Ionic Liquid-Based Quartz Crystal Microbalance Sensors for Organic Vapors: A Tutorial Review
Abstract
:1. Introduction
2. Operating Principle of a QCM Sensor
3. Ionic Liquids
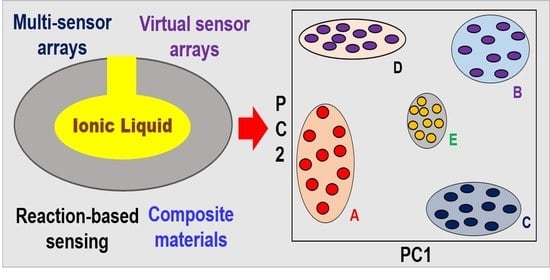
4. Advancements in Ionic Liquid-Based Materials for QCM Sensors
4.1. Ionic Liquid-Based QCM Sensors and Multi-Sensor Arrays
4.2. Reaction-Based Sensing Ionic Liquids for QCM Sensors
4.3. Ionic Liquid-Based QCM Virtual Sensor Arrays
4.4. Ionic Liquid-Like Materials for QCM Sensors and Multi-Sensor Arrays
5. Conclusions and Future Perspectives
Funding
Conflicts of Interest
Appendix A
| VOCs or Complex Mixtures Analyzed | QCM Sensor Type | Reference |
|---|---|---|
| Acetone, acetonitrile, 1-butanol, chloroform, dichloromethane, ethanol, ethyl acetate, methanol, 2-propanol, tetrahydrofuran, and toluene. | Single sensor | Liang et al., 2002 [37] |
| Acetonitrile, cyclohexane, isooctane, methanol, tetrahydrofuran, and toluene. | Single sensor | Goubaidoulline et al., 2005 [73] |
| Methanol, ethanol, n-propanol, and n-butanol. | Multi-sensor array | Seyama et al., 2006 [74] |
| Benzene, dichloromethane, ethanol, and heptane. | Multi-sensor array | Jin et al., 2006 [75] |
| Methane | Single sensor | Jin et al., 2008 [84] |
| Acetone, benzene, dichloromethane, ethanol, methanol, propanol, and toluene. | Single sensor | Xu et al., 2008 [78] |
| Acetone, dichloromethane, ethanol, and toluene. | Multi-sensor array | Xu et al., 2009 [79] |
| Dichloromethane, ethanol, hexane, and water. | Multichannel monolithic sensor array | Jin et al., 2009 [77] |
| Acetone, benzene, cyclohexane, ethanol, hexane, pyridine, and toluene. | Single sensor | Ji et al., 2010 [86] |
| Benzene, formaldehyde, methane, and natural gas. | Multi-sensor array | Hou et al., 2011 [85] |
| Acetic acid, acetone, butylamine, butylformate, butyric acid, p-cresol, p-cymene, ethanol, ethylacetate, ethylbutanoate, eugenol, hexane, hexanal, guaiacol, limonene, linalool, menthol, methanol, methylacetate, 3-methyl-2-butanone, methyl-isopropylketone, i-octane, octylamine, pentanal, phenol, propanal, 1-propanol, 2-propanol, propionic acid, propylamine, and α-terpineol; headspace from Cinnamon zeylanicum and Cinnamon cassia. | Multi-sensor array | Toniolo et al., 2013 [80] |
| Benzaldehyde, 2-butanone, butyraldehyde, formaldehyde, formic acid, propionaldehyde, and propylamine | Single sensor | Tseng and Chu 2010 [87] |
| Acetone, 2-butanone, cycloheptanone, cylohexanone, cyclopentanone, 3-pentanone, and propionaldehyde. | Single sensor | Liu et al., 2013 [89] |
| Allyl azide, benzyl azide, butyl azide, pentyl azide, phenyl azide, and propyl azide. | Single sensor | Tseng and Chu 2014 [88] |
| Acetone, acetophenone, acrolein, benzaldehyde, butyraldehyde, crotonaldehyde, cyclohexanone, 2-cyclohexen-1-one, cyclopentanone, p-fluorobenzaldehyde, formaldehyde, isobutyraldehyde, 3-methylcrotonaldehyde, pivalaldehyde, p-tolualdehyde, and valeraldehyde. | Single sensor | Li et al., 2015 [91] |
| Acrolein, acryloyl chloride, cyclopentadiene, cyclopentene, methyl acrylate, 1-pentene, and propylamine. | Single sensor | Hsu et al., 2016 [92] |
| Dimethylamine, ethanol, ethylamine, ethylmethylamine, isoamylamine, isobutylamine, isopropylamine, 2-methoxyethylamine, and propylamine. | Single sensor | Li and Chu 2020 [93] |
| Acetone, acetonitrile, 1-butanol, chloroform, ethanol, methanol, 3-methyl-1-butanol, nitromethane, 1-propanol, 2-propanol, and toluene. | Single sensor | Regmi et al., 2015 [107] |
| Benzene, chloroform, dichloromethane, ethanol, hexane, heptane, methanol, 1-propanol, toluene, and xylenes. | Multi-sensor array | Vaughan et al., 2018 [108] |
