Rottlerin Stimulates Exosome/Microvesicle Release Via the Increase of Ceramide Levels Mediated by Ampk in an In Vitro Model of Intracellular Lipid Accumulation
Abstract
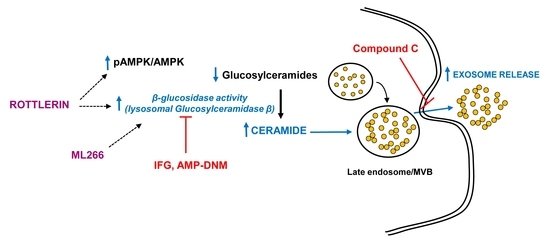
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Immunofluorescence Microscopy
2.3. Western Blotting
2.4. Exosome/microvesicle Isolation and Analysis
2.5. Lipidomic Analysis
2.6. Determination of β-Galactosidase and β-Glucosidase Activities by Flow Cytometry
2.7. RNA Isolation and Quantitative RT-PCR (qRT-PCR)
2.8. Statistical Analysis
3. Results
3.1. Rottlerin Reduces Lysosomal LDL-Cholesterol Accumulation
3.2. Rottlerin Stimulates Exosome/Microvesicle Secretion
3.3. Rottlerin Alters Sphingolipid Cell Content and Composition
3.4. Rottlerin Alters Galactosylceramidase and Glucosylceramidase Activities
3.5. Inhibition of β-Glucosidase Prevents the Effects of Rottlerin on Exosome/Microvesicle Release and Cellular Sphingolipid Levels
3.6. Activation of Enzyme β-Glucosidase Stimulates Exosome/Microvesicle Release through the Increase in Ceramide
3.7. Effects of Rottlerin on the Gene Expression of Enzymes Involved in Glucosylceramide Metabolism
3.8. The Role of the AMPK Pathway on Rottlerin Effects on Exosome Secretion
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arenaccio, C.; Federico, M. The Multifaceted Functions of Exosomes in Health and Disease: An Overview. Adv. Exp. Med. Biol. 2017, 998, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Thery, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.P.; Lin, Z.X.; Jiang, X.Y.; Yu, X.Y. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol. Sin. 2018, 39, 542–551. [Google Scholar] [CrossRef] [Green Version]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef] [Green Version]
- Mittelbrunn, M.; Gutierrez-Vazquez, C.; Villarroya-Beltri, C.; Gonzalez, S.; Sanchez-Cabo, F.; Gonzalez, M.A.; Bernad, A.; Sanchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef] [Green Version]
- Yuyama, K.; Sun, H.; Mitsutake, S.; Igarashi, Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J. Biol. Chem. 2012, 287, 10977–10989. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Seisdedos, D.; Babiy, B.; Lerma, M.; Casado, M.E.; Martinez-Botas, J.; Lasuncion, M.A.; Pastor, O.; Busto, R. Curcumin stimulates exosome/microvesicle release in an in vitro model of intracellular lipid accumulation by increasing ceramide synthesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158638. [Google Scholar] [CrossRef]
- Wu, C.Y.; Jhang, J.G.; Lin, W.S.; Chuang, P.H.; Lin, C.W.; Chu, L.A.; Chiang, A.S.; Ho, H.C.; Chan, C.C.; Huang, S.Y. Dihydroceramide desaturase promotes the formation of intraluminal vesicles and inhibits autophagy to increase exosome production. iScience 2021, 24, 103437. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.M.; Prieto, M.; Silva, L.C. Ceramide: A simple sphingolipid with unique biophysical properties. Prog. Lipid Res. 2014, 54, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brugger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Maioli, E.; Daveri, E.; Maellaro, E.; Ietta, F.; Cresti, L.; Valacchi, G. Non-conventional rottlerin anticancer properties. Arch. Biochem. Biophys. 2018, 645, 50–53. [Google Scholar] [CrossRef]
