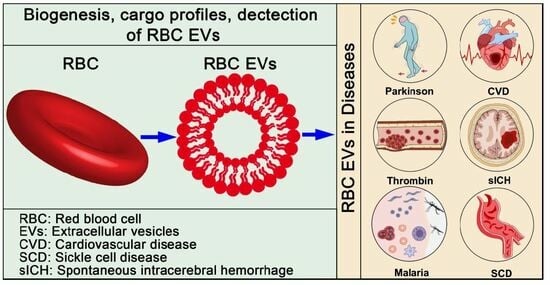
Red Blood Cell-Derived Extracellular Vesicles: An Overview of Current Research Progress, Challenges, and Opportunities
Abstract
:1. Introduction
2. Biogenesis of RBC EVs
3. Cargo Profiles of RBC EVs
4. Detection of RBC EVs
5. RBC EVs in Diseases
5.1. Procoagulant Activity and Hemostasis
5.2. Transfusion-Related Adverse Effects
5.3. Parkinson’s Disease (PD)
5.4. Cardiovascular Disease (CVD)
5.5. Sickle Cell Disease (SCD)
5.6. Malaria
6. The Applications of RBC EVs
6.1. RBC EVs as a Diagnostic and Prognostic Biomarker in Diseases
6.2. RBC EVs as Drug Delivery Vectors
7. Current Challenges and Opportunities
7.1. Selective Purification of RBC EVs
7.2. The Heterogeneity of RBC EVs
7.3. Application of RBC EVs in Stored RBC Products: Eliminate Them or Exploit Them
7.4. The Legal Framework of Using RBC EVs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Tian, J.W.; Zhang, H.J.; Li, S.Y.; Guo, Y.L.; Chen, G.; Yu, Z.L. Tumor Cell-derived Extracellular Vesicles in Modulating Phenotypes and Immune Functions of Macrophages: Mechanisms and Therapeutic Applications. J. Cancer 2023, 14, 1321–1334. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.L.; Liu, J.Y.; Chen, G. Small extracellular vesicle PD-L1 in cancer: The knowns and unknowns. NPJ Precis. Oncol. 2022, 6, 42. [Google Scholar] [CrossRef]
- Xie, Q.H.; Zheng, J.Q.; Ding, J.Y.; Wu, Y.F.; Liu, L.; Yu, Z.L.; Chen, G. Exosome-Mediated Immunosuppression in Tumor Microenvironments. Cells 2022, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Rubin, O.; Delobel, J.; Prudent, M.; Lion, N.; Kohl, K.; Tucker, E.I.; Tissot, J.D.; Angelillo-Scherrer, A. Red blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion 2013, 53, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Bakkour, S.; Acker, J.P.; Chafets, D.M.; Inglis, H.C.; Norris, P.J.; Lee, T.H.; Busch, M.P. Manufacturing method affects mitochondrial DNA release and extracellular vesicle composition in stored red blood cells. Vox Sang. 2016, 111, 22–32. [Google Scholar] [CrossRef]
- Nantakomol, D.; Dondorp, A.M.; Krudsood, S.; Udomsangpetch, R.; Pattanapanyasat, K.; Combes, V.; Grau, G.E.; White, N.J.; Viriyavejakul, P.; Day, N.P.; et al. Circulating red cell-derived microparticles in human malaria. J. Infect. Dis. 2011, 203, 700–706. [Google Scholar] [CrossRef]
- Nantakomol, D.; Palasuwan, A.; Chaowanathikhom, M.; Soogarun, S.; Imwong, M. Red cell and platelet-derived microparticles are increased in G6PD-deficient subjects. Eur. J. Haematol. 2012, 89, 423–429. [Google Scholar] [CrossRef]
- Punyadee, N.; Mairiang, D.; Thiemmeca, S.; Komoltri, C.; Pan-Ngum, W.; Chomanee, N.; Charngkaew, K.; Tangthawornchaikul, N.; Limpitikul, W.; Vasanawathana, S.; et al. Microparticles provide a novel biomarker to predict severe clinical outcomes of dengue virus infection. J. Virol. 2015, 89, 1587–1607. [Google Scholar] [CrossRef]
