1. Introduction
Thin films of ligand-stabilized or cross-linked noble metal nanoparticles are interesting materials for applications as highly responsive physical and chemical sensors. For example, thin films of gold or silver nanoparticles deposited onto flexible substrates or prepared as freestanding membranes can be used as highly sensitive resistive strain [
1,
2,
3,
4] or pressure sensors [
5,
6,
7]. Recently, such sensors have been proposed for applications in modern health care and medical devices, e.g., for human motion detection, pulse wave analysis, and voice recognition [
8,
9,
10,
11]. Furthermore, resistive gas sensors made from thin films of gold nanoparticles (GNPs) are well-suited for the detection of volatile organic compounds (VOCs) released from the human body and exhaled with breath. Thus, these sensors have been proposed for applications in medical diagnosis [
12,
13,
14].
The high sensitivity of GNP-based chemiresistors is attributed to the high permeability of nanoparticle assemblies and a perturbation-sensitive charge transport mechanism based on thermally activated charge carrier tunneling [
15]. Further advantages of these sensors are their short response and recovery times and the possibility to operate them at room temperature. Thus, GNP-based chemiresistors can be operated with low power consumption and they can be fabricated on various polymer substrates, such as polyethylene (PE), polyethylene terephthalate (PET), or polyimide (PI) [
13,
16,
17,
18,
19]. Moreover, the chemical selectivity of GNP-based chemiresistors can be tuned by modifying the GNP surface with differently functionalized ligands or cross-linkers. For example, Cooper et al. fabricated chemiresistors based on differently functionalized GNPs which were drop-casted onto glass substrates with lithographically structured electrodes. They demonstrated that these sensors can be used to selectively detect different hydrocarbon fuels in seawater [
20]. In addition, it was shown that the chemical sensitivity and selectivity of flexible GNP-based chemiresistors can be adjusted by straining the transducing element [
13,
16,
21]. The use of flexible polymer substrates also enables the design of multifunctional sensor patches which can be attached directly onto the skin to monitor motion, pulse, temperature and metabolites transpired through the skin [
22,
23].
The above-mentioned examples highlight numerous advantages of flexible GNP-based sensing elements. However, the development of facile procedures for their efficient fabrication, which eventually requires patterning of GNP films and their integration into sensor arrays and complex electronics on polymer substrates, remains a challenging task. A feasible approach is to structure particle assemblies on flexible substrates using lithographic techniques [
4]. Li et al. used block copolymer photolithography to selectively deposit periodic arrays of silver nanoparticles onto PET substrates [
24]. Another approach is to transfer nanoparticle structures from a rigid to a flexible substrate using contact printing [
25,
26]. However, as GNPs are usually prepared as colloidal solutions, the most straightforward approach is the direct patterning of GNP films via ink-based printing techniques. For example, Andres and Kotov reported the fabrication of patterned GNP films by depositing negatively charged GNPs and positively charged polymers via layer-by-layer inkjet printing [
27]. In general, inkjet printing offers the advantage of being more resource-efficient than conventional subtractive manufacturing methods. Furthermore, on demand adjustments of geometric features can easily be implemented.
Some recent studies presented fully inkjet-printed chemiresistive sensing devices. For example, Song et al. presented a fully printed chemiresistive glucose sensor based on PET-supported polyaniline [
28]. Silva and co-workers reported a fully printed gas sensor based on carbon nanotubes with enhanced sensitivity to ethanol vapor [
29]. Recently, we reported the fabrication of highly responsive humidity sensors based on polyethyleneglycol- (PEG-) stabilized GNPs via inkjet printing [
30]. Major achievements and state of the art techniques for the manufacturing of inkjet-printed chemiresistive devices and their intended applications in wireless electronics have recently been reviewed by Hartwig et al. [
31].
