmonic Blood Glucose Monitor Based on Enzymatic Etching of Gold Nanorods. Chem. Commun. 2013, 49, 1856–1858. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Gong, H.; Li, Y.; Li, Y.; Lin, W.; Tang, D.; Knopp, D. CRISPR-Cas12a-Derived Photoelectrochemical Biosensor for Point-Of-Care Diagnosis of Nucleic Acid. Anal. Chem. 2022, 94, 7442–7448. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, T.; Li, W.; Wang, Z.; Liu, Y.; Wang, F.; Wang, X.; Zhang, G.; Zhang, Z. Homocytosine-Templated Gold Nanoclusters as a Label-Free Fluorescent Probe: Ferrous Ions and Glucose Detection Based on Fenton and Enzyme-Fenton Reaction. Colloids Surface A 2021, 628, 127229. [Google Scholar] [CrossRef]
- Lai, W.; Wei, Q.; Zhuang, J.; Lu, M.; Tang, D. Fenton Reaction-Based Colorimetric Immunoassay for Sensitive Detection of Brevetoxin B. Biosens. Bioelectron. 2016, 80, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.J.; Yan, H.H.; Yuan, D.; Zhang, Q.; Li, C.M.; Huang, C.Z.; Wang, J. The Synergistic Effect Enhanced Chemical Etching of Gold Nanorods for the Rapid and Sensitive Detection of Biomarks. Talanta 2020, 219, 121203. [Google Scholar] [CrossRef]
- Lee, C.; Yoon, J. Temperature Dependence of Hydroxyl Radical Formation in the hv/Fe3+/H2O2 and Fe3+/H2O2 Systems. Chemosphere 2004, 56, 923–934. [Google Scholar] [CrossRef]
- Mao, Z.Y.; Zhu, L.N.; Gao, J.; Liu, J.J.; Wei, Y.H.; Li, X.Y.; Yin, B.C.; Wang, J. A Coooh Nanoflake-Based Light Scattering Probe for the Simple and Selective Detection of Uric Acid in Human Serum. Anal. Methods 2018, 10, 4951–4957. [Google Scholar] [CrossRef]
- Chandra, S.; Singh, V.K.; Yadav, P.K.; Bano, D.; Kumar, V.; Pandey, V.K.; Talat, M.; Hasan, S.H. Mustard Seeds Derived Fluorescent Carbon Quantum Dots and Their Peroxidase-Like Activity for Colorimetric Detection of H2O2 and Ascorbic Acid in a Real Sample. Anal. Chim. Acta 2019, 1054, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, W.; Han, Y.; Chen, X.; Zhu, L.; Tang, W.; Wang, J.; Yue, T.; Li, Z. N,S Co-Doped Carbon Dots Based Fluorescent “On-Off-On” Sensor for Determination of Ascorbic Acid in Common Fruits. Food Chem. 2018, 258, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Deng, L.; Alsaiari, S.; Zhang, D.; Khashab, N.M. “Light-on” Sensing of Antioxidants Using Gold Nanoclusters. Anal. Chem. 2014, 86, 4989–4994. [Google Scholar] [CrossRef] [PubMed]
- Darabdhara, G.; Sharma, B.; Das, M.R.; Boukherroub, R.; Szunerits, S. Cu-Ag Bimetallic Nanoparticles on Reduced Graphene Oxide Nanosheets as Peroxidase Mimic for Glucose and Ascorbic Acid Detection. Sensor. Actuat. B Chem. 2017, 238, 842–851. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, S.; Huang, M.; Cheng, R.; Gao, J.; Wang, J. Silver-Coated Gold Nanorods as Optical Probes for the Sensitive Detection of Ascorbic Acid in Tablets. Chemosensors 2022, 10, 543. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10120543
Wen S, Huang M, Cheng R, Gao J, Wang J. Silver-Coated Gold Nanorods as Optical Probes for the Sensitive Detection of Ascorbic Acid in Tablets. Chemosensors. 2022; 10(12):543. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10120543
Chicago/Turabian StyleWen, Shuai, Min Huang, Ru Cheng, Jie Gao, and Jian Wang. 2022. "Silver-Coated Gold Nanorods as Optical Probes for the Sensitive Detection of Ascorbic Acid in Tablets" Chemosensors 10, no. 12: 543. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10120543