- Gschwendt, M.; Muller, H.J.; Kielbassa, K.; Zang, R.; Kittstein, W.; Rincke, G.; Marks, F. Rottlerin, a novel protein kinase inhibitor. Biochem. Biophys. Res. Commun. 1994, 199, 93–98. [Google Scholar] [CrossRef]
- Soltoff, S.P. Rottlerin: An inappropriate and ineffective inhibitor of PKCdelta. Trends Pharmacol. Sci. 2007, 28, 453–458. [Google Scholar] [CrossRef]
- Mischitelli, M.; Jemaa, M.; Almasry, M.; Faggio, C.; Lang, F. Stimulation of Suicidal Erythrocyte Death by Rottlerin. Cell. Physiol. Biochem. 2016, 40, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M.; Ladilov, Y. Emerging Role of cAMP/AMPK Signaling. Cells 2022, 11, 308. [Google Scholar] [CrossRef]
- Yan, C.; Tian, X.; Li, J.; Liu, D.; Ye, D.; Xie, Z.; Han, Y.; Zou, M.H. A High-Fat Diet Attenuates AMPK alpha1 in Adipocytes to Induce Exosome Shedding and Nonalcoholic Fatty Liver Development In Vivo. Diabetes 2021, 70, 577–588. [Google Scholar] [CrossRef]
- Ren, X.; Lv, J.; Wang, N.; Liu, J.; Gao, C.; Wu, X.; Yu, Y.; Teng, Q.; Dong, W.; Kong, H.; et al. Thioredoxin upregulation delays diabetes-induced photoreceptor cell degeneration via AMPK-mediated autophagy and exosome secretion. Diabetes Res. Clin. Pract. 2022, 185, 109788. [Google Scholar] [CrossRef]
- Kumar, D.; Shankar, S.; Srivastava, R.K. Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett. 2014, 343, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.I.; Mahajan, P.; Jan, S.; Jain, S.K.; Tiwari, H.; Sandey, J.; Bharate, S.; Nargotra, A.; Syed, S.H. Rottlerin is a pan phosphodiesterase inhibitor and can induce neurodifferentiation in IMR-32 human neuroblastoma cells. Eur. J. Pharmacol. 2019, 857, 172448. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Botas, J.; Ferruelo, A.J.; Suarez, Y.; Fernandez, C.; Gomez-Coronado, D.; Lasuncion, M.A. Dose-dependent effects of lovastatin on cell cycle progression. Distinct requirement of cholesterol and non-sterol mevalonate derivatives. Biochim. Biophys. Acta 2001, 1532, 185–194. [Google Scholar] [CrossRef]
- Calvo, D.; Gomez-Coronado, D.; Suarez, Y.; Lasuncion, M.A.; Vega, M.A. Human CD36 is a high affinity receptor for the native lipoproteins HDL, LDL, and VLDL. J. Lipid Res. 1998, 39, 777–788. [Google Scholar] [CrossRef]
- Canfran-Duque, A.; Pastor, O.; Quintana-Portillo, R.; Lerma, M.; de la Pena, G.; Martin-Hidalgo, A.; Fernandez-Hernando, C.; Lasuncion, M.A.; Busto, R. Curcumin promotes exosomes/microvesicles secretion that attenuates lysosomal cholesterol traffic impairment. Mol. Nutr. Food Res. 2014, 58, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Babiy, B.; Busto, R.; Pastor, O. A normalized signal calibration with a long-term reference improves the robustness of RPLC-MRM/MS lipidomics in plasma. Anal. Bioanal. Chem. 2021, 413, 4077–4090. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Peng, B.; Ahrends, R. Adaptation of Skyline for Targeted Lipidomics. J. Proteome Res. 2016, 15, 291–301. [Google Scholar] [CrossRef]
- Peng, B.; Kopczynski, D.; Pratt, B.S.; Ejsing, C.S.; Burla, B.; Hermansson, M.; Benke, P.I.; Tan, S.H.; Chan, M.Y.; Torta, F.; et al. LipidCreator workbench to probe the lipidomic landscape. Nat. Commun. 2020, 11, 2057. [Google Scholar] [CrossRef]