- Fischer, D.; Thies, F.; Awad, O.; Brat, C.; Meybohm, P.; Baer, P.C.; Muller, M.M.; Urbschat, A.; Maier, T.J.; Zacharowski, K.; et al. Red Blood Cell-Derived Microparticles Exert No Cancer Promoting Effects on Colorectal Cancer Cells In Vitro. Int. J. Mol. Sci. 2022, 23, 9323. [Google Scholar] [CrossRef]
- Noulsri, E.; Lerdwana, S.; Palasuwan, D.; Palasuwan, A. Cell-Derived Microparticles in Blood Products from Blood Donors Deficient in Glucose-6-Phosphate Dehydrogenase. Lab. Med. 2021, 52, 528–535. [Google Scholar] [CrossRef]
- Usman, W.M.; Pham, T.C.; Kwok, Y.Y.; Vu, L.T.; Ma, V.; Peng, B.; Chan, Y.S.; Wei, L.; Chin, S.M.; Azad, A.; et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 2018, 9, 2359. [Google Scholar] [CrossRef]
- Tang, T.T.; Wang, B.; Li, Z.L.; Wen, Y.; Feng, S.T.; Wu, M.; Liu, D.; Cao, J.Y.; Yin, Q.; Yin, D.; et al. Kim-1 Targeted Extracellular Vesicles: A New Therapeutic Platform for RNAi to Treat AKI. J. Am. Soc. Nephrol. 2021, 32, 2467–2483. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Yang, Y.; Wu, Z.; Tan, R.; Pham, T.T.; Yeo, E.Y.M.; Pirisinu, M.; Jayasinghe, M.K.; Pham, T.C.; Liang, K.; et al. Red blood cell extracellular vesicles deliver therapeutic siRNAs to skeletal muscles for treatment of cancer cachexia. Mol. Ther. 2023, 31, 1418–1436. [Google Scholar] [CrossRef] [PubMed]
- Chiangjong, W.; Netsirisawan, P.; Hongeng, S.; Chutipongtanate, S. Red Blood Cell Extracellular Vesicle-Based Drug Delivery: Challenges and Opportunities. Front. Med. 2021, 8, 761362. [Google Scholar] [CrossRef]
- Diaz-Varela, M.; de Menezes-Neto, A.; Perez-Zsolt, D.; Gamez-Valero, A.; Segui-Barber, J.; Izquierdo-Useros, N.; Martinez-Picado, J.; Fernandez-Becerra, C.; Del Portillo, H.A. Proteomics study of human cord blood reticulocyte-derived exosomes. Sci. Rep. 2018, 8, 14046. [Google Scholar] [CrossRef]
- Ciana, A.; Achilli, C.; Gaur, A.; Minetti, G. Membrane Remodelling and Vesicle Formation During Ageing of Human Red Blood Cells. Cell Physiol. Biochem. 2017, 42, 1127–1138. [Google Scholar] [CrossRef]
- Bebesi, T.; Kitka, D.; Gaal, A.; Szigyarto, I.C.; Deak, R.; Beke-Somfai, T.; Koprivanacz, K.; Juhasz, T.; Bota, A.; Varga, Z.; et al. Storage conditions determine the characteristics of red blood cell derived extracellular vesicles. Sci. Rep. 2022, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.; Kostova, E.; Bloem, E.; Hilarius-Stokman, P.; Meijer, A.B.; van den Berg, T.K.; Verhoeven, A.J.; de Korte, D.; van Bruggen, R. Potassium leakage primes stored erythrocytes for phosphatidylserine exposure and shedding of pro-coagulant vesicles. Br. J. Haematol. 2013, 160, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.B.; Ly, T.B.; Wesseling, M.C.; Hittinger, M.; Torge, A.; Devitt, A.; Perrie, Y.; Bernhardt, I. Characterization of Microvesicles Released from Human Red Blood Cells. Cell Physiol. Biochem. 2016, 38, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Cloos, A.S.; Ghodsi, M.; Stommen, A.; Vanderroost, J.; Dauguet, N.; Pollet, H.; D’Auria, L.; Mignolet, E.; Larondelle, Y.; Terrasi, R.; et al. Interplay Between Plasma Membrane Lipid Alteration, Oxidative Stress and Calcium-Based Mechanism for Extracellular Vesicle Biogenesis from Erythrocytes During Blood Storage. Front. Physiol. 2020, 11, 712. [Google Scholar] [CrossRef]
