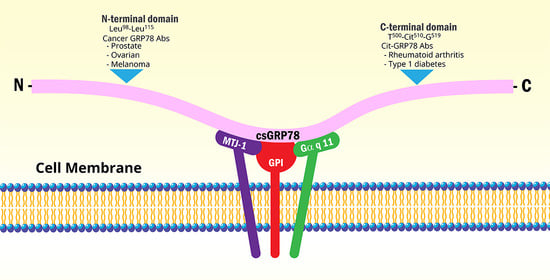
Physiological Roles of the Autoantibodies to the 78-Kilodalton Glucose-Regulated Protein (GRP78) in Cancer and Autoimmune Diseases
Abstract
:1. Introduction
2. Molecular Mechanisms and ER Stress Conditions That Promote GRP78 Autoimmunity in Cancer
3. Mechanisms and ER Stress Conditions That Promote GRP78 Autoimmunity in RA
4. GRP78 Autoantibodies in Immune-Mediated Neurological Diseases
5. GRP78 Autoimmunity in Type 1 Diabetes
6. Molecular Mechanisms Involved in GRP78 Autoantigenicity and Migration to the Cell Surface
7. Antibodies and Synthetic Peptides Targeting GRP78 for Therapeutic Purposes
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abs | Antibodies |
| α2M* | activated alpha2-macroglobulin |
| Akt | protein kinase B |
| ACPA | anti-cyclic citrullinated peptide antibodies |
| AMOGAD | anti-myelin oligodendrocyte glycoprotein antibody-associated disorder |
| AMOP | isthmin C-terminal adhesion associated domain |
| AQP4-Ab | aquaporin-4 water channel antibody |
| ATF6 | activating transcription factor 6 |
| BBB | blood-brain barrier |
| csGRP78 | cell surface GRP78 |
| CNS | central nervous system |
| cit-GRP78 | citrullinated GRP78 |
| ER | endoplasmic reticulum |
| ERK | extracellular signal-regulated protein |
| GRP78 | 78 kDa glucose-regulated protein |
| ICAM-1 | intercellular adhesion molecule-1 |
| IgG | immunoglobulin G |
| IgG1 | immunoglobulin G, subclass 1 |
| Ire1 | inositol requiring enzyme 1 |
| ISM | isthmin |
| JNK | Jun N-terminal kinase |
| LEMS | Lambert-Eaton myasthenic syndrome |
| MHC-I | major histocompatibility complex class I |
| MAPK | mitogen-activated protein kinase |
| MS | multiple sclerosis |
| NMO | neuromyelitis optica |
| NPSLE | neuropsychiatric systemic lupus erythematosus |
| NF-κB | nuclear factor kappa B |
| PADI | peptidylarginine deiminase |
| PI3K | phosphoinositide 3-kinase |
| PERK | protein kinase RNA-like ER kinase |
| RA | rheumatoid arthritis; RA-FLS |
| RA | fibroblast-like synoviocytes |
| RF | rheumatoid factor |
| SubA | subtilase cytotoxin A subunit |
| SBS | substrate binding site |
| T1D | type 1 diabetes |
| TNF-α | tumor necrosis factor alpha |
| UPR | unfolded protein response. |
References
- Kiang, J.G.; Tsokos, G.C. Heat shock protein 70 kDa: Molecular biology, biochemistry and physiology. Pharmacol. Ther. 1998, 80, 183–201. [Google Scholar] [CrossRef]
- Lee, A.S. The glucose-regulated proteins: Stress induction and clinical applications. Trends Biochem. Sci. 2001, 26, 504–510. [Google Scholar] [CrossRef]
- Wiersma, V.R.; Michalak, M.; Abdullah, T.M.; Bremer, E.; Eggleton, P. Mechanisms of translocation of ER chaperones to the cell surface and immunomodulatory roles in cancer and autoimmunity. Front. Oncol. 2015, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gronow, M.; Selim, M.A.; Papalas, J.; Pizzo, S.V. GRP78: A multifunctional receptor on the cell surface. Antioxid. Redox Signal. 2009, 11, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Raiter, A.; Vilkin, A.; Gingold, R.; Levi, Z.; Halpern, M.; Niv, Y.; Britta-Hardy, B. The presence of anti-GRP78 antibodies in the serum of patients with colorectal carcinoma: A potential biomarker for early cancer detection. Int. J. Biol. Mark. 2014, 43, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Bodman-Smith, M.D.; Corrigal, V.M.; Berglin, E.; Cornell, H.R.; Tzioufas, A.G.; Magravani, C.P.; Chan, C.; Rantapää-Dahiqvist, S.; Panayi, G.S. Antibody response to the human stress protein BiP in rheumatoid arthritis. Rheumatology 2004, 43, 1283–1287. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, F.; Takeshita, Y.; Hamamoto, Y.; Yasuteru, Y.; Honda, M.; Sato, R.; Maeda, T.; Takahashi, T.; Fujikawa, S. GRP78 antibodies are associated with clinical phenotype in neuromyelitis optica. Ann. Clin. Transl. Neurol. 2019, 142, 2253–2264. [Google Scholar]