Here, we present a new protocol for printing flexible GNP-based chemiresistors with tunable chemical selectivity. First, interdigitated silver electrodes were fabricated on PI foil using dispenser printing. Afterwards, films of cross-linked GNPs were inkjet-printed in a layer-by-layer fashion using ink formulations containing alkylamine-stabilized GNPs and dithiol cross-linkers. Furthermore, we show that the chemical selectivity of these chemiresistors can be tuned by blending functionalized monothiols into the cross-linker ink. Using this approach, several sensors were produced and individually printed sensors were combined to form a sensor array. The sensitivities of the individual sensors as well as the signal patterns produced by the array were studied by dosing the sensors with vapors of solvents ranging from nonpolar hydrophobic to polar hydrophilic.
2. Materials and Methods
Materials. 1-Dodecylamine (12A, 98%), 1-dodecanethiol (12T, ≥98%), tert-butylamine-borane complex (TBAB, 98%), 4-mercapto-1-butanol (4M1OH, 95%), 3-mercaptopropionic acid (3MPA, ≥99%), 1-thioglycerol (TG, ≥97%), 4-methyl-2-pentanone (4M2P, 98.5%), and n-octane (99%) were purchased from Merck (Darmstadt, Germany) and used as received. Ethanol (EtOH, 99.8%) and toluene (99.5%) were purchased from VWR (Hannover, Germany). n-Heptane (99%) and 1-propanol (99.5%) were purchased from Grüssing (Filsum, Germany). Tetrachloroauric(III) acid (HAuCl4, 99.99%), 1,9-nonanedithiol (9DT, 97%), and 1-butanol (99%) were purchased from Alfa Aesar (Kandel, Germany). Purified water (18.2 MΩ cm) was provided using a PURELAB flex System (ELGA LabWater). Polytetrafluoroethylene (PTFE) syringe filters (pore size of 0.2 µm) were purchased from Carl Roth (Karlsruhe, Germany). Polyimide (PI) foil (Kapton, 200QR1; thickness: ∼50 μm) and conductive silver paste (PE873) were supplied by DuPont (Neu-Isenburg, Germany). Nylon syringe filters (PureTech Nylon030N0451) with a pore size of 0.45 µm were purchased from Finetech (Taipei, Taiwan).
Synthesis of dodecylamine-stabilized gold nanoparticle (GNP) ink. GNPs with a diameter of ~7 nm were synthesized according to the method of Peng et al. [
32] with slight modifications. Ethanol was used instead of acetone to precipitate the GNPs for purification. 12A was chosen as stabilizing agent instead of oleylamine. After centrifugation, the GNPs were re-dispersed in octane instead of hexane, resulting in a GNP ink with a particle concentration of ~4.5 µmol L
−1. The particle concentration was determined according to the method of Haiss [
33]. Transmission electron microscopy (TEM) images of the GNPs, size statistics, as well as UV/Vis data of the prepared GNP ink can be found in the
Supplementary Materials.