| Acetone, acetonitrile, chloroform, ethanol, methanol, 1-propanol, tetrachloromethane, and toluene. | Virtual sensor array | Regmi et al., 2012 [99] |
| Acetone, acetonitrile, chloroform, dichloromethane, ethanol, ethyl acetate, methanol, nitromethane, 2-propanol, toluene, and p-xylene. | Virtual sensor array | Regmi et al., 2014 [100] |
| Methanol, ethanol, 1-propanol, 1-butanol, 1-pentanol, 1-hexanol, 1-octanol, dichloromethane, chloroform, tetrachloromethane, 1,2-dichloroethane, 1-chlorohexane, toluene, p-xylene, cyclohexane, n-hexane, acetonitrile, and acrylonitrile; petroleum ether and kerosene. | Virtual sensor array | Speller et al., 2015 [98] |
| Lemon, lemon eucalyptus, lemongrass, lime, and orange essential oils. | Virtual multi-sensor array | Speller et al., 2016 [102] |
| Methanol, ethanol, 1-propanol, 2-propanol, 1-butanol, 2-butanol, 3-methyl-1-butanol, and 1-hexanol. | Virtual sensor array | Speller et al., 2017 [101] |
| Diesel, gasoline, kerosene, and petroleum ether; three gasoline grades including Exxon plus, Exxon regular, and Exxon supreme; samples of gasoline adulterated with methanol, ethanol, toluene, and xylene. | Virtual multi-sensor array | Speller et al., 2017 [103] |
| Dichloromethane, chloroform, chloropropane, chlorobutane, and tetrachloromethane. | Multi-sensor array and virtual sensor array | Vaughan 2020 [104] |
References
- U.S. Environmental Protection Agency. Technical Overview of Volatile Organic Compound. 2017. Available online: https://www.epa.gov/indoor-air-quality-iaq/technical-overview-volatile-organic-compounds (accessed on 29 June 2021).
- Helen, G.S.; Jacob, P.; Peng, M.; Dempsey, D.A.; Hammond, S.K.; Benowitz, N.L. Intake of toxic and carcinogenic volatile organic compounds from secondhand smoke in motor vehicles. Cancer Epidemiol. Prev. Biomark. 2014, 23, 2774–2782. [Google Scholar] [CrossRef] [Green Version]
- Schlosser, P.M.; Bale, A.S.; Gibbons, C.F.; Wilkins, A.; Cooper, G.S. Human health effects of dichloromethane: Key findings and scientific issues. Environ. Health Perspect. 2015, 123, 114–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohura, T.; Amagai, T.; Shen, X.; Li, S.; Zhang, P.; Zhu, L. Comparative study on indoor air quality in Japan and China: Characteristics of residential indoor and outdoor VOCs. Atmos. Environ. 2009, 43, 6352–6359. [Google Scholar] [CrossRef]
- Thazin, Y.; Eamsa-Ard, T.; Pobkrut, T.; Kerdcharoen, T. Formalin Adulteration Detection in Food Using E-Nose Based on Nanocomposite Gas Sensors; IEEE: Piscataway, NJ, USA, 2019; pp. 64–67. [Google Scholar]
- Agarwal, S.; Prajapati, Y.K.; Mishra, V. Thinned fibre Bragg grating as a fuel adulteration sensor: Simulation and experimental study. Opto Electron. Rev. 2015, 23, 231–238. [Google Scholar] [CrossRef]
- Torres-Tello, J.; Guaman, A.V.; Ko, S.-B. Improving the Detection of Explosives in a MOX Chemical Sensors Array with LSTM Networks. IEEE Sens. J. 2020, 20, 14302–14309. [Google Scholar] [CrossRef]
- Palma, S.I.C.J.; Traguedo, A.P.; Porteira, A.R.; Frias, M.J.; Gamboa, H.; Roque, A.C.A. Machine learning for the meta-analyses of microbial pathogens’ volatile signatures. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Paul, R.; Tis, T.B.; Saville, A.C.; Hansel, J.C.; Yu, T.; Ristaino, J.B.; Wei, Q. Non-invasive plant disease diagnostics enabled by smartphone-based fingerprinting of leaf volatiles. Nat. Plants 2019, 5, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Nakhleh, M.K.; Broza, Y.Y.; Haick, H. Monolayer-capped gold nanoparticles for disease detection from breath. Nanomedicine 2014, 9, 1991–2002. [Google Scholar] [CrossRef]