- Shimizu, F.; Takeshita, Y.; Sano, Y.; Hamamoto, Y.; Shiraishi, H.; Sato, T.; Yoshimura, S.; Maeda, T.; Fujikawa, S.; Nishihara, H.; et al. GRP78 antibodies damage the blood-brain barrier and relate to cerebellar degeneration in Lamber-Eaton myasthenic syndrome. Brain 2019, 142, 2253–2264. [Google Scholar] [CrossRef]
- Rondas, D.; Crèvecoeur, I.; D’Hertrog, W.; Ferreira, G.B.; Staes, A.; Garg, A.D.; Eizirik, D.L.; Agostinis, P.; Gevaert, K.; Overbergh, L.; et al. Citrullinated glucose-regulated protein 78 is an autoantigen in type 1 diabetes. Diabetes 2015, 64, 573–586. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, F.; Nishihara, H.; Kanda, T. Blood-brain barrier dysfunction in immune-mediated neurological diseases. Immunol. Med. 2018, 41, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasnain, S.Z.; Lourie, R.; Das, I.; Chen, A.C.; McGuckin, M.A. The interplay between endoplasmic reticulum stress and inflammation. Immunol. Cell. Biol. 2012, 90, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Ruiz, J.R.; Bettigole, S.E.; Glimcher, L.H. Tumorigenic and immunosuppresive effects of endoplasmic reticulum stress in cancer. Cell 2017, 168, 692–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.-J.; Yoo, S.-A.; Kim, W.-U. Role of the endoplasmic reticulum stress in rheumatoid arthritis pathogenesis. J. Korean Med. Sci. 2014, 29, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Gidalevitz, T.; Stevens, F.; Argon, Y. Orchestration of secretory protein folding by ER chaperones. Biochim. Biophys. Acta 2013, 1833, 2410–2424. [Google Scholar] [CrossRef] [Green Version]
- Blond-Elguindi, S.; Fourie, A.M.; Sambrook, J.E.; Gething, M.J. Peptide-dependent stimulation of the ATPase activity of the molecular chaperone BiP is the result of conversion of oligomers to active monomers. J. Biol. Chem. 1993, 268, 12735–12739. [Google Scholar] [CrossRef]
- Gardner, B.M.; Pincus, D.; Gotthardt, K.; Gallagher, C.M.; Walter, P. Endoplasmic resticulum stress sensing in the unfolded protein response. Cold Spring Harb. Perspect. Biol. 2013, 5, a013169. [Google Scholar] [CrossRef] [Green Version]
- Baumeister, P.; Luo, S.; Skarnes, W.C.; Sui, G.; Seto, E.; Shi, Y.; Lee, A.S. Endoplasmic reticulum stress induction of the Grp78/BiP promoter: Activating mechanisms mediated by YY1 and its interactive chromatin modifiers. Mol. Cell. Biol. 2005, 25, 4529–4540. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.S. Glucose-regulated proteins in cancer: Molecular mechanisms and therapeutic potential. Nat. Rev. Cancer 2014, 14, 263–276. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, R.; Ni, M.; Gill, P.; Lee, A.S. Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J. Biol. Chem. 2010, 285, 15065–15075. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, T.; Simmen, T. Endoplasmic reticulum chaperones and oxidoreductases: Critical regulators of tumor cell survival and immunorecognition. Front. Oncol. 2014, 4, 27–37. [Google Scholar]