Fabrication of chemiresistors. First, interdigitated silver electrodes, consisting of four fingers with a length of ~4.5 mm and a spacing of ~250 µm, were printed on PI foil, using a robotic dispensing system (DT-200F, Dispenser Tech Co., Ltd., New Taipei City 23585, Taiwan). Two 3 × 3 mm
2 electrode pads with a distance of 5 mm were subsequently printed the same way. The conductive silver paste was treated with a homogeniser (Omni Ruptor 4000, Kennesaw, GA 30144, USA) for 3 min before being dispensed through a stainless-steel nozzle with an inner diameter of 110 µm. After printing, the silver paste electrodes were sintered for 1 h at 100 °C on a heating plate. Finally, the PI foil with the electrode structures was treated with air plasma (Delta AC driver TP04, 90 s, 310 mTorr, 10 A). Plasma cleaning enabled a homogenous deposition of the GNP ink onto the substrate with the electrodes. For printing the GNP film transducers, the PI foil with the electrodes was placed on a heating stage adjusted to 37 °C. The GNP ink was agitated and filtered using a nylon syringe filter (0.45 µm pore size) before being printed using an inkjet printing system (JetLab 4, MicroFab Technologies Inc., Plano, TX 75074, USA). The ink containing the pure 9DT cross-linker was prepared by dissolving 9DT in n-octane. Inks containing mixtures of 9DT with various monothiols (L) were prepared by dissolving these compounds in 1-propanol. In this study the following 9DT+L formulations were used: 9DT+TG, 9DT+3MPA, 9DT+4M1OH, with each mixture in 9DT:L ratios of 3:1 (i.e., 25% L) and 1:1 (i.e., 50% L). Taking into account the footprint of thiol ligands on gold of 0.214 nm
2 a complete monolayer coverage would require ~800 thiol molecules per nanoparticle [
34]. Assuming similar droplet sizes for, both, the GNP ink as well as the 9DT/9DT+L inks, the concentrations of the latter were adjusted to 4.5 mmol L
−1 in order to provide ~10
3 thiol molecules per nanoparticle. The GNP ink and the 9DT/9DT+L inks were printed in an alternating sequence, i.e., GNP-(9DT/9DT+L)-GNP-(9DT/9DT+L)-GNP, etc. Each of the deposited GNP, 9DT, or 9DT+L coatings consisted of four printed layers. The moving direction of the printer stage was rotated by 90° after each printed layer. In order to enable the formation of fairly homogenous coatings the droplet spacing was set to 40 µm for every first, 50 µm for every second, 45 µm for every third, and 37 µm for every fourth printed layer. This was achieved by maintaining a consistent ejection frequency of 500 Hz and setting the moving speed of the printer stage to 20 mm s
−1 for every first, 25 mm s
−1 for every second, 22.5 mm s
−1 for every third, and 18.5 mm s
−1 for every forth layer. For each printed layer the droplet spacing was used as input parameter to set the distance between printed lines. The 50 µm piezoelectric nozzle was driven by a bipolar (positive, negative) pulse. A first rise time of 4 µs, dwell time, fall time, and echo time of 3 µs, second rise time of 2 µs as well as echo voltage of −30 V, and dwell voltage of 20 and 25 V were used as default. Droplets of 45 to 65 µm in diameter were ejected at speeds of 1–2 m s
−1. After the printing process, the sensors fabricated with the pure 9DT cross-linker (without L) were immersed in the 9DT ink for 12 h at room temperature. The sensors fabricated with the 9DT+L inks were immersed for 1 h in their respective ink mixture. Finally, the sensors were washed with n-octane and ethanol, dried and stored under nitrogen until use.
Characterization of GNP chemiresistive devices. Scanning electron microscopy (SEM) images were acquired using a Leo 1550 Gemini microscope (Zeiss, Jena, Germany) operated at 5 kV. To test the sensing performance of the inkjet-printed sensors they were placed into a custom-made metal cell with a total volume of ~10 mL. Test vapors of n-octane, toluene, 4M2P, 1-butanol, 1-propanol, ethanol, and water were generated using a programmable gas calibration system (Kalibriersystem Model CGM 2000, Umwelttechnik MCZ GmbH, Bad Nauheim, Germany). Nitrogen of quality 5.0 was used as carrier gas and the flow rate through the test cell was adjusted to 400 mL min
−1 and kept constant whilst switching between carrier gas and analyte vapors. The sensors were operated at ambient temperature and dosed with analyte concentrations ranging from 25 and 2000 ppm. The exposure to analyte was set to 240 s followed by purging for 480 s with carrier gas. In order to determine the resistive responses of the sensors to analyte exposure each sensor was connected in series with a commercial SMD resistor acting as shunt. A constant voltage of 5 V was applied to all sensor/shunt pairs using a Keithley 2601A sourcemeter (SMU, Keithley Instruments, Solon, OH, USA). A custom-built multiplexer was used to successively read out the voltage drop at each shunt resistor using a Keithley 2002 multimeter (MM; Solon, OH, USA). The resistive responses of the sensors were determined from the measured voltage drop at each shunt and the shunt resistance. Details can be found in the
Supplementary Materials. IV-Characteristics of the sensors were recorded using a Keithley 2601A sourcemeter by sweeping the voltage from −5 to 5 V. In order to monitor the formation of the sensor films during the layer-by-layer print process, the resistances of the films were measured directly after depositing the GNP and 9DT coatings. These measurements were done using a KLA DM2630 multimeter (Taichung City 401, Taiwan).