- Vorselen, D.; MacKintosh, F.C.; Roos, W.H.; Wuite, G.J. Competition between Bending and Internal Pressure Governs the Mechanics of Fluid Nanovesicles. ACS Nano 2017, 11, 2628–2636. [Google Scholar] [CrossRef]
- Vorselen, D.; van Dommelen, S.M.; Sorkin, R.; Piontek, M.C.; Schiller, J.; Dopp, S.T.; Kooijmans, S.A.A.; van Oirschot, B.A.; Versluijs, B.A.; Bierings, M.B.; et al. The fluid membrane determines mechanics of erythrocyte extracellular vesicles and is softened in hereditary spherocytosis. Nat. Commun. 2018, 9, 4960. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Kriebardis, A.G.; Rinalducci, S.; Antonelou, M.H.; Hansen, K.C.; Papassideri, I.S.; Zolla, L. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion 2015, 55, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Rubin, O.; Crettaz, D.; Canellini, G.; Tissot, J.D.; Lion, N. Microparticles in stored red blood cells: An approach using flow cytometry and proteomic tools. Vox Sang. 2008, 95, 288–297. [Google Scholar] [CrossRef]
- Cho, C.H.; Yun, S.G.; Koh, Y.E.; Lim, C.S. Effect of Irradiation on Microparticles in Red Blood Cell Concentrates. Ann. Lab. Med. 2016, 36, 362–366. [Google Scholar] [CrossRef]
- Buerck, J.P.; Burke, D.K.; Schmidtke, D.W.; Snyder, T.A.; Papavassiliou, D.V.; O’Rear, E.A. Production of erythrocyte microparticles in a sub-hemolytic environment. J. Artif. Organs 2021, 24, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Acker, J.P.; Almizraq, R.J.; Millar, D.; Maurer-Spurej, E. Screening of red blood cells for extracellular vesicle content as a product quality indicator. Transfusion 2018, 58, 2217–2226. [Google Scholar] [CrossRef]
- Almizraq, R.J.; Holovati, J.L.; Acker, J.P. Characteristics of Extracellular Vesicles in Red Blood Concentrates Change with Storage Time and Blood Manufacturing Method. Transfus. Med. Hemother 2018, 45, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Falcon-Pérez, J.M.; López-Sarrio, S.; González, E.; Alkhraisat, M.H. Advances in Platelet Rich Plasma-Derived Extracellular Vesicles for Regenerative Medicine: A Systematic-Narrative Review. Int. J. Mol. Sci. 2023, 24, 13043. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, J.; Fan, L.; Lin, Q.; Fu, D.; Wei, B.; Wei, S. MicroRNA Profiling of Exosomes Derived from Red Blood Cell Units: Implications in Transfusion-Related Immunomodulation. BioMed Res. Int. 2019, 2019, 2045915. [Google Scholar] [CrossRef]
- Oh, J.Y.; Marques, M.B.; Xu, X.; Li, J.; Genschmer, K.R.; Phillips, E.; Chimento, M.F.; Mobley, J.; Gaggar, A.; Patel, R.P. Different-sized extracellular vesicles derived from stored red blood cells package diverse cargoes and cause distinct cellular effects. Transfusion 2023, 63, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Stamoulis, K.E.; Anastasiadi, A.T.; Papassideri, I.S.; Kriebardis, A.G.; Rinalducci, S.; Antonelou, M.H. Leukoreduction makes