- Gonzalez-Gronow, M.; Cuchacovich, M.; Llanos, C.; Urzua, C.; Gawdi, G.; Pizzo, S.V. Prostate cancer cell proliferation in vitro is modulated by antibodies against glucose-regulated protein 78 isolated from patient serum. Cancer Res. 2006, 66, 11424–11431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selim, M.A.; Burchette, J.M.; Bowers, E.V.; de Ridder, G.G.; Mo, L.; Pizzo, S.V.; Gonzalez-Gronow, M. Changes in oligosaccharide chains of autoantibodies to GRP78 expressed during progression of malignant melanoma stimulate melanoma cell growth and survival. Melanoma Res. 2011, 21, 323–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Tseng, C.C.; Tsai, Y.L.; Fu, X.; Schiff, R.; Lee, A.S. Cancer cells resistant to therapy promote cell surface relocalization of GRP78 which complexes with PI3K and enhances PI (3, 4, 5) P3 production. PLoS ONE 2013, 8, e80071. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Gao, W.; Zhou, Y.; Wey, S.; Mitra, S.K.; Krasnoperov, V.; Dong, D.; Liu, S.; Li, D.; et al. Monoclonal antibody against cell surface GRP78 as a novel agent in suppressing PI3K/AKT signaling, tumor growth, and metastasis. Clin. Cancer Res. 2013, 19, 6802–6811. [Google Scholar] [CrossRef] [Green Version]
- Triantafilou, M.; Fradelizi, D.; Triantafilou, K. Major histocompatibility class one molecule associates with glucose related protein (GRP)78 on the cell surface. Hum. Immunol. 2001, 62, 764–770. [Google Scholar] [CrossRef]
- Ulianich, L.; Terrazzano, G.; Annunziatella, M.; Ruggiero, G.; Beguinot, F.; Di Jeso, B. ER stress impairs MHC class I surface expression and increases susceptibilioty of thyroid cells to NK-mediated cytotoxicity. Biochim. Biophys. Acta 2011, 4, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Marincola, F.M.; Jafee, E.M.; Hicklin, D.J.; Ferrone, S. Escape of human solid tumors from T-cell recognition: Molecular mechanisms and functional significance. Adv. Immunol. 2000, 74, 181–273. [Google Scholar]
- Seliger, B.; Atkins, D.; Bock, M.; Ritz, U.; Ferrone, S.; Huber, C.; Storkel, S. Characterization of human lymphocyte antigen class I antigen-processing machinery defects in renal cell carcinoma lesions with special emphasis on transporter-associated with antigen-processing down-regulation. Clin. Cancer Res. 2003, 9, 1721–1727. [Google Scholar]
- Mintz, P.J.; Kim, J.; Do, K.A.; Wang, X.; Zinnere, R.G.; Cristofanilli, M.; Arap, M.A.; Hong, W.K.; Logothetis, C.J.; Pasqualini, R.; et al. Fingerprinting the circulating repertoire of antibodies from cancer patients. Nat. Biotechnol. 2003, 21, 57–63. [Google Scholar] [CrossRef]
- Misra, U.K.; Gonzalez-Gronow, M.; Gawdi, G.; Wang, F.; Pizzo, S.V. A novel receptor function for the heat shock protein GRP78 gene expression attenuates alpha-2M* induced signaling. Cell Signal. 2004, 16, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Gerçel-Taylor, C.; Bazzett, L.B.; Taylor, D.D. Presence of aberrant tumor-reactive immunoglobulins in the circulation of patients with ovarian cancer. Gynecol. Oncol. 2001, 81, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, Y.; Ohara, T.; Mashiko, T.; Abe, T.; Masuda, N.; Akahoski, T. Relationship between N-linked oligosaccharide chains of human serum immunoglobulin G and serum tumor markers with non-small cell lung cancer progression. Anticancer Res. 2006, 26, 4293–4298. [Google Scholar] [PubMed]
- Kanoh, Y.; Ohara, T.; Tadano, T.; Kanoh, M.; Akahoski, T. Changes to N-linked oligosaccharide chains of human serum immunoglobulin G and matrix metalloproteinase-2 with cancer progression. Anticancer Res. 2008, 28, 715–720. [Google Scholar]