- Canfran-Duque, A.; Barrio, L.C.; Lerma, M.; de la Pena, G.; Serna, J.; Pastor, O.; Lasuncion, M.A.; Busto, R. First-Generation Antipsychotic Haloperidol Alters the Functionality of the Late Endosomal/Lysosomal Compartment in Vitro. Int. J. Mol. Sci. 2016, 17, 404. [Google Scholar] [CrossRef] [Green Version]
- Pfrieger, F.W.; Ungerer, N. Cholesterol metabolism in neurons and astrocytes. Prog. Lipid Res. 2011, 50, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Cenedella, R.J. Cholesterol synthesis inhibitor U18666A and the role of sterol metabolism and trafficking in numerous pathophysiological processes. Lipids 2009, 44, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, C.; Kamiyama, O.; Ikeda, K.; Sanae, F.; Kato, A.; Adachi, I.; Imahori, T.; Takahata, H.; Okamoto, T.; Asano, N. In vitro inhibition of glycogen-degrading enzymes and glycosidases by six-membered sugar mimics and their evaluation in cell cultures. Bioorg. Med. Chem. 2008, 16, 7330–7336. [Google Scholar] [CrossRef] [PubMed]
- Overkleeft, H.S.; Renkema, G.H.; Neele, J.; Vianello, P.; Hung, I.O.; Strijland, A.; van der Burg, A.M.; Koomen, G.J.; Pandit, U.K.; Aerts, J.M. Generation of specific deoxynojirimycin-type inhibitors of the non-lysosomal glucosylceramidase. J. Biol. Chem. 1998, 273, 26522–26527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aerts, J.M.; Hollak, C.; Boot, R.; Groener, A. Biochemistry of glycosphingolipid storage disorders: Implications for therapeutic intervention. Philos. Trans. R Soc. Lond. B Biol. Sci. 2003, 358, 905–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, S.; Patnaik, S.; Schoenen, F.; Zheng, W.; Choi, J.; Motabar, O.; Southall, N.; Westbroek, W.; Goldin, E.; Sidransky, E.; et al. Discovery, SAR, and Biological Evaluation of Non-inhibitory Chaperones of Glucocerebrosidase. In Probe Reports from the NIH Molecular Libraries Program; National Center for Biotechnology Information: Bethesda, MD, USA, 2010; Bookshelf ID: NBK143537. [Google Scholar]
- Tran, M.L.; Genisson, Y.; Ballereau, S.; Dehoux, C. Second-Generation Pharmacological Chaperones: Beyond Inhibitors. Molecules 2020, 25, 3145. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.; Goebel, C.; Runz, H.; Mobius, W.; Weiss, S.; Feussner, I.; Simons, M.; Schneider, A. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J. Biol. Chem. 2010, 285, 26279–26288. [Google Scholar] [CrossRef] [Green Version]
- Canfran-Duque, A.; Pastor, O.; Reina, M.; Lerma, M.; Cruz-Jentoft, A.J.; Lasuncion, M.A.; Busto, R. Curcumin Mitigates the Intracellular Lipid Deposit Induced by Antipsychotics In Vitro. PLoS ONE 2015, 10, e0141829. [Google Scholar] [CrossRef] [Green Version]
- Canfran-Duque, A.; Pastor, O.; Garcia-Seisdedos, D.; Molina, Y.L.; Babiy, B.; Lerma, M.; Sanchez-Castellano, C.; Martinez-Botas, J.; Gomez-Coronado, D.; Lasuncion, M.A.; et al. The Antipsychotic Risperidone Alters Dihydroceramide and Ceramide Composition and Plasma Membrane Function in Leukocytes In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 3919. [Google Scholar] [CrossRef]