- Nakhleh, M.K.; Amal, H.; Jeries, R.; Broza, Y.Y.; Aboud, M.; Gharra, A.; Ivgi, H.; Khatib, S.; Badarneh, S.; Har-Shai, L. Diagnosis and classification of 17 diseases from 1404 subjects via pattern analysis of exhaled molecules. ACS Nano 2017, 11, 112–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, J.W.; Bartlett, P.N. A brief history of electronic noses. Sens. Actuators B Chem. 1994, 18, 210–211. [Google Scholar] [CrossRef]
- Martın, Y.G.; Oliveros, M.C.C.; Pavón, J.L.P.; Pinto, C.G.; Cordero, B.M. Electronic nose based on metal oxide semiconductor sensors and pattern recognition techniques: Characterisation of vegetable oils. Anal. Chim. Acta 2001, 449, 69–80. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020. [Google Scholar] [CrossRef]
- Julian, T.; Hidayat, S.N.; Rianjanu, A.; Dharmawan, A.B.; Wasisto, H.S.; Triyana, K. Intelligent Mobile Electronic Nose System Comprising a Hybrid Polymer-Functionalized Quartz Crystal Microbalance Sensor Array. ACS Omega 2020, 5, 29492–29503. [Google Scholar] [CrossRef]
- Fan, X.; Du, B. Selective detection of trace p-xylene by polymer-coated QCM sensors. Sens. Actuators B Chem. 2012, 166, 753–760. [Google Scholar] [CrossRef]
- Khot, L.R.; Panigrahi, S.; Lin, D. Development and evaluation of piezoelectric-polymer thin film sensors for low concentration detection of volatile organic compounds related to food safety applications. Sens. Actuators B Chem. 2011, 153, 1–10. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Uno, T. Molecular imprinting strategy for solvent molecules and its application for QCM-based VOC vapor sensing. Sens. Actuators B Chem. 2006, 113, 94–99. [Google Scholar] [CrossRef]
- Jha, S.K.; Liu, C.; Hayashi, K. Molecular imprinted polyacrylic acids based QCM sensor array for recognition of organic acids in body odor. Sens. Actuators B Chem. 2014, 204, 74–87. [Google Scholar] [CrossRef]
- Xu, F.; Sun, L.; Huang, P.; Sun, Y.; Zheng, Q.; Zou, Y.; Chu, H.; Yan, E.; Zhang, H.; Wang, J. A pyridine vapor sensor based on metal-organic framework-modified quartz crystal microbalance. Sens. Actuators B Chem. 2018, 254, 872–877. [Google Scholar] [CrossRef]
- Ma, Z.; Yuan, T.; Fan, Y.; Wang, L.; Duan, Z.; Du, W.; Zhang, D.; Xu, J. A benzene vapor sensor based on a metal-organic framework-modified quartz crystal microbalance. Sens. Actuators B Chem. 2020, 311, 127365. [Google Scholar] [CrossRef]
- Penza, M.; Cassano, G.; Aversa, P.; Cusano, A.; Cutolo, A.; Giordano, M.; Nicolais, L. Carbon nanotube acoustic and optical sensors for volatile organic compound detection. Nanotechnology 2005, 16, 2536. [Google Scholar] [CrossRef]
- Consales, M.; Cutolo, A.; Penza, M.; Aversa, P.; Cassano, G.; Giordano, M.; Cusano, A. Carbon nanotubes coated acoustic and optical VOCs sensors: Towards the tailoring of the sensing performances. IEEE Trans. Nanotechnol. 2007, 6, 601–612. [Google Scholar] [CrossRef]
- Schlupp, M.; Weil, T.; Berresheim, A.J.; Wiesler, U.M.; Bargon, J.; Müllen, K. Polyphenylene dendrimers as sensitive and selective sensor layers. Angew. Chem. Int. Ed. 2001, 40, 4011–4015. [Google Scholar] [CrossRef]
- Lubczyk, D.; Siering, C.; Lörgen, J.; Shifrina, Z.B.; Müllen, K.; Waldvogel, S.R. Simple and sensitive online detection of triacetone triperoxide explosive. Sens. Actuators B Chem. 2010, 143, 561–566. [Google Scholar] [CrossRef]
- Brunink, J.A.J.; Di Natale, C.; Bungaro, F.; Davide, F.A.M.; D’Amico, A.; Paolesse, R.; Boschi, T.; Faccio, M.; Ferri, G. The application of metalloporphyrins as coating material for quartz microbalance-based chemical sensors. Anal. Chim. Acta 1996, 325, 53–64. [Google Scholar] [CrossRef]
- Paolesse, R.; Tortora, L.; Monti, D.; Nardis, S.; Stefanelli, M.; D’Amico, A.; Di Natale, C. Dip and wait: A facile route to nanostructured porphyrin films for QCM functionalization. Procedia Chem. 2009, 1, 180–183. [Google Scholar] [CrossRef]