- Margni, R.A.; Borel, I.M. Paradoxical behavior of asymmetric IgG antibodies. Immunol. Rev. 1998, 163, 77–87. [Google Scholar] [CrossRef]
- Van de Boyenkamp, F.S.; Hafkenscheid, L.; Rispens, T.; Rombouts, Y. The emerging importance of IgG Fab glycosylation in immunity. J. Immunol. 2016, 196, 1435–1441. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.; Morrison, S.L. Antibody variable region glycosylation: Biochemical and clinical effects. Springer Semin. Immunopathol. 1993, 15, 259–273. [Google Scholar] [CrossRef]
- Russel, A.; Adua, E.; Ugrina, I.; Laws, S.; Wang, W. Unravelling immunoglobulin G Fc N-glycosylation: A dynamic marker potentiating predictive, preventive and personalized medicine. Int. J. Mol. Sci. 2018, 19, 390. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.M.; Weinblatt, M.E. Rheumatoid arthritis. Lancet 2001, 358, 903–911. [Google Scholar] [CrossRef]
- Stevens, C.R.; Williams, R.B.; Farrell, A.J.; Blake, D.R. Hypoxia and inflammatory synovitis: Observations and speculation. Ann. Rheum. Dis. 1991, 50, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.-A.; You, S.; Yoon, H.-J.; Kim, D.-H.; Kim, H.-S.; Lee, K.; Ahn, J.H.; Hwang, D.; Lee, A.S.; Kim, K.-J.; et al. A novel pathogenic role of ER chaperone GRP78/BiP in rheumatoid arthritis. J. Exp. Med. 2012, 209, 871–886. [Google Scholar] [CrossRef] [PubMed]
- Masson-Bessière, C.; Sabbag, M.; Durieux, J.J.; Nogueira, L.; Vincent, C.; Girbal-Neuhausere, E.; Durroux, R.; Cantagrel, A.; Serre, G. In the rheumatoid pannus, anti-filaggrin autoantibodies are produced by local plasma cells and constitute a higher proportion of IgG than in synovial fluid and serum. Clin. Exp. Immunol. 2000, 119, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R. Anti-citrullinated antibodies in rheumatoid arthritis: A bridge between genetic predisposition and autoimmunity. Korean J. Intern. Med. 2013, 28, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R. Peptidylarginine deiminase type 4, anticitrullinated peptide antibodies, and rheumatoid arthritis. Autoimmun. Rev. 2005, 4, 201–206. [Google Scholar] [CrossRef]
- Suzuki, A.; Yamada, R.; Chang, X.; Tokuhiro, S.; Sawada, T.; Suzuki, M.; Nagasaki, M.; Nakayama-Hamada, M.; Kawaida, R.; Ono, M.; et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat. Genet. 2003, 34, 395–402. [Google Scholar] [CrossRef]
- Shoda, H.; Fujio, K.; Shibuya, M.; Okamura, T.; Sumitomo, S.; Okamoto, A.; Sawada, T.; Yamamoto, K. Detection of autoantibodies to citrullinated BiP in rheumatoid arthritis patients and pro-inflammatory role of citrullinated BiP in collagen-induced arthritis. Arthritis. Res. Ther. 2011, 13, R191. [Google Scholar] [CrossRef] [Green Version]
- Rombouts, Y.; Willemze, A.; van Beers, J.B.C.; Shi, J.; Kerkman, P.F.; van Toorn, L.; Janssen, G.M.C.; Zaldumbide, A.; Hoeben, R.C.; Pruijn, G.J.M.; et al. Extensive glycosylation of ACPA-IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, 578–585. [Google Scholar] [CrossRef]
- Van Zeben, D.; Rook, G.A.; Hazes, J.M.; Zwinderman, A.H.; Zhang, Y.; Ghelani, S.; Rademacher, T.W.; Breedveld, F.C. Early agalactosylation of Igg is associated with a more progressive disease course in patients with rheumatoid arthritis: Results of a follow-up study. Rheumatology 1994, 33, 36–43. [Google Scholar] [CrossRef]