3. Results and Discussion
The protocol for the fabrication of flexible GNP-based chemiresistors developed in this study is based on the combination of standard printing technologies without the need of additional lithographic process steps. In the first major step, silver electrodes consisting of two interdigitated finger pairs were printed onto PI foil using a robotic dispensing system.
Figure 1a depicts a schematic drawing of a sensor with the electrode structure. The printed electrodes are clearly seen in
Figure 2 presenting two micrographs of a sensor device.
In the second major process step, the GNP film transducers were inkjet-printed using the layer-by-layer approach detailed in the Materials and Methods section.
Figure 1b presents a transmission electron microscopy (TEM) image of the GNPs used in this study. Four layers of GNPs were printed on top of each other, as schematically depicted in
Figure 1a. In order to obtain a fairly homogeneous coating of the substrate the printing direction was rotated by 90° after each completed layer and the moving speed of the printer was altered between 18.5–25.0 mm s
−1. After printing four GNP layers, the ink containing the 1,9-nonanedithiol (9DT) cross-linker in n-octane was applied by subsequently printing four layers using the same scheme as for the GNP ink. The concentration of the 9DT ink was adjusted to yield a 10
3-fold excess of linker molecules with respect to GNPs. During the whole printing process the substrates were kept on a heating stage at 37 °C to enhance solvent evaporation and cross-linking reactions. It is to note that organic thiols easily replace amine ligands on the surface of GNPs due to the formation of strong Au-S bonds [
35]. Thus, the reaction of 9DT with dodecylamine-stabilized GNPs leads to a cross-linked network of GNPs with enhanced mechanical robustness [
36].
Figure 1a schematically presents the molecular structure of fabricated GNP films and indicates the mesh-like texture of the films resulting from above-described print process. This mesh-like pattern is confirmed by the optical micrograph shown in
Figure 2b.
In order to produce chemiresistors with baseline resistances in the low megohm range it was necessary to repeat above described printing of GNPs and cross-linkers. For example, to produce the sensor shown in
Figure 2 the deposition of GNPs was performed thrice (i.e., 3 × 4 layers GNPs) and the deposition of cross-linker was done twice (i.e., 2 × 4 layers 9DT), as indicated by the schematic drawing in
Figure 1a. The sensor produced according to this protocol had a baseline resistance of ~0.7 MΩ.
Figure 3a shows how the baseline resistance of four sensor devices decreased with increasing number of printed GNP and 9DT coatings. Note, each coating consisted of four printed layers of GNPs and 9DT, respectively. After printing the first GNP coating the resistance was still too high to be measured with our equipment. However, after the second GNP coating, the resistances decreased to the low megohm range. After printing the third GNP coating all four sensors had very similar resistances below 1 MΩ. Based on these findings, all sensors considered in the following sections were routinely fabricated by printing three GNP coatings. To achieve ligand/linker exchange of the third GNP coating and to enhance cross-linking of GNPs throughout the whole film all sensors were immersed in the cross-linker ink before exploring their vapor sensing properties. The data points plotted in
Figure 3a represent resistance values measured before this immersion step, whereas the IV-curves displayed in the inset were measured after the immersion step. The slope of these curves corresponds to a resistance of ~0.7 MΩ, which is very similar to the resistances measured before the immersion step. Hence, it is concluded that the immersion step had no significant influence on the final baseline resistance of the sensors.