- Selyanchyn, R.; Korposh, S.; Wakamatsu, S.; Lee, S.-W. Respiratory monitoring by porphyrin modified quartz crystal microbalance sensors. Sensors 2011, 11, 1177–1191. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Brunet, J.; Varenne, C.; Ndiaye, A.; Pauly, A.; Penza, M.; Alvisi, M. Tetra-tert-butyl copper phthalocyanine-based QCM sensor for toluene detection in air at room temperature. Sens. Actuators B Chem. 2015, 210, 398–407. [Google Scholar] [CrossRef]
- Harbeck, M.; Erbahar, D.D.; Gürol, I.; Musluoğlu, E.; Ahsen, V.; Öztürk, Z.Z. Phthalocyanines as sensitive coatings for QCM sensors: Comparison of gas and liquid sensing properties. Sens. Actuators B Chem. 2011, 155, 298–303. [Google Scholar] [CrossRef]
- Harbeck, S.; Göçmen, S.; Emirik, Ö.; Öztürk, Z.Z.; Ahsen, V.; Gürek, A.G. Synthesis of branched alkoxy side chains containing phthalocyanine derivates and their application in mass sensitive QCM sensors. Sens. Actuators B Chem. 2016, 233, 55–62. [Google Scholar] [CrossRef]
- Temel, F.; Tabakci, M. Calix [4] arene coated QCM sensors for detection of VOC emissions: Methylene chloride sensing studies. Talanta 2016, 153, 221–227. [Google Scholar] [CrossRef]
- Holloway, A.F.; Nabok, A.; Hashim, A.; Penders, J. The use of calixarene thin films in the sensor array for VOCs detection and olfactory navigation. Sens. Transducers 2010, 113, 71–81. [Google Scholar]
- Ishii, R.; Naganawa, R.; Nishioka, M.; Hanaoka, T.-A. Microporous organic–inorganic nanocomposites as the receptor in the QCM sensing of toluene vapors. Anal. Sci. 2013, 29, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Quy, N.; Hung, T.M.; Thong, T.Q.; Huy, T.Q.; Hoa, N.D. Novel synthesis of highly ordered mesoporous Fe2O3/SiO2 nanocomposites for a room temperature VOC sensor. Curr. Appl. Phys. 2013, 13, 1581–1588. [Google Scholar] [CrossRef]
- Zhang, K.; Hu, R.; Fan, G.; Li, G. Graphene oxide/chitosan nanocomposite coated quartz crystal microbalance sensor for detection of amine vapors. Sens. Actuators B Chem. 2017, 243, 721–730. [Google Scholar] [CrossRef]
- Liang, C.; Yuan, C.-Y.; Warmack, R.J.; Barnes, C.E.; Dai, S. Ionic liquids: A new class of sensing materials for detection of organic vapors based on the use of a quartz crystal microbalance. Anal. Chem. 2002, 74, 2172–2176. [Google Scholar] [CrossRef]
- Yu, L.; Garcia, D.; Ren, R.; Zeng, X. Ionic liquid high temperature gas sensors. Chem. Commun. 2005, 17, 2277–2279. [Google Scholar] [CrossRef]
- Fauzi, F.; Rianjanu, A.; Santoso, I.; Triyana, K. Gas and humidity sensing with quartz crystal microbalance (QCM) coated with graphene-based materials–A mini review. Sens. Actuators A Phys. 2021, 330, 112837. [Google Scholar] [CrossRef]
- Pérez, R.L.; Ayala, C.E.; Park, J.-Y.; Choi, J.-W.; Warner, I.M. Coating-Based Quartz Crystal Microbalance Detection Methods of Environmentally Relevant Volatile Organic Compounds. Chemosensors 2021, 9, 153. [Google Scholar] [CrossRef]
- Wang, L. Metal-organic frameworks for QCM-based gas sensors: A review. Sens. Actuators A Phys. 2020, 307, 111984. [Google Scholar] [CrossRef]
- Hekiem, N.L.L.; Ralib, A.A.M.; Ahmad, F.; Nordin, A.N.; Ab Rahim, R.; Za’bah, N.F. Advanced vapour sensing materials: Existing and latent to acoustic wave sensors for VOCs detection as the potential exhaled breath biomarkers for lung cancer. Sens. Actuators A Phys. 2021, 329, 112792. [Google Scholar] [CrossRef]
- Torad, N.L.; Zhang, S.; Amer, W.A.; Ayad, M.M.; Kim, M.; Kim, J.; Ding, B.; Zhang, X.; Kimura, T.; Yamauchi, Y. Advanced nanoporous material–based QCM devices: A new horizon of interfacial mass sensing technology. Adv. Mater. Interfaces 2019, 6, 1900849. [Google Scholar] [CrossRef]