- Rombouts, Y.; Ewing, E.; van de Stadt, L.A.; Selman, M.H.J.; Trouw, L.A.; Deelder, A.M.; Huizinga, T.W.J.; Wuhrer, M.; Van Schaardenburg, D.; Toes, R.; et al. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann. Rheum. Dis. 2015, 74, 234–241. [Google Scholar] [CrossRef]
- Ercan, A.; Cui, J.; Chatterton, D.E.W.; Deane, K.D.; Hazen, M.M.; Brintnell, W.; O’Donnell, C.I.; Derber, L.A.; Weinblatt, M.E.; Shadick, N.A.; et al. Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2010, 62, 2239–2248. [Google Scholar] [CrossRef] [Green Version]
- Aghamollaei, H.; Gargari, S.L.M.; Ghanei, M.; Rasaee, M.J.; Amani, J.; Bakherad, H.; Famoosh, G. Structure prediction, expression and antigenicity of c-terminal of GRP78. Biochem. Mol. Biol. Inc. 2017, 64, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.C.; Lai, N.S.; Yu, H.C.; Huang, H.B.; Hsieh, S.C.; Yu, C.L. Anti-citrullinated protein antibodies bind surface-expressed citrullinated grp78 on monocyte-macrophages and stimulate tumor necrosis factor alpha production. Arthritis Rheum. 2010, 62, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.C.; Lai, N.S.; Yin, W.Y.; Yu, H.C.; Huang, H.B.; Tung, C.H.; Huang, K.Y.; Yu, C.L. Anti-citrullinated protein antibodies activated ERK1/2 and JNK antigen-activated protein kinases via binding to surface-expressed citrullinated GRP78 on mononuclear cells. J. Clin. Immunol. 2013, 33, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Gharesi-Fard, B.; Zare, M.; Kamali-Sarvestani, E. The reaction of placerntal GRP78 protein with sera from women with multiple sclerosis. Iran. J. Immunol. 2017, 14, 306–315. [Google Scholar] [PubMed]
- Matsueda, Y.; Arinuma, Y.; Nagai, T.; Hirohata, S. Elevation of sereum anti-glucose-regulated protein 78 antibodies in neuropsychiatric systemic lupus erythematosus. Lupus Sci. Med. 2018, 5, e000281. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Ogawa, R.; Mizukami, Y.; Watanabe, K.; Hara, K.; Kadono, C.; Takahashi, T.; Misu, T.; Takeshita, Y.; Sano, Y.; et al. GRP78 antibodies are associated with blood-brain barrier breakdown in anti-myelin oligodendrocyte glycoprotein antibody-associated disorder. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1038. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Schaller, K.L.; Owens, G.P.; Clotleur, A.C.; Kellner, D.; Takeshita, Y.; Obermeier, B.; Kryzer, T.J.; Sano, Y.; Kanda, T.; et al. Glucose-regulated protein 78 autoantibody associates with blood-brain barrier disruption in neuromyelitis optica. Sci. Transl. Med. 2017, 9, eaai9111. [Google Scholar] [CrossRef] [Green Version]
- Noseworthy, F.D.; Lucchinetti, C.; Rodriguez, M.; Weinshenker, B.G. Multiple sclerosis. N. Engl. J. Med. 2000, 343, 938–952. [Google Scholar] [CrossRef]
- Wuhrer, M.; Selman, M.H.; McDonnell, L.A.; Kümpfel, T.; Derfuss, T.; Khademi, M.; Olsson, T.; Hohlfeld, R.; Meinl, E.; Krumbholz, M. Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J. Neuroinflamm. 2015, 12, 235. [Google Scholar] [CrossRef] [Green Version]
- Liang, M.H.; Corzillius, M.; Bae, S.C.; Lew, R.A.; Fortin, P.R.; Gordon, C.; Isenberg, D.; Alarcón, G.S.; Straaton, K.V.; Denburg, J. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999, 42, 599–608. [Google Scholar]
- Vučković, F.; Krištić, J.; Gudelj, I.; Teruel, M.; Keser, T.; Pezer, M.; Pučić-Baković, M.; Štambuk, J.; Trbojević-Akmačić, I.; Barrios, C.; et al. Association of Systemic Lupus Erythematosus With Decreased Immunosuppressive Potential of the IgG Glycome. Arthritis Rheumatol. 2015, 67, 2978–2989. [Google Scholar] [CrossRef] [PubMed]