Scanning electron microscopy (SEM) images of a printed GNP film consisting of three GNP coatings and two 9DT coatings are presented in
Figure 4. On the micrometer scale a rather homogeneous coating of the substrate is observed. However, higher magnifications reveal a granular structure on the sub-micrometer scale, presumably due to clustering of GNPs. Similar results were reported earlier for 9DT cross-linked GNP films deposited onto polyethylene (PE) substrates via layer-by-layer self-assembly [
16].
The chemiresistive responses of the 9DT cross-linked GNP films were initially studied by dosing them with toluene vapor. Vapor concentrations ranging from 25–2000 ppm were generated using a programmable gas calibration system with nitrogen as carrier gas.
Figure 5a shows the transient responses to vapor pulses of 240 s duration revealing very short response and recovery times, and almost ideal rectangular response profiles. Considering a test cell volume of ~10 mL and a flow rate of 400 mL min
−1 the time required to fully exchange the carrier gas by analyte vapor is ~8 s (see
Supplementary Materials). As the t
90 times of the measured transients were in the same time range, we conclude that the actual t
90 times of the sensors were ≤8 s.
Figure 5b shows the response isotherm obtained by plotting the response amplitudes vs. applied vapor concentrations. The data points represent the mean values of three different devices. Thus, the standard deviations shown as error bars underline the good reproducibility of the sensors’ performance. A first-order Langmuir isotherm according to Equation (1) could smoothly be fitted to these data:
Here, (∆
R/
R0)
sat represents the relative change of resistance at saturation, [
A] is the concentration of toluene vapor, and
Kb is the Langmuir binding constant. The values extracted as fit parameters for (∆
R/
R0)
sat and
Kb are 6% and ~10
4 L mol
−1, respectively. The binding constant is similar to that reported previously for chemiresistors based on 9DT cross-linked GNP films which were deposited onto PE substrates via layer-by-layer self-assembly [
16]. However, the value is approximately three times higher than that reported for similar GNP films deposited onto glass substrates [
37]. The maximum sensitivity of the sensor is given by the initial slope of the response isotherm, corresponding to
ppm
−1. Very similar sensitivities were reported for above mentioned chemiresistors based on 9DT cross-linked GNP-films on PE substrates which were contacted by electrodes with a 400 µm gap [
16].
In order to test the mechanical robustness of our printed sensors we repeatedly flexed one sensor using radii of curvature down to 5 mm. Afterwards, the baseline resistance and the response to toluene vapor were measured. These experiments did not indicate any significant changes, neither of the baseline resistance nor of the response characteristics. Additionally, optical microscopy images did not indicate any delamination or degradation of the GNP film or of the electrodes. Details of these experiments can be found in the
Supplementary Materials.
After having established the protocol for printing GNP-based chemiresistors, we focused on tuning their chemical selectivity. To this end, monothiols (ligands, L) with polar functional groups were blended into the ink containing the 9DT cross-linker at different ratios. An overview of used monothiols and the 9DT cross-linker, their molecular structures and mixing ratios, as well as the abbreviations used for the sensors fabricated in this study, is presented in
Table 1. Essentially, the sensors were printed using the same protocol as described above. However, instead of the pure 9DT cross-linker ink, 9DT+L mixtures were applied as detailed in the Materials and Methods section. It is expected that, in addition to the 9DT cross-linker, the added monothiols bind to the GNP surface via their thiol head-group. Thus, hydroxyl or carboxyl groups are inserted into the organic matrix surrounding the GNPs. Consequently, this should tune the selectivity more toward polar analytes.
