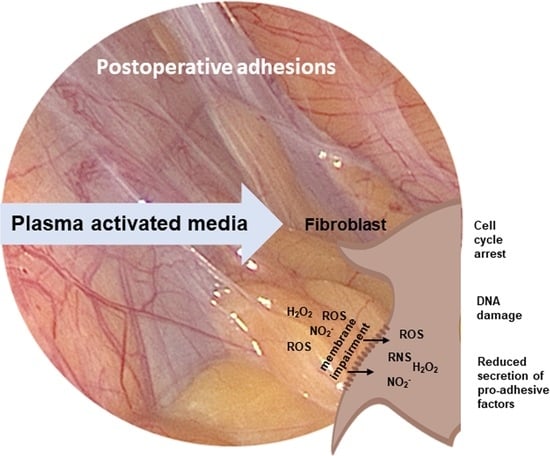
Cell Type-Specific Anti-Adhesion Properties of Peritoneal Cell Treatment with Plasma-Activated Media (PAM)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of Plasma-Activated Medium (PAM)
2.3. Cell Confluency Assay
2.4. Raman Imaging
2.5. Principal Component Analysis (PCA)
2.6. Viability Assay
2.7. Flow Cytometry
2.8. Protein Expression Analysis by DigiWest Multiplex Protein Profiling
2.9. Apoptosis; Caspase 3/7 Assay
2.10. DNA Methylation; 5mC Staining
2.11. Western Blot
2.12. Hydroxyproline Assay
2.13. Soluble Collagen Assay
2.14. Matrix Metalloproteinases (MMPs) Assay
2.15. Cytokine Multiplex Assay
2.16. Statistical Analysis
3. Results
3.1. Cell Type-Specific Anti-Proliferation
3.2. PAM Treatment Induces Molecular Alterations of Essential Cell Components while Maintaining Cell Morphology
3.3. PAM Induces Cell Type-Specific Anti-Proliferative Signaling
3.4. PAM Treatment Reduces Pro-Adhesive Protein Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paolo, N.D.; Nicolai, G.A.; Garosi, G. The Peritoneum: From Histological Studies to Mesothelial Transplant through Animal Experimentation. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2008, 28, 5–9. [Google Scholar] [CrossRef]
- Capobianco, A.; Cottone, L.; Monno, A.; Manfredi, A.A.; Rovere-Querini, P. The peritoneum: Healing, immunity, and diseases. J. Pathol. 2017, 243, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Beyene, R.T.; Kavalukas, S.L.; Barbul, A. Intra-abdominal adhesions: Anatomy, physiology, pathophysiology, and treatment. Curr. Probl. Surg. 2015, 52, 271–319. [Google Scholar] [CrossRef]
- Bruggmann, D.; Tchartchian, G.; Wallwiener, M.; Munstedt, K.; Tinneberg, H.R.; Hackethal, A. Intra-abdominal adhesions: Definition, origin, significance in surgical practice, and treatment options. Dtsch. Arztebl. Int. 2010, 107, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.; Weiser, T.G.; Hider, P.; Wilson, L.; Gruen, R.L.; Bickler, S.W. Estimated need for surgery worldwide based on prevalence of diseases: A modelling strategy for the WHO Global Health Estimate. Lancet Glob. Health 2015, 3, S13–S20. [Google Scholar] [CrossRef]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxidative Med. Cell. Longev. 2019, 2019, 3873928. [Google Scholar] [CrossRef]
- Weiss, M.; Barz, J.; Ackermann, M.; Utz, R.; Ghoul, A.; Weltmann, K.D.; Stope, M.B.; Wallwiener, D.; Schenke-Layland, K.; Oehr, C.; et al. Dose-Dependent Tissue-Level Characterization of a Medical Atmospheric Pressure Argon Plasma Jet. ACS Appl. Mater. Interfaces 2019, 11, 19841–19853. [Google Scholar] [CrossRef]
- Holl, M.; Becker, L.; Keller, A.L.; Feuerer, N.; Marzi, J.; Carvajal Berrio, D.A.; Jakubowski, P.; Neis, F.; Pauluschke-Frohlich, J.; Brucker, S.Y.; et al. Laparoscopic Peritoneal Wash Cytology-Derived Primary Human Mesothelial Cells for In Vitro Cell Culture and Simulation of Human Peritoneum. Biomedicines 2021, 9, 176. [Google Scholar] [CrossRef]
- Weiss, M.; Utz, R.; Ackermann, M.; Taran, F.A.; Krämer, B.; Hahn, M.; Wallwiener, D.; Brucker, S.; Haupt, M.; Barz, J. Characterization of a non-thermally operated electrosurgical argon plasma source by electron spin resonance spectroscopy. Plasma Process. Polym. 2019, 16, 1800150. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.-D. The kINPen—A review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef]