- Regmi, B.P. GUMBOS-and Ionic Liquid-Coated Quartz Crystal Microbalance Sensors for Detection and Molecular Weight Determination of Organic Vapors. Ph.D. Dissertation, Louisiana State University, Baton Rouge, LA, USA, 2014. [Google Scholar]
- Lin, Z.; Ward, M.D. The role of longitudinal waves in quartz crystal microbalance applications in liquids. Anal. Chem. 1995, 67, 685–693. [Google Scholar] [CrossRef]
- Muralidharan, V.S. Critical review on electrochemical quartz crystal micro balance-principles and applications to corrosion research. Bull. Electrochem. 2001, 17, 183–192. [Google Scholar]
- Ogi, H. Wireless-electrodeless quartz-crystal-microbalance biosensors for studying interactions among biomolecules: A review. Proc. Jpn. Acad. Ser. B 2013, 89, 401–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauerbrey, G. The use of quarts oscillators for weighing thin layers and for microweighing. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Alassi, A.; Benammar, M.; Brett, D. Quartz crystal microbalance electronic interfacing systems: A review. Sensors 2017, 17, 2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanazawa, K.K.; Gordon Ii, J.G. The oscillation frequency of a quartz resonator in contact with liquid. Anal. Chim. Acta 1985, 175, 99–105. [Google Scholar] [CrossRef]
- Dixon, M.C. Quartz crystal microbalance with dissipation monitoring: Enabling real-time characterization of biological materials and their interactions. J. Biomol. Tech. JBT 2008, 19, 151. [Google Scholar]
- Rodahl, M.; Höök, F.; Krozer, A.; Brzezinski, P.; Kasemo, B. Quartz crystal microbalance setup for frequency and Q-factor measurements in gaseous and liquid environments. Rev. Sci. Instrum. 1995, 66, 3924–3930. [Google Scholar] [CrossRef] [Green Version]
- Bandey, H.L.; Martin, S.J.; Cernosek, R.W.; Hillman, A.R. Modeling the responses of thickness-shear mode resonators under various loading conditions. Anal. Chem. 1999, 71, 2205–2214. [Google Scholar] [CrossRef]
- Voinova, M.V.; Jonson, M.; Kasemo, B. Internal and interfacial friction in the dynamics of soft/solid interfaces. arXiv 1999, arXiv:cond-mat/9906415. [Google Scholar]
- Çınar, S.; Schulz, M.D.; Oyola-Reynoso, S.; Bwambok, D.K.; Gathiaka, S.M.; Thuo, M. Application of Ionic Liquids in Pot-in-Pot Reactions. Molecules 2016, 21, 272. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, S.; Kavanagh, A.; Zíołkowski, B.; Florea, L.; MacFarlane, D.R.; Fraser, K.; Diamond, D. Ionic liquid modulation of swelling and LCST behavior of N-isopropylacrylamide polymer gels. Phys. Chem. Chem. Phys. 2014, 16, 3610–3616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowmiah, S.; Srinivasadesikan, V.; Tseng, M.-C.; Chu, Y.-H. On the chemical stabilities of ionic liquids. Molecules 2009, 14, 3780–3813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Mu, T. Comprehensive investigation on the thermal stability of 66 ionic liquids by thermogravimetric analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Freemantle, M. New frontiers for ionic liquids. Chem. Eng. News 2007, 85, 23–26. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Hantao, L.W.; Najafi, A.; Zhang, C.; Augusto, F.; Anderson, J.L. Tuning the selectivity of ionic liquid stationary phases for enhanced separation of nonpolar analytes in kerosene using multidimensional gas chromatography. Anal. Chem. 2014, 86, 3717–3721. [Google Scholar] [CrossRef]
- Gao, T.; Andino, J.M.; Alvarez-Idaboy, J.R. Computational and experimental study of the interactions between ionic liquids and volatile organic compounds. Phys. Chem. Chem. Phys. 2010, 12, 9830–9838. [Google Scholar] [CrossRef]
- Anderson, J.L.; Armstrong, D.W. High-stability ionic liquids. A new class of stationary phases for gas chromatography. Anal. Chem. 2003, 75, 4851–4858. [Google Scholar] [CrossRef] [Green Version]
- Poole, C.F.; Poole, S.K. Ionic liquid stationary phases for gas chromatography. J. Sep. Sci. 2011, 34, 888–900. [Google Scholar] [CrossRef]
- Collin, W.R.; Bondy, A.; Paul, D.; Kurabayashi, K.; Zellers, E.T. μGC × μGC: Comprehensive two-dimensional gas chromatographic separations with microfabricated components. Anal. Chem. 2015, 87, 1630–1637. [Google Scholar] [CrossRef]
- Regmi, B.P.; Chan, R.; Agah, M. Ionic liquid functionalization of semi-packed columns for high-performance gas chromatographic separations. J. Chromatogr. A 2017, 1510, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Cagliero, C.; Bicchi, C. Ionic liquids as gas chromatographic stationary phases: How can they change food and natural product analyses? Anal. Bioanal. Chem. 2020, 412, 17–25. [Google Scholar] [CrossRef]
- Wei, D.; Ivaska, A. Applications of ionic liquids in electrochemical sensors. Anal. Chim. Acta 2008, 607, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.W.; Zhang, L.-K.; He, L.; Gross, M.L. Ionic liquids as matrixes for matrix-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 2001, 73, 3679–3686. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wang, H.; Zhao, Y.; Guan, P.; Li, Z.; Zhang, H.; Gao, H.; Zhang, S.; Wang, J. Hierarchically porous covalent organic frameworks assembled in ionic liquids for highly effective catalysis of C–C coupling reactions. Green Chem. 2020, 22, 2605–2612. [Google Scholar] [CrossRef]
- Skoronski, E.; Fernandes, M.; Malaret, F.J.; Hallett, J.P. Use of phosphonium ionic liquids for highly efficient extraction of phenolic compounds from water. Sep. Purif. Technol. 2020, 248, 117069. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Goubaidoulline, I.; Vidrich, G.; Johannsmann, D. Organic vapor sensing with ionic liquids entrapped in alumina nanopores on quartz crystal resonators. Anal. Chem. 2005, 77, 615–619. [Google Scholar] [CrossRef]
- Seyama, M.; Iwasaki, Y.; Tate, A.; Sugimoto, I. Room-temperature ionic-liquid-incorporated plasma-deposited thin films for discriminative alcohol-vapor sensing. Chem. Mater. 2006, 18, 2656–2662. [Google Scholar] [CrossRef]
- Jin, X.; Yu, L.; Garcia, D.; Ren, R.X.; Zeng, X. Ionic liquid high-temperature gas sensor array. Anal. Chem. 2006, 78, 6980–6989. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Hamilton, A.; Chung, A.; Baker, G.A.; Wang, Z.; Zeng, X. Differential solute gas response in ionic-liquid-based QCM arrays: Elucidating design factors responsible for discriminative explosive gas sensing. Anal. Chem. 2011, 83, 7823–7833. [Google Scholar] [CrossRef]
- Jin, X.; Huang, Y.; Mason, A.; Zeng, X. Multichannel monolithic quartz crystal microbalance gas sensor array. Anal. Chem. 2009, 81, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, C.; Pei, K.; Zhao, K.; Zhao, Z.K.; Li, H. Ionic liquids used as QCM coating materials for the detection of alcohols. Sens. Actuators B Chem. 2008, 134, 258–265. [Google Scholar] [CrossRef]
- Xu, X.; Cang, H.; Li, C.; Zhao, Z.K.; Li, H. Quartz crystal microbalance sensor array for the detection of volatile organic compounds. Talanta 2009, 78, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, R.; Pizzariello, A.; Dossi, N.; Lorenzon, S.; Abollino, O.; Bontempelli, G. Room temperature ionic liquids as useful overlayers for estimating food quality from their odor analysis by quartz crystal microbalance measurements. Anal. Chem. 2013, 85, 7241–7247. [Google Scholar] [CrossRef]
- Kachoosangi, R.T.; Musameh, M.M.; Abu-Yousef, I.; Yousef, J.M.; Kanan, S.M.; Xiao, L.; Davies, S.G.; Russell, A.; Compton, R.G. Carbon nanotube−ionic liquid composite sensors and biosensors. Anal. Chem. 2009, 81, 435–442. [Google Scholar] [CrossRef]
- Bara, J.E.; Camper, D.E.; Gin, D.L.; Noble, R.D. Room-temperature ionic liquids and composite materials: Platform technologies for CO2 capture. Acc. Chem. Res. 2010, 43, 152–159. [Google Scholar] [CrossRef]
- Correia, D.M.; Fernandes, L.C.; Martins, P.M.; García-Astrain, C.; Costa, C.M.; Reguera, J.; Lanceros-Méndez, S. Ionic liquid–polymer composites: A new platform for multifunctional applications. Adv. Funct. Mater. 2020, 30, 1909736. [Google Scholar] [CrossRef]
- Jin, X.; Yu, L.; Zeng, X. Enhancing the sensitivity of ionic liquid sensors for methane detection with polyaniline template. Sens. Actuators B Chem. 2008, 133, 526–532. [Google Scholar] [CrossRef]
- Hou, K.-Y.; Rehman, A.; Zeng, X. Study of ionic liquid immobilization on polyvinyl ferrocene substrates for gas sensor arrays. Langmuir 2011, 27, 5136–5146. [Google Scholar] [CrossRef]
- Ji, Q.; Honma, I.; Paek, S.M.; Akada, M.; Hill, J.P.; Vinu, A.; Ariga, K. Layer-by-layer films of graphene and ionic liquids for highly selective gas sensing. Angew. Chem. Int. Ed. 2010, 49, 9737–9739. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.-C.; Chu, Y.-H. Chemoselective gas sensing ionic liquids. Chem. Commun. 2010, 46, 2983–2985. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.-C.; Chu, Y.-H. Reaction-based azide gas sensing with tailored ionic liquids measured by quartz crystal microbalance. Anal. Chem. 2014, 86, 1949–1952. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Tseng, M.-C.; Chu, Y.-H. Sensing ionic liquids for chemoselective detection of acyclic and cyclic ketone gases. Chem. Commun. 2013, 49, 2560–2562. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-P.; Liu, W.-C.; Tseng, M.-C.; Chu, Y.-H. Ionic liquids tailored for reaction-based gas sensing on quartz crystal microbalance. Rev. Anal. Chem. 2015, 34, 77–86. [Google Scholar] [CrossRef]
- Li, H.-Y.; Hsu, T.-H.; Chen, C.-Y.; Tseng, M.-C.; Chu, Y.-H. Exploring silver ionic liquids for reaction-based gas sensing on a quartz crystal microbalance. Analyst 2015, 140, 6245–6249. [Google Scholar] [CrossRef] [Green Version]
- Hsu, T.-H.; Chiang, S.-J.; Chu, Y.-H. Quartz Crystal Microbalance Analysis of Diels–Alder Reactions of Alkene Gases to Functional Ionic Liquids on Chips. Anal. Chem. 2016, 88, 10837–10841. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Chu, Y.-H. Reaction-Based Amine and Alcohol Gases Detection with Triazine Ionic Liquid Materials. Molecules 2020, 25, 104. [Google Scholar] [CrossRef] [Green Version]
- Peveler, W.J.; Yazdani, M.; Rotello, V.M. Selectivity and specificity: Pros and cons in sensing. ACS Sens. 2016, 1, 1282–1285. [Google Scholar] [CrossRef] [Green Version]
- Länge, K. Bulk and surface acoustic wave sensor arrays for multi-analyte detection: A review. Sensors 2019, 19, 5382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potyrailo, R.A. Multivariable sensors for ubiquitous monitoring of gases in the era of internet of things and industrial internet. Chem. Rev. 2016, 116, 11877–11923. [Google Scholar] [CrossRef] [PubMed]
- Potyrailo, R.A. Toward high value sensing: Monolayer-protected metal nanoparticles in multivariable gas and vapor sensors. Chem. Soc. Rev. 2017, 46, 5311–5346. [Google Scholar] [CrossRef] [PubMed]
- Speller, N.C.; Siraj, N.; Regmi, B.P.; Marzoughi, H.; Neal, C.; Warner, I.M. Rational Design of QCM-D Virtual Sensor Arrays Based on Film Thickness, Viscoelasticity, and Harmonics for Vapor Discrimination. Anal. Chem. 2015, 87, 5156–5166. [Google Scholar] [CrossRef]
- Regmi, B.P.; Monk, J.; El-Zahab, B.; Das, S.; Hung, F.R.; Hayes, D.J.; Warner, I.M. A novel composite film for detection and molecular weight determination of organic vapors. J. Mater. Chem. 2012, 22, 13732–13741. [Google Scholar] [CrossRef]
- Regmi, B.P.; Speller, N.C.; Anderson, M.J.; Brutus, J.O.; Merid, Y.; Das, S.; El-Zahab, B.; Hayes, D.J.; Murray, K.K.; Warner, I.M. Molecular weight sensing properties of ionic liquid-polymer composite films: Theory and experiment. J. Mater. Chem. C 2014, 2, 4867–4878. [Google Scholar] [CrossRef]
- Speller, N.C.; Siraj, N.; McCarter, K.S.; Vaughan, S.; Warner, I.M. QCM virtual sensor array: Vapor identification and molecular weight approximation. Sens. Actuators B Chem. 2017, 246, 952–960. [Google Scholar] [CrossRef]
- Speller, N.C.; Siraj, N.; Vaughan, S.; Speller, L.N.; Warner, I.M. Assessment of QCM array schemes for mixture identification: Citrus scented odors. RSC Adv. 2016, 6, 95378–95386. [Google Scholar] [CrossRef]
- Speller, N.C.; Siraj, N.; Vaughan, S.; Speller, L.N.; Warner, I.M. QCM virtual multisensor array for fuel discrimination and detection of gasoline adulteration. Fuel 2017, 199, 38–46. [Google Scholar] [CrossRef]
- Vaughan, S.R.; Pérez, R.L.; Chhotaray, P.; Warner, I.M. Quartz Crystal Microbalance Based Sensor Arrays for Detection and Discrimination of VOCs Using Phosphonium Ionic Liquid Composites. Sensors 2020, 20, 615. [Google Scholar] [CrossRef] [Green Version]
- Seddon, K.R.; Stark, A.; Torres, M.-J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2275–2287. [Google Scholar] [CrossRef]
- Aleixandre, M.; Nakamoto, T. Study of Room Temperature Ionic Liquids as Gas Sensing Materials in Quartz Crystal Microbalances. Sens. Basel 2020, 20, 4026. [Google Scholar] [CrossRef]
- Regmi, B.P.; Galpothdeniya, W.I.S.; Siraj, N.; Webb, M.H.; Speller, N.C.; Warner, I.M. Phthalocyanine-and porphyrin-based GUMBOS for rapid and sensitive detection of organic vapors. Sens. Actuators B Chem. 2015, 209, 172–179. [Google Scholar] [CrossRef]
- Vaughan, S.R.; Speller, N.C.; Chhotaray, P.; McCarter, K.S.; Siraj, N.; Pérez, R.L.; Li, Y.; Warner, I.M. Class specific discrimination of volatile organic compounds using a quartz crystal microbalance based multisensor array. Talanta 2018, 188, 423–428. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, X.; Zhao, W.; Sun, J.; Tang, Q.; Du, Y.; Jia, H.; Ji, Q. Highly Sensitive Gas-Sensing Films for Volatile Organic Acids from Imidazolium-Based Poly (ionic liquid) s. J. Nanosci. Nanotechnol. 2020, 20, 3588–3597. [Google Scholar] [CrossRef]
- Sipka, R.; Vlcek, J.; Otta, J.; Hruska, M.; Tomecek, D.; Fitl, P.; Vrnata, M. Polymer Ionic Liquids as Perspective Materials for Chemiresistors and QCM Sensors; IOP Publishing: Bristol, UK, 2020; p. 2145. [Google Scholar]
- Çapan, İ.; Tarımcı, Ç.; Capan, R. Fabrication of Langmuir–Blodgett thin films of porphyrins and investigation on their gas sensing properties. Sens. Actuators B Chem. 2010, 144, 126–130. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regmi, B.P.; Adhikari, P.L.; Dangi, B.B. Ionic Liquid-Based Quartz Crystal Microbalance Sensors for Organic Vapors: A Tutorial Review. Chemosensors 2021, 9, 194. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9080194
Regmi BP, Adhikari PL, Dangi BB. Ionic Liquid-Based Quartz Crystal Microbalance Sensors for Organic Vapors: A Tutorial Review. Chemosensors. 2021; 9(8):194. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9080194
Chicago/Turabian StyleRegmi, Bishnu P., Puspa L. Adhikari, and Beni B. Dangi. 2021. "Ionic Liquid-Based Quartz Crystal Microbalance Sensors for Organic Vapors: A Tutorial Review" Chemosensors 9, no. 8: 194. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9080194