a difference: A pair proteomics study of extracellular vesicles in red blood cell units. Transfus. Apher. Sci. 2021, 60, 103166. [Google Scholar] [CrossRef]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Yu, Z.L.; Liu, X.C.; Wu, M.; Shi, S.; Fu, Q.Y.; Jia, J.; Chen, G. Untouched isolation enables targeted functional analysis of tumour-cell-derived extracellular vesicles from tumour tissues. J. Extracell. Vesicles 2022, 11, e12214. [Google Scholar] [CrossRef] [PubMed]
- Canellini, G.; Rubin, O.; Delobel, J.; Crettaz, D.; Lion, N.; Tissot, J.D. Red blood cell microparticles and blood group antigens: An analysis by flow cytometry. Blood Transfus. 2012, 10 (Suppl. 2), s39–s45. [Google Scholar] [CrossRef]
- Grisendi, G.; Finetti, E.; Manganaro, D.; Cordova, N.; Montagnani, G.; Spano, C.; Prapa, M.; Guarneri, V.; Otsuru, S.; Horwitz, E.M.; et al. Detection of microparticles from human red blood cells by multiparametric flow cytometry. Blood Transfus. 2015, 13, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Oriss, T.B.; Cavaretta, J.P.; Rosengart, M.R.; Lee, J.S. Red cell microparticle enumeration: Validation of a flow cytometric approach. Vox Sang. 2012, 103, 42–48. [Google Scholar] [CrossRef]
- Nantakomol, D.; Imwong, M.; Soontarawirat, I.; Kotjanya, D.; Khakhai, C.; Ohashi, J.; Nuchnoi, P. The absolute counting of red cell-derived microparticles with red cell bead by flow rate based assay. Cytometry B Clin. Cytom. 2009, 76, 191–198. [Google Scholar] [CrossRef]
- Bozic, D.; Hocevar, M.; Kisovec, M.; Pajnic, M.; Paden, L.; Jeran, M.; Bedina Zavec, A.; Podobnik, M.; Kogej, K.; Iglic, A.; et al. Stability of Erythrocyte-Derived Nanovesicles Assessed by Light Scattering and Electron Microscopy. Int. J. Mol. Sci. 2021, 22, 12772. [Google Scholar] [CrossRef]
- Almizraq, R.J.; Seghatchian, J.; Holovati, J.L.; Acker, J.P. Extracellular vesicle characteristics in stored red blood cell concentrates are influenced by the method of detection. Transfus. Apher. Sci. 2017, 56, 254–260. [Google Scholar] [CrossRef]
- Noubouossie, D.F.; Henderson, M.W.; Mooberry, M.; Ilich, A.; Ellsworth, P.; Piegore, M.; Skinner, S.C.; Pawlinski, R.; Welsby, I.; Renne, T.; et al. Red blood cell microvesicles activate the contact system, leading to factor IX activation via 2 independent pathways. Blood 2020, 135, 755–765. [Google Scholar] [CrossRef]
- Levin, G.Y.; Sukhareva, E. Antithrombin activity in microvesicles derived from stored red blood cells. Blood Transfus. 2015, 13, 688–689. [Google Scholar] [CrossRef]
- Devalet, B.; Wannez, A.; Bailly, N.; Alpan, L.; Gheldof, D.; Douxfils, J.; Deneys, V.; Bihin, B.; Chatelain, B.; Dogne, J.M.; et al. Application of a clot-based assay to measure the procoagulant activity of stored allogeneic red blood cell concentrates. Blood Transfus. 2018, 16, 163–172. [Google Scholar] [CrossRef]
- Levin, G.Y.; Sukhareva, E.G. Antithrombin Activity of Erythrocyte Microvesicles. Bull. Exp. Biol. Med. 2017, 162, 718–721. [Google Scholar] [CrossRef]