- Titulaer, M.J.; Lang, B.; Verschuuren, J.J. Lambert-Eaton myathenic syndrome from clinical characteristics to therapeutic strategies. Lancet 2011, 10, 1098–1107. [Google Scholar] [CrossRef]
- Jarius, S.; Wildemann, B. Thed history of neuromyelitis optica. J. Neuroinflamm. 2013, 10, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennon, V.A.; Kryzer, T.J.; Pittock, S.J.; Verkman, A.S.; Hinson, S.R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005, 202, 473–477. [Google Scholar] [CrossRef] [Green Version]
- Waters, P.J.; McKeon, A.; Leite, M.I.; Rajasekharan, S.; Lennon, V.A.; Villalobos, A.; Palace, J.; Mandrekar, J.N.; Vincent, A.; Bar-Or, A.; et al. Serologic diagnosis of NMO: A multicenter comparison of aquaporin-4-IgG assays. Neurology 2012, 78, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Roemer, S.F.; Parisi, J.E.; Lennon, V.A.; Benarroch, E.E.; Lassmann, H.; Bruck, W.; Mandler, R.N.; Weinshenker, B.G.; Pittock, S.J.; Wingerchuk, D.M.; et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 2007, 10, 1194–1205. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, F.; Sano, Y.; Takahashi, T.; Haruki, H.; Saito, K.; Koga, M.; Kanda, T. Sera from neuromyelitis optica patients disrupt the blood-brain barrier. J. Neurol. Neurosurg. Psychiatry 2012, 83, 288–297. [Google Scholar] [CrossRef]
- Renaudineau, Y.; Duguè, C.; Dueymes, M.; Youinou, P. Antiendothelial cell antibodies in systemic lupus erythematosus. Autoimmun. Rev. 2002, 1, 365–372. [Google Scholar] [CrossRef]
- Yu, Y.J.; Watts, R.J. Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics 2013, 10, 459–472. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Eizirik, D.L.; Colli, M.L.; Ortis, F. The role of inflammation in insulin and beta-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 2009, 5, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Engin, F. ER stress and development of type 1 diabetes. J. Investig. Med. 2016, 64, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, D.L.; Cardozo, A.K.; Cnop, M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 2008, 29, 42–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buitinga, M.; Callebaut, A.; Sodré, F.M.C.; Crèvocoeur, I.; Blahnik-Fagan, G.; Yang, M.-L.; Bugliani, M.; Arribas-Layton, D.; Marré, M.; Cook, D.P.; et al. Inflammation-Induced Citrullinated Glucose-regulated Protein 78 Elicits Immune Responses in Human Type 1 Diabetes. Diabetes 2018, 67, 2337–2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardozo, A.K.; Ortis, F.; Storling, J.; Feng, Y.-M.; Rasschaert, J.; Tonnesen, M.; Van Eylen, F.; Mandrup-Poulsen, T.; Herchuelz, A.; Eizirik, D.L. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes 2005, 54, 452–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, R.; de Ridder, G.G.; Eu, J.P.; Paton, A.W.; Paton, J.C.; Pizzo, S.V. The Escherichia coli subtilase cytotoxin a subunit specifically cleaves cell-surface GRP78 protein and abolishes COOH-terminal-dependent signaling. J. Biol. Chem 2012, 287, 32755–32769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, S.; Hiramatsu, N.; Hayakawa, K.; Saito, Y.; Kato, H.; Huang, T.; Yao, J.; Paton, A.W.; Paton, J.C.; Kitamura, M.; et al. Selective Abrogation of BiP/GRP78 Blunts Activation of NF-κB through the ATF6 Branch of the UPR: Involvement of C/EBPβ and mTOR-Dependent Dephosphorylation of Akt. Mol. Cell. Biol. 2011, 31, 1710–1718. [Google Scholar] [CrossRef] [Green Version]
- Limso, C.; Ngo, J.M.; Nguyen, P.; Leal, S.; Husain, A.; Sahoo, D.; Ghosh, P.; Bhandari, D. The Gα-interacting vesicle-associated protein interacts with and promotes cell surface localization of GRP78 during endoplasmic reticulum stress. FEBS Lett. 2020, 594, 1088–1100. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Zhang, Y.; Tseng, C.-C.; Stanciauskas, R.; Pinaud, F.; Lee, A.S. Characterization and Mechanism of Stress-Induced Translocation of 78-Kilodalton Glucose-regulated Protein (GRP78) to the Cell Surface. J. Biol. Chem. 2015, 290, 8049–8064. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Li, X.; Feng, Y.; Fan, C.; Chen, Z.; Zhu, P. The differential expressions of 78-kDa glucose-regulated protein of infiltrating plasma cells in peripheral joints with the histopathological variants of rheumatoid synovitis. Arthritis Res. Ther. 2009, 11, R4. [Google Scholar] [CrossRef] [Green Version]
- Katayama, H.; Kobayashi, M.; Irajizad, E.; Sevillano, A.M.; Patel, N.; Mao, X.; Rustling, L.; Vykoukal, J.; Cai, Y.; Hsiao, F.; et al. Protein citrullination as a source of cancer neoantigens. J. Immunother. Cancer 2021, 9, e002549. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, I.; Cohen, M. Linking cell-surface GRP78 to cancer: From basic research to clinical value of GRP78 antibodies. Cancer Lett. 2022, 524, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashimi, A.; Won, K.D.; Pham, E.; Mariano, N.; Bailis, J.; Austin, R.; Shayegan, B. Investigating a novel recombinant antibody to attenuate prostate cancer progression by targeting cell surface GRP78. J. Clin. Oncol. 2019, 37, 206. [Google Scholar] [CrossRef]
- Rauschert, N.; Brändlein, S.; Holzinger, E.; Hensel, F.; Müller-Hermelink, H.K.; Vollmers, H.P. A new tumor-specific variant of GRP78 as a target for antibody-based therapy. Lab. Investig. 2008, 88, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Hensel, F.; Eckstein, M.; Rosenwald, A.; Brändlein, S. Early development of PAT-SM6 for the treatment of melanoma. Melanoma Res. 2013, 23, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, Y.; Yu, V.C.; Chong, Y.S.; Yoshioka, T.; Ge, R. Isthmin targets cell-surface GRP78 and triggers apoptosis via induction of mitochondrial dysfunction. Cell Death Differ. 2014, 21, 797–810. [Google Scholar] [CrossRef] [Green Version]
- Kao, C.; Chandna, R.; Ghode, A.; Dsouza, C.; Chen, M.; Larsson, A.; Lim, S.H.; Wang, M.; Cao, Z.; Zhu, Y.; et al. Proapoptotic cyclic peptide BC71 targets cell-surface GRP78 and functions as an anticancer therapeutic in mice. EBioMedicine 2018, 33, 22–32. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Gronow, M.; Pizzo, S.V. Physiological Roles of the Autoantibodies to the 78-Kilodalton Glucose-Regulated Protein (GRP78) in Cancer and Autoimmune Diseases. Biomedicines 2022, 10, 1222. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10061222
Gonzalez-Gronow M, Pizzo SV. Physiological Roles of the Autoantibodies to the 78-Kilodalton Glucose-Regulated Protein (GRP78) in Cancer and Autoimmune Diseases. Biomedicines. 2022; 10(6):1222. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10061222
Chicago/Turabian StyleGonzalez-Gronow, Mario, and Salvatore Vincent Pizzo. 2022. "Physiological Roles of the Autoantibodies to the 78-Kilodalton Glucose-Regulated Protein (GRP78) in Cancer and Autoimmune Diseases" Biomedicines 10, no. 6: 1222. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10061222