Figure 3b shows IV-curves of GNP-based sensors printed with the different ink formulations, as indicated. All sensors showed ohmic conductivity. Further, as readily seen by the slopes of the IV-curves all sensors printed with the 9DT+L mixtures had higher resistances than the film printed with the pure 9DT ink. In agreement with findings reported by Lindsay and coworkers [
38], this finding suggests that replacing the cross-linker to some extent by monothiols leads to a less interconnected network of GNPs and, hence, to a composite material with lower conductivity. Further, this interpretation is supported by the observation that the films printed with inks containing the lowest 9DT concentration, i.e., the 50 vol% 9DT+L formulations, showed the highest resistances (see inset of
Figure 3b).
The sensors printed with different ink formulations were combined to form an array of seven sensors, which was connected to a custom-made multiplexer for signal readout. Details of the setup can be found in the
Supplementary Materials. To characterize their chemical selectivity the sensors were dosed using volatile analytes with hydrophobic (n-octane, toluene, 4M2P), amphiphilic (1-butanol, 1-propanol), and hydrophilic (ethanol, water) character.
Figure 6a presents the response transients recorded with these analytes at concentrations of 2000 ppm. All sensors showed remarkably fast and fully reversible responses. The sensitivities of the sensors to the different analytes were determined as the initial slopes of respective response isotherms (
Supplementary Materials, Figure S3) and are presented in
Figure 6b. Although the response isotherms show nonlinear characteristics the comparison of data presented in
Figure 6a,b reveals that the sensitivities basically follow the same trends as the response amplitudes measured at relatively high vapor concentrations.
The sensitivities of a GNP-based chemiresistor to different volatile analytes depend on various parameters. It is generally accepted that the sensing mechanism of these transducers is based on the perturbation of thermally activated electron tunneling between neighboring GNPs [
2]. On the one hand, analyte sorption usually causes swelling of the GNP film and, thereby, the interparticle distances increase. Thus, this effect increases the resistance of the transducer [
15,
39]. On the other hand, sorption of polar analytes with high permittivity can decrease the charging energy of the GNPs. Consequently, this effect decreases the transducer’s resistance [
40]. Usually, however, GNP-based chemiresistors respond to analyte sorption with an overall increase in their resistance, suggesting that swelling of the GNP film is usually the dominating contribution to the sensing mechanism. Furthermore, the sensitivity of a GNP-based chemiresistor to a given analyte critically depends on the amount of analyte absorbed within the organic matrix of the film, i.e., the sensitivity depends on the partition coefficient of this particular analyte. In general, the partition coefficients are high for analytes with solubility properties matching those of the ligand/linker matrix of the GNP film. For example, if the GNPs are embedded within a non-polar hydrophobic ligand/linker matrix the partition coefficients are high for analytes with the same non-polar hydrophobic character, but low for analytes with polar hydrophilic properties [
15]. Additionally, the vapor pressure of the analytes plays an important role. Analytes with low vapor pressure tend to have higher partition coefficients and, thus, give rise to more sensitive responses. Further, the molecular volume of the analytes may also affect the sensitivity as larger analyte molecules may more effectively cause swelling of the sensor film [
39].
Because of above outlined complexity of the sensing mechanism a quantitative analysis of the sensitivities to different analytes shown in
Figure 6b is beyond the scope of this study. However, in the following paragraphs we will qualitatively discuss some of their most striking trends.
First of all, the transients presented in
Figure 6a show that all seven sensors responded to the applied analytes with an increase in resistance. In agreement with previous studies [
15,
39], this behavior suggests that for each sensor/analyte pair sorption-induced swelling of the film material was the dominating contribution to the sensing mechanism. Compared to the other analytes all sensors showed relatively high sensitivity to 1-butanol, which has an amphiphilic character and intermediate polarity. This seems especially remarkable in the case of sensor S-9DT which was printed using the ink containing the pure 9DT cross-linker with its hydrophobic alkylene backbone. However, it is well-known that 9DT cross-linked GNP films contain numerous non-reacted free thiol groups [
36] which are available to form hydrogen bonds with protic (1-butanol, 1-propanol, ethanol) and aprotic (4M2P) polar compounds. Furthermore, the GNP films possibly contained some residual amphiphilic dodecylamine (12A), which was the initial stabilizer of the GNP ink. Hence, as all GNP films contained 9DT as cross-linker (and possibly residual 12A) it is conceivable that sorption of analyte molecules was primarily governed by an overall amphiphilic character of the GNP films, which explains their relatively high sensitivity to 1-butanol. Additionally, the relatively low vapor pressure of 1-butanol may contribute to the overall enhanced sensitivity to this analyte. However, by blending the monothiol ligands with polar functional groups into the 9DT ink the amphiphilic character of the sensor films could be tuned toward a more polar hydrophilic character.