- Levin, G.Y.; Sukhareva, E.G.; Egorikhina, M.N. Effects of erythrocyte microvesicles on the coagulation process stages. Bull. Exp. Biol. Med. 2013, 156, 32–34. [Google Scholar] [CrossRef]
- Avenick, D.; Kidd, L.; Istvan, S.; Dong, F.; Richter, K.; Edwards, N.; Hisada, Y.; Posma, J.J.N.; Massih, C.A.; Mackman, N. Effects of storage and leukocyte reduction on the concentration and procoagulant activity of extracellular vesicles in canine packed red cells. J. Vet. Emerg. Crit. Care 2021, 31, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Sowy, S.; Rutter, C.R.; Jeffery, U. Extracellular vesicle concentration and procoagulant activity of canine haemoperitoneum fluid and packed red blood cells. J. Small Anim. Pract. 2019, 60, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Xia, B.T.; Jung, A.D.; Chang, A.L.; Abplanalp, W.A.; Caldwell, C.C.; Goodman, M.D.; Pritts, T.A. Microparticles from stored red blood cells promote a hypercoagulable state in a murine model of transfusion. Surgery 2018, 163, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.; Sukhareva, E.; Lavrentieva, A. Impact of microparticles derived from erythrocytes on fibrinolysis. J. Thromb. Thrombolysis 2016, 41, 452–458. [Google Scholar] [CrossRef]
- Levin, G.; Sukhareva, E. The influence of thermal trauma on pro- and anticoagulant activity of erythrocyte-derived microvesicles. Burns 2016, 42, 1528–1533. [Google Scholar] [CrossRef]
- Jy, W.; Johansen, M.E.; Bidot, C., Jr.; Horstman, L.L.; Ahn, Y.S. Red cell-derived microparticles (RMP) as haemostatic agent. Thromb. Haemost. 2013, 110, 751–760. [Google Scholar] [CrossRef]
- Tripisciano, C.; Weiss, R.; Karuthedom George, S.; Fischer, M.B.; Weber, V. Extracellular Vesicles Derived From Platelets, Red Blood Cells, and Monocyte-Like Cells Differ Regarding Their Ability to Induce Factor XII-Dependent Thrombin Generation. Front. Cell Dev. Biol. 2020, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, E.X. Thromboinflammatory effects of RBC microvesicles. Blood 2020, 135, 708–709. [Google Scholar] [CrossRef]
- Kim, Y.; Goodman, M.D.; Jung, A.D.; Abplanalp, W.A.; Schuster, R.M.; Caldwell, C.C.; Lentsch, A.B.; Pritts, T.A. Microparticles from aged packed red blood cell units stimulate pulmonary microthrombus formation via P-selectin. Thromb. Res. 2020, 185, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Bussow, J.; Meybohm, P.; Weber, C.F.; Zacharowski, K.; Urbschat, A.; Muller, M.M.; Jennewein, C. Microparticles from stored red blood cells enhance procoagulant and proinflammatory activity. Transfusion 2017, 57, 2701–2711. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, N.D.; Nadali, J.; Divani, A.; Hatefimoadab, N. Ways To Enhance Blood Transfusion Safety: A Systematic Review. Florence Nightingale J. Nurs. 2022, 30, 288–300. [Google Scholar] [CrossRef]