- Fink, S.; Ruoff, F.; Stahl, A.; Becker, M.; Kaiser, P.; Traenkle, B.; Junker, D.; Weise, F.; Ruetalo, N.; Horber, S.; et al. Multiplexed Serum Antibody Screening Platform Using Virus Extracts from Endemic Coronaviridae and SARS-CoV-2. ACS Infect. Dis. 2021, 7, 1596–1606. [Google Scholar] [CrossRef]
- Milutinovic, S.; Zhuang, Q.; Niveleau, A.; Szyf, M. Epigenomic stress response. Knockdown of DNA methyltransferase 1 triggers an intra-S-phase arrest of DNA replication and induction of stress response genes. J. Biol. Chem. 2003, 278, 14985–14995. [Google Scholar] [CrossRef]
- Capella-Monsonís, H.; Coentro, J.Q.; Graceffa, V.; Wu, Z.; Zeugolis, D.I. An experimental toolbox for characterization of mammalian collagen type I in biological specimens. Nat. Protoc. 2018, 13, 507–529. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Liedek, A.; Shendi, D.; Bach, M.; Tovar, G.E.M.; Kluger, P.J.; Southan, A. Eclectic characterisation of chemically modified cell-derived matrices obtained by metabolic glycoengineering and re-assessment of commonly used methods. RSC Adv. 2020, 10, 35273–35286. [Google Scholar] [CrossRef]
- Wenzel, T.; Carvajal Berrio, D.A.; Daum, R.; Reisenauer, C.; Weltmann, K.D.; Wallwiener, D.; Brucker, S.Y.; Schenke-Layland, K.; Brauchle, E.M.; Weiss, M. Molecular Effects and Tissue Penetration Depth of Physical Plasma in Human Mucosa Analyzed by Contact- and Marker-Independent Raman Microspectroscopy. ACS Appl. Mater. Interfaces 2019, 11, 42885–42895. [Google Scholar] [CrossRef]
- Wenzel, T.; Carvajal Berrio, D.A.; Reisenauer, C.; Layland, S.; Koch, A.; Wallwiener, D.; Brucker, S.Y.; Schenke-Layland, K.; Brauchle, E.M.; Weiss, M. Trans-Mucosal Efficacy of Non-Thermal Plasma Treatment on Cervical Cancer Tissue and Human Cervix Uteri by a Next Generation Electrosurgical Argon Plasma Device. Cancers 2020, 12, 267. [Google Scholar] [CrossRef]
- Shetty, G.K.; Kendall, C.W.C.; Shepherd, N.A.; Stone, N.; Barr, H. Raman spectroscopy: Elucidation of biochemical changes in carcinogenesis of oesophagus. Br. J. Cancer 2006, 94, 1460–1464. [Google Scholar] [CrossRef]
- Chan, J.W.; Taylor, D.S.; Zwerdling, T.; Lane, S.M.; Ihara, K.; Huser, T. Micro-Raman Spectroscopy Detects Individual Neoplastic and Normal Hematopoietic Cells. Biophys. J. 2006, 90, 648–656. [Google Scholar] [CrossRef]
- Krafft, P.R.; Yonas, H.; Carlson, A.P.; Information, P.E.K.F.C. Near-Complete Resolution of Clinical and Radiographic Findings After Endovascular Embolization of a Giant Serpentine A1 Aneurysm. World Neurosurg. 2016, 86, 512.e9–512.e14. [Google Scholar] [CrossRef]
- Sajan, D.; Binoy, J.; Pradeep, B.; Krishna, K.V.; Kartha, V.; Joe, I.; Jayakumar, V. NIR-FT Raman and infrared spectra and ab initio computations of glycinium oxalate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 173–180. [Google Scholar] [CrossRef]
- Stone, N.; Kendall, C.; Smith, J.; Crow, P.; Barr, H. Raman spectroscopy for identification of epithelial cancers. Faraday Discuss. 2004, 126, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; McWilliams, A.; Lui, H.; McLean, D.I.; Lam, S.; Zeng, H. Near-infrared Raman spectroscopy for optical diagnosis of lung cancer. Int. J. Cancer 2003, 107, 1047–1052. [Google Scholar] [CrossRef]