- Honda, T.; Motoyoshi, K.; Kasahara, J.; Yamagata, K.; Takahashi, H.; Nakamura, H.; Murayama, T. Tyrosine-phosphorylation and activation of glucosylceramide synthase by v-Src: Its role in survival of HeLa cells against ceramide. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158817. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Kohyama-Koganeya, A.; Hirabayashi, Y. New insights on glucosylated lipids: Metabolism and functions. Biochim. Biophys. Acta 2013, 1831, 1475–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korschen, H.G.; Yildiz, Y.; Raju, D.N.; Schonauer, S.; Bonigk, W.; Jansen, V.; Kremmer, E.; Kaupp, U.B.; Wachten, D. The non-lysosomal beta-glucosidase GBA2 is a non-integral membrane-associated protein at the endoplasmic reticulum (ER) and Golgi. J. Biol. Chem. 2013, 288, 3381–3393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reza, S.; Ugorski, M.; Suchanski, J. Glucosylceramide and galactosylceramide, small glycosphingolipids with significant impact on health and disease. Glycobiology 2021, 31, 1416–1434. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Zhan, X.; Ye, J.; Han, L.; Qiu, W.; Gu, X.; Zhang, H. A rare form of Gaucher disease resulting from saposin C deficiency. Blood Cells Mol. Dis. 2018, 68, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Dinkins, M.B.; Dasgupta, S.; Wang, G.; Zhu, G.; Bieberich, E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 1792–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishibashi, Y.; Hirabayashi, Y. AMP-activated Protein Kinase Suppresses Biosynthesis of Glucosylceramide by Reducing Intracellular Sugar Nucleotides. J. Biol. Chem. 2015, 290, 18245–18260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.Y.; Lu, W.J.; Changou, C.A.; Hsiung, Y.C.; Trang, N.T.T.; Lee, C.Y.; Chang, T.H.; Jayakumar, T.; Hsieh, C.Y.; Yang, C.H.; et al. Platelet autophagic machinery involved in thrombosis through a novel linkage of AMPK-MTOR to sphingolipid metabolism. Autophagy 2021, 17, 4141–4158. [Google Scholar] [CrossRef]







| Sphingolipid | Control | Rottlerin | ||
|---|---|---|---|---|
| 2 μM | 5 μM | 10 μM | ||
| Ceramide | 0.71 ± 0.04 | 0.97 ± 0.03 ** | 0.91 ± 0.02 ** | 0.91 ± 0.04 ** |
| Hexosylceramide | 2.18 ± 0.05 | 1.85 ± 0.06 ** | 1.82 ± 0.02 ** | 1.70 ± 0.01 *** |
| Sphingomyelin | 20.13 ± 1.28 | 18.77 ± 0.16 | 17.49 ± 0.35 ** | 18.09 ± 1.07 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, Y.L.; García-Seisdedos, D.; Babiy, B.; Lerma, M.; Martínez-Botas, J.; Casarejos, M.J.; Vallejo, M.T.; Gómez-Coronado, D.; Lasunción, M.A.; Pastor, Ó.; et al. Rottlerin Stimulates Exosome/Microvesicle Release Via the Increase of Ceramide Levels Mediated by Ampk in an In Vitro Model of Intracellular Lipid Accumulation. Biomedicines 2022, 10, 1316. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10061316
Molina YL, García-Seisdedos D, Babiy B, Lerma M, Martínez-Botas J, Casarejos MJ, Vallejo MT, Gómez-Coronado D, Lasunción MA, Pastor Ó, et al. Rottlerin Stimulates Exosome/Microvesicle Release Via the Increase of Ceramide Levels Mediated by Ampk in an In Vitro Model of Intracellular Lipid Accumulation. Biomedicines. 2022; 10(6):1316. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10061316
Chicago/Turabian StyleMolina, Yessenia L., David García-Seisdedos, Bohdan Babiy, Milagros Lerma, Javier Martínez-Botas, María J. Casarejos, María T. Vallejo, Diego Gómez-Coronado, Miguel A. Lasunción, Óscar Pastor, and et al. 2022. "Rottlerin Stimulates Exosome/Microvesicle Release Via the Increase of Ceramide Levels Mediated by Ampk in an In Vitro Model of Intracellular Lipid Accumulation" Biomedicines 10, no. 6: 1316. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10061316