- van Manen, L.; Peters, A.L.; van der Sluijs, P.M.; Nieuwland, R.; van Bruggen, R.; Juffermans, N.P. Clearance and phenotype of extracellular vesicles after red blood cell transfusion in a human endotoxemia model. Transfus. Apher. Sci. 2019, 58, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Voss, S.C.; Yassin, M.; Grivel, J.C.; Al Hmissi, S.; Allahverdi, N.; Nashwan, A.; Merenkov, Z.; Abdulla, M.; Al Malki, A.; Raynaud, C.; et al. Red blood cell derived extracellular vesicles during the process of autologous blood doping. Drug Test. Anal. 2022, 14, 1984–1994. [Google Scholar] [CrossRef]
- Peters, A.L.; Vlaar, A.P.J.; van Bruggen, R.; de Korte, D.; Meijers, J.C.M.; Nieuwland, R.; Juffermans, N.P. Transfusion of autologous extracellular vesicles from stored red blood cells does not affect coagulation in a model of human endotoxemia. Transfusion 2018, 58, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Belizaire, R.M.; Prakash, P.S.; Richter, J.R.; Robinson, B.R.; Edwards, M.J.; Caldwell, C.C.; Lentsch, A.B.; Pritts, T.A. Microparticles from stored red blood cells activate neutrophils and cause lung injury after hemorrhage and resuscitation. J. Am. Coll. Surg. 2012, 214, 648–655. [Google Scholar] [CrossRef]
- Xie, R.; Yang, Y.; Zhu, Y.; Gao, L.; Jiang, X.; Sun, J.; Bian, M.; Yang, J. Microparticles in red cell concentrates prime polymorphonuclear neutrophils and cause acute lung injury in a two-event mouse model. Int. Immunopharmacol. 2018, 55, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Straat, M.; van Hezel, M.E.; Boing, A.; Tuip-De Boer, A.; Weber, N.; Nieuwland, R.; van Bruggen, R.; Juffermans, N.P. Monocyte-mediated activation of endothelial cells occurs only after binding to extracellular vesicles from red blood cell products, a process mediated by beta-integrin. Transfusion 2016, 56, 3012–3020. [Google Scholar] [CrossRef]
- Gao, Y.; Jin, H.; Tan, H.; Wang, Y.; Wu, J.; Wang, Y.; Zhang, J.; Yang, Y.; Tian, W.; Hou, R. The role of extracellular vesicles from stored RBC units in B lymphocyte survival and plasma cell differentiation. J. Leukoc. Biol. 2020, 108, 1765–1776. [Google Scholar] [CrossRef]
- Paul, E.; George, J.; Ward, S.; Fitzgerald, K.; Jones, G.; Magana, K.; Modi, J.; Magee, T.; Hughes, G.; Ford, A.I.; et al. Assessing Uptake of the Core Outcome Set in Randomized Controlled Trials for Parkinson’s Disease: A Systematic Review. Ageing Res. Rev. 2023, 91, 102081. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Stewart, T.; Sheng, L.; Li, N.; Bullock, K.; Song, N.; Shi, M.; Banks, W.A.; Zhang, J. Transmission of alpha-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: Another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol. Commun. 2017, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chan, R.B.; Cai, Z.; Liu, X.; Wu, Y.; Yu, Z.; Feng, T.; Yang, Y.; Zhang, J. alpha-Synuclein-containing erythrocytic extracellular vesicles: Essential contributors to hyperactivation of monocytes in Parkinson’s disease. J. Neuroinflamm. 2022, 19, 53. [Google Scholar] [CrossRef]
- Yuan, Y.; Maitusong, M.; Muyesai, N. Association of endothelial and red blood cell microparticles with acute myocardial infarction in Chinese: A retrospective study. Ann. Palliat. Med. 2020, 9, 1564–1570. [Google Scholar] [CrossRef]