Compared to sensor S-9DT printed with the pure 9DT ink, all sensors printed with 9DT+L ink formulations responded less sensitively to the hydrophobic analytes 4M2P, toluene, and n-octane. For example, sensors S-3MPA.25 and S-3MPA.50 printed with the 9DT+3MPA ink clearly showed the trend of decreasing sensitivity with increasing monothiol concentration. Accordingly, we attribute the observed decreased sensitivity to the increased hydrophilic character of the monothiol-blended GNP films.
The data presented in
Figure 6b also show that the sensitivities of all sensors to alcohols decrease in the order 1-butanol > 1-propanol > ethanol. We attribute this general trend mainly to the different vapor pressures, which increase significantly in the same order (1-butanol: 8.85 hPa; 1-propanol: 28.2 hPa; ethanol: 78.7 hPa) [
41,
42]. Additionally, the decreasing molecular size of these analytes as well as their increasing relative permittivity (1-butanol: 17.8; 1-propanol: 20.1; ethanol: 25.3) [
41,
42] may contribute to the observed overall decrease in sensitivity. However, a closer inspection of the data reveals that the sensitivities of the sensors printed with the 9DT+L inks to 1-butanol, 1-propanol, and ethanol, increase in this order with respect to that of sensor S-9DT. For example, sensor S-TG.50, printed with the 9DT+TG (1:1) ink formulation, shows even higher sensitivities to 1-propanol and ethanol than sensor S-9DT. In general, this behavior confirms the expected increasing hydrophilic character of the GNP films printed with the monothiol-blended inks. This trend is even more obvious when considering the responses to water vapor. Here, both transducers printed with the 9DT+TG inks show significantly higher sensitivity to water than sensor S-9DT. Obviously, blending the TG ligand functionalized with two hydroxyl groups into the 9DT ink (cf.
Table 1) tuned the chemical selectivity of the chemiresistor most effectively toward polar hydrophilic analytes.
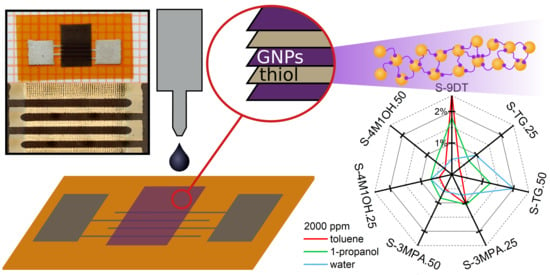
Figure 7 presents radar plots of the sensor responses to toluene, 1-propanol, and water at vapor concentrations of 2000 ppm and 100 ppm. The radar plots clearly demonstrate that the selectivity of the sensors was successfully tuned by blending different monothiols into the 9DT ink. Note, if the selectivity of the sensors would have been unaffected by using different ink formulations and if only the sensitivities of the sensors would have been affected, the radar plots would form distorted heptagons, which would have different sizes but still the same shape for the different analytes. Obviously, the radar plots presented in
Figure 7 show very distinct shapes for the three analytes. Thus, these radar plots clearly demonstrate the successful tuning of the sensors’ selectivity as well as the capability of the sensor array to differentiate between the analytes. Furthermore, although above discussed response isotherms are non-linear, the radar plots referring to the same analyte at very different concentrations are still very similar and can clearly be recognized (
Figure 7a,b).