- Stone, N.; Kendall, C.; Shepherd, N.; Crow, P.; Barr, H. Near-infrared Raman spectroscopy for the classification of epithelial pre-cancers and cancers. J. Raman Spectrosc. 2002, 33, 564–573. [Google Scholar] [CrossRef]
- Dukor, R.K. Vibrational Spectroscopy in the Detection of Cancer. In Handbook of Vibrational Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Malini, R.; Venkatakrishna, K.; Kurien, J.; Pai, K.; Rao, L.; Kartha, V.B.; Krishna, C.M. Discrimination of normal, inflammatory, premalignant, and malignant oral tissue: A Raman spectroscopy study. Biopolym. Orig. Res. Biomol. 2006, 81, 179–193. [Google Scholar] [CrossRef]
- Faoláin, E.Ó.; Hunter, M.B.; Byrne, J.M.; Kelehan, P.; McNamara, M.; Byrne, H.J.; Lyng, F.M. A study examining the effects of tissue processing on human tissue sections using vibrational spectroscopy. Vib. Spectrosc. 2005, 38, 121–127. [Google Scholar] [CrossRef]
- Notingher, I.; Green, C.; Dyer, C.; Perkins, E.; Hopkins, N.; Lindsay, C.; Hench, L.L. Discrimination between ricin and sulphur mustard toxicity in vitro using Raman spectroscopy. J. R. Soc. Interface 2004, 1, 79–90. [Google Scholar] [CrossRef]
- Jyothi Lakshmi, R.; Kartha, V.B.; Murali Krishna, C.; Solomon, J.G.R.; Ullas, G.; Uma Devi, P. Tissue Raman Spectroscopy for the Study of Radiation Damage: Brain Irradiation of Mice. Radiat. Res. 2002, 157, 175–182. [Google Scholar] [CrossRef]
- Kachrimanis, K.; Braun, D.; Griesser, U.J. Quantitative analysis of paracetamol polymorphs in powder mixtures by FT-Raman spectroscopy and PLS regression. J. Pharm. Biomed. Anal. 2007, 43, 407–412. [Google Scholar] [CrossRef]
- Lau, D.P.; Huang, Z.; Lui, H.; Man, C.S.; Berean, K.; Morrison, M.D.; Zeng, H. Raman spectroscopy for optical diagnosis in normal and cancerous tissue of the nasopharynx?preliminary findings. Lasers Surg. Med. 2003, 32, 210–214. [Google Scholar] [CrossRef]
- Frank, C.J.; McCreery, R.L.; Redd, D.C.B. Raman Spectroscopy of Normal and Diseased Human Breast Tissues. Anal. Chem. 1995, 67, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Koljenović, S.; Schut, T.B.; Vincent, A.; Kros, J.M.; Puppels, G.J. Detection of Meningioma in Dura Mater by Raman Spectroscopy. Anal. Chem. 2005, 77, 7958–7965. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Chica, A.J.; Medina, M.A.; Sánchez-Jiménez, F.; Ramírez, F.J. Characterization by Raman spectroscopy of conformational changes on guanine–cytosine and adenine–thymine oligonucleotides induced by aminooxy analogues of spermidine. J. Raman Spectrosc. 2004, 35, 93–100. [Google Scholar] [CrossRef]
- Wood, B.R.; Quinn, M.A.; Tait, B.; Ashdown, M.; Hislop, T.; Romeo, M.; McNaughton, D. FTIR microspectroscopic study of cell types and potential confounding variables in screening for cervical malignancies. Biospectroscopy 1998, 4, 75–91. [Google Scholar] [CrossRef]
- Sigurdsson, S.; Philipsen, P.A.; Hansen, L.K.; Larsen, J.; Gniadecka, M.; Wulf, H.C. Detection of Skin Cancer by Classification of Raman Spectra. IEEE Trans. Biomed. Eng. 2004, 51, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Tandon, P.; Gupta, V.D. Phonon dispersion in poly(dimethylsilane). J. Organomet. Chem. 2006, 691, 2902–2908. [Google Scholar] [CrossRef]
- Hanlon, E.B.; Manoharan, R.; Koo, T.-W.; Shafer, K.E.; Motz, J.T.; Fitzmaurice, M.; Kramer, J.R.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Prospects for in vivo Raman spectroscopy. Phys. Med. Biol. 2000, 45, R1–R59. [Google Scholar] [CrossRef]
- Mordechai, S.; Sahu, R.K.; Hammody, Z.; Mark, S.; Kantarovich, K.; Guterman, H.; Podshyvalov, A.; Goldstein, J.; Argov, S. Possible common biomarkers from FTIR microspectroscopy of cervical cancer and melanoma. J. Microsc. 2004, 215, 86–91. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Brauchle, E.; Thude, S.; Brucker, S.Y.; Schenke-Layland, K. Cell death stages in single apoptotic and necrotic cells monitored by Raman microspectroscopy. Sci. Rep. 2014, 4, 4698. [Google Scholar] [CrossRef]
- Daum, R.; Brauchle, E.M.; Berrio, D.A.C.; Jurkowski, T.P.; Schenke-Layland, K. Non-invasive detection of DNA methylation states in carcinoma and pluripotent stem cells using Raman microspectroscopy and imaging. Sci. Rep. 2019, 9, 7014. [Google Scholar] [CrossRef] [PubMed]
- Kurita, H.; Haruta, N.; Uchihashi, Y.; Seto, T.; Takashima, K. Strand breaks and chemical modification of intracellular DNA induced by cold atmospheric pressure plasma irradiation. PLoS ONE 2020, 15, e0232724. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.O. Cyclin-dependent kinases: Engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 1997, 13, 261–291. [Google Scholar] [CrossRef] [PubMed]
- Hans, F.; Dimitrov, S. Histone H3 phosphorylation and cell division. Oncogene 2001, 20, 3021–3027. [Google Scholar] [CrossRef]
- Bauer, G.; Sersenová, D.; Graves, D.B.; Machala, Z. Cold Atmospheric Plasma and Plasma-Activated Medium Trigger RONS-Based Tumor Cell Apoptosis. Sci. Rep. 2019, 9, 14210. [Google Scholar] [CrossRef]
- Bochicchio, B.; Laurita, A.; Heinz, A.; Schmelzer, C.E.; Pepe, A. Investigating the role of (2S,4R)-4-hydroxyproline in elastin model peptides. Biomacromolecules 2013, 14, 4278–4288. [Google Scholar] [CrossRef]
- Piez, K.A. The amino acid chemistry of some calcified tissues. Ann. N. Y. Acad. Sci. 1963, 109, 256–268. [Google Scholar] [CrossRef]
- Keller, S.; Wörgötter, K.; Liedek, A.; Kluger, P.J.; Bach, M.; Tovar, G.E.M.; Southan, A. Azide-Functional Extracellular Matrix Coatings as a Bioactive Platform for Bioconjugation. ACS Appl. Mater. Interfaces 2020, 12, 26868–26879. [Google Scholar] [CrossRef]
- Blackert, S.; Haertel, B.; Wende, K.; von Woedtke, T.; Lindequist, U. Influence of non-thermal atmospheric pressure plasma on cellular structures and processes in human keratinocytes (HaCaT). J. Derm. Sci. 2013, 70, 173–181. [Google Scholar] [CrossRef]
- Saed, G.M.; Diamond, M.P. Differential expression of alpha smooth muscle cell actin in human fibroblasts isolated from intraperitoneal adhesions and normal peritoneal tissues. Fertil. Steril. 2004, 82 (Suppl. 3), 1188–1192. [Google Scholar] [CrossRef]
- Xu, X.; Rivkind, A.; Pappo, O.; Pikarsky, A.; Levi-Schaffer, F. Role of mast cells and myofibroblasts in human peritoneal adhesion formation. Ann. Surg. 2002, 236, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, K. Mesothelial cell: A multifaceted model of aging. Ageing Res. Rev. 2013, 12, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Saed, G.M.; Diamond, M.P. Hypoxia-induced irreversible up-regulation of type I collagen and transforming growth factor-beta1 in human peritoneal fibroblasts. Fertil. Steril. 2002, 78, 144–147. [Google Scholar] [CrossRef]
- O’Connor, J.W.; Gomez, E.W. Cell Adhesion and Shape Regulate TGF-Beta1-Induced Epithelial-Myofibroblast Transition via MRTF-A Signaling. PLoS ONE 2013, 8, e83188. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011, 17, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Lucas, P.A.; Warejcka, D.J.; Young, H.E.; Lee, B.Y. Formation of abdominal adhesions is inhibited by antibodies to transforming growth factor-beta1. J. Surg. Res. 1996, 65, 135–138. [Google Scholar] [CrossRef]
- Nie, J.; Hao, W.; Dou, X.; Wang, X.; Luo, N.; Lan, H.Y.; Yu, X. Effects of Smad7 overexpression on peritoneal inflammation in a rat peritoneal dialysis model. Perit. Dial. Int. 2007, 27, 580–588. [Google Scholar] [CrossRef]
- Guo, H.; Leung, J.C.; Cheung, J.S.; Chan, L.Y.; Wu, E.X.; Lai, K.N. Non-viral Smad7 gene delivery and attenuation of postoperative peritoneal adhesion in an experimental model. Br. J. Surg. 2009, 96, 1323–1335. [Google Scholar] [CrossRef]
- Kang, S.U.; Kim, Y.S.; Kim, Y.E.; Park, J.K.; Lee, Y.S.; Kang, H.Y.; Jang, J.W.; Ryeo, J.B.; Lee, Y.; Shin, Y.S.; et al. Opposite effects of non-thermal plasma on cell migration and collagen production in keloid and normal fibroblasts. PLoS ONE 2017, 12, e0187978. [Google Scholar] [CrossRef]
- Dik, W.A.; McAnulty, R.J.; Versnel, M.A.; Naber, B.A.; Zimmermann, L.J.; Laurent, G.J.; Mutsaers, S.E. Short course dexamethasone treatment following injury inhibits bleomycin induced fibrosis in rats. Thorax 2003, 58, 765–771. [Google Scholar] [CrossRef]
- Brodsky, B.; Persikov, A.V. Molecular structure of the collagen triple helix. Adv. Protein Chem. 2005, 70, 301–339. [Google Scholar] [CrossRef] [PubMed]
- Eastoe, J.E.; Leach, A.A. Recent Advances in Gelatin and Glue Research; Stainsby, G., Ed.; Pergamon Press Inc.: New York, NY, USA, 1958; Volume 31. [Google Scholar]
- Sun, Y.; Liang, Y.; Hu, J.; Wang, J.; Wang, D.; Li, X.; Yan, L. Reduction of intraarticular adhesion by topical application of colchicine following knee surgery in rabbits. Sci. Rep. 2014, 4, 6405. [Google Scholar] [CrossRef]
- Chen, C.; Fan, H.; Kuo, S.P.; Chang, J.; Pedersen, T.; Mills, T.J.; Huang, C. Blood Clotting by Low-Temperature Air Plasma. IEEE Trans. Plasma Sci. 2009, 37, 993–999. [Google Scholar] [CrossRef]
- Smithmyer, M.E.; Cassel, S.E.; Kloxin, A.M. Bridging 2D and 3D culture: Probing impact of extracellular environment on fibroblast activation in layered hydrogels. AIChE J. 2019, 65, e16837. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holl, M.; Rasch, M.-L.; Becker, L.; Keller, A.-L.; Schultze-Rhonhof, L.; Ruoff, F.; Templin, M.; Keller, S.; Neis, F.; Keßler, F.; et al. Cell Type-Specific Anti-Adhesion Properties of Peritoneal Cell Treatment with Plasma-Activated Media (PAM). Biomedicines 2022, 10, 927. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10040927
Holl M, Rasch M-L, Becker L, Keller A-L, Schultze-Rhonhof L, Ruoff F, Templin M, Keller S, Neis F, Keßler F, et al. Cell Type-Specific Anti-Adhesion Properties of Peritoneal Cell Treatment with Plasma-Activated Media (PAM). Biomedicines. 2022; 10(4):927. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10040927
Chicago/Turabian StyleHoll, Myriam, Marie-Lena Rasch, Lucas Becker, Anna-Lena Keller, Laura Schultze-Rhonhof, Felix Ruoff, Markus Templin, Silke Keller, Felix Neis, Franziska Keßler, and et al. 2022. "Cell Type-Specific Anti-Adhesion Properties of Peritoneal Cell Treatment with Plasma-Activated Media (PAM)" Biomedicines 10, no. 4: 927. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10040927