- Giannopoulos, G.; Oudatzis, G.; Paterakis, G.; Synetos, A.; Tampaki, E.; Bouras, G.; Hahalis, G.; Alexopoulos, D.; Tousoulis, D.; Cleman, M.W.; et al. Red blood cell and platelet microparticles in myocardial infarction patients treated with primary angioplasty. Int. J. Cardiol. 2014, 176, 145–150. [Google Scholar] [CrossRef]
- Valkov, N.; Das, A.; Tucker, N.R.; Li, G.; Salvador, A.M.; Chaffin, M.D.; Pereira De Oliveira Junior, G.; Kur, I.; Gokulnath, P.; Ziegler, O.; et al. SnRNA sequencing defines signaling by RBC-derived extracellular vesicles in the murine heart. Life Sci. Alliance 2021, 4, e202101048. [Google Scholar] [CrossRef]
- Khan, S.S.; Meyer, M. Atrial Fibrillation and Heart Failure with Preserved Ejection Fraction: Two Dishes-Same Ingredients. JACC Heart Fail. 2023. [Google Scholar] [CrossRef]
- Yang, L.; Huang, S.; Zhang, Z.; Liu, Z.; Zhang, L. Roles and Applications of Red Blood Cell-Derived Extracellular Vesicles in Health and Diseases. Int. J. Mol. Sci. 2022, 23, 5927. [Google Scholar] [CrossRef] [PubMed]
- Dastur, C.K.; Yu, W. Current management of spontaneous intracerebral haemorrhage. Stroke Vasc. Neurol. 2017, 2, 21–29. [Google Scholar] [CrossRef]
- Rehni, A.K.; Cho, S.; Zhang, Z.; Khushal, P.; Raval, A.P.; Koch, S.; Perez-Pinzon, M.A.; Zhao, W.; Jy, W.; Dave, K.R. Red Cell Microparticles Suppress Hematoma Growth Following Intracerebral Hemorrhage in Chronic Nicotine-Exposed Rats. Int. J. Mol. Sci. 2022, 23, 15167. [Google Scholar] [CrossRef] [PubMed]
- Rehni, A.K.; Cho, S.; Quero, H.N.; Shukla, V.; Zhang, Z.; Dong, C.; Zhao, W.; Perez-Pinzon, M.A.; Koch, S.; Jy, W.; et al. Red Blood Cell Microparticles Limit Hematoma Growth in Intracerebral Hemorrhage. Stroke 2022, 53, 3182–3191. [Google Scholar] [CrossRef]
- Kavanagh, P.L.; Fasipe, T.A.; Wun, T. Sickle Cell Disease: A Review. JAMA 2022, 328, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Mankelow, T.J.; Drizou, D.; Bullock, T.; Latham, T.; Trompeter, S.; Blair, A.; Anstee, D.J. Large red cell-derived membrane particles are major contributors to hypercoagulability in sickle cell disease. Sci. Rep. 2021, 11, 11035. [Google Scholar] [CrossRef]
- Hierso, R.; Lemonne, N.; Villaescusa, R.; Lalanne-Mistrih, M.L.; Charlot, K.; Etienne-Julan, M.; Tressieres, B.; Lamarre, Y.; Tarer, V.; Garnier, Y.; et al. Exacerbation of oxidative stress during sickle vaso-occlusive crisis is associated with decreased anti-band 3 autoantibodies rate and increased red blood cell-derived microparticle level: A prospective study. Br. J. Haematol. 2017, 176, 805–813. [Google Scholar] [CrossRef]
- Nader, E.; Romana, M.; Guillot, N.; Fort, R.; Stauffer, E.; Lemonne, N.; Garnier, Y.; Skinner, S.C.; Etienne-Julan, M.; Robert, M.; et al. Association Between Nitric Oxide, Oxidative Stress, Eryptosis, Red Blood Cell Microparticles, and Vascular Function in Sickle Cell Anemia. Front. Immunol. 2020, 11, 551441. [Google Scholar] [CrossRef]
- An, R.; Man, Y.; Cheng, K.; Zhang, T.; Chen, C.; Wang, F.; Abdulla, F.; Kucukal, E.; Wulftange, W.J.; Goreke, U.; et al. Sickle red blood cell-derived extracellular vesicles activate endothelial cells and enhance sickle red cell adhesion mediated by von Willebrand factor. Br. J. Haematol. 2023, 201, 552–563. [Google Scholar] [CrossRef]
- Olatunya, O.S.; Lanaro, C.; Longhini, A.L.; Penteado, C.F.F.; Fertrin, K.Y.; Adekile, A.; Saad, S.T.O.; Costa, F.F. Red blood cells microparticles are associated with hemolysis markers and may contribute to clinical events among sickle cell disease patients. Ann. Hematol. 2019, 98, 2507–2521. [Google Scholar] [CrossRef]
- Vimonpatranon, S.; Roytrakul, S.; Phaonakrop, N.; Lekmanee, K.; Atipimonpat, A.; Srimark, N.; Sukapirom, K.; Chotivanich, K.; Khowawisetsut, L.; Pattanapanyasat, K. Extracellular Vesicles Derived from Early and Late Stage Plasmodium falciparum-Infected Red Blood Cells Contain Invasion-Associated Proteins. J. Clin. Med. 2022, 11, 4250. [Google Scholar] [CrossRef] [PubMed]
- Mantel, P.Y.; Hoang, A.N.; Goldowitz, I.; Potashnikova, D.; Hamza, B.; Vorobjev, I.; Ghiran, I.; Toner, M.; Irimia, D.; Ivanov, A.R.; et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 2013, 13, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Babatunde, K.A.; Mbagwu, S.; Hernandez-Castaneda, M.A.; Adapa, S.R.; Walch, M.; Filgueira, L.; Falquet, L.; Jiang, R.H.Y.; Ghiran, I.; Mantel, P.Y. Malaria infected red blood cells release small regulatory RNAs through extracellular vesicles. Sci. Rep. 2018, 8, 884. [Google Scholar] [CrossRef]
- Mantel, P.Y.; Hjelmqvist, D.; Walch, M.; Kharoubi-Hess, S.; Nilsson, S.; Ravel, D.; Ribeiro, M.; Gruring, C.; Ma, S.; Padmanabhan, P.; et al. Infected erythrocyte-derived extracellular vesicles alter vascular function via regulatory Ago2-miRNA complexes in malaria. Nat. Commun. 2016, 7, 12727. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Barnes, T.; Hafalla, J.C.; Combes, V.; Ryffel, B.; Secher, T.; Grau, G.E.; Riley, E.M.; de Souza, J.B. Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS Pathog. 2010, 6, e1000744. [Google Scholar] [CrossRef]
- Ye, W.; Chew, M.; Hou, J.; Lai, F.; Leopold, S.J.; Loo, H.L.; Ghose, A.; Dutta, A.K.; Chen, Q.; Ooi, E.E.; et al. Microvesicles from malaria-infected red blood cells activate natural killer cells via MDA5 pathway. PLoS Pathog. 2018, 14, e1007298. [Google Scholar] [CrossRef]
- Sampaio, N.G.; Emery, S.J.; Garnham, A.L.; Tan, Q.Y.; Sisquella, X.; Pimentel, M.A.; Jex, A.R.; Regev-Rudzki, N.; Schofield, L.; Eriksson, E.M. Extracellular vesicles from early stage Plasmodium falciparum-infected red blood cells contain PfEMP1 and induce transcriptional changes in human monocytes. Cell Microbiol. 2018, 20, e12822. [Google Scholar] [CrossRef] [PubMed]
- Regev-Rudzki, N.; Wilson, D.W.; Carvalho, T.G.; Sisquella, X.; Coleman, B.M.; Rug, M.; Bursac, D.; Angrisano, F.; Gee, M.; Hill, A.F.; et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 2013, 153, 1120–1133. [Google Scholar] [CrossRef]
- Khowawisetsut, L.; Vimonpatranon, S.; Lekmanee, K.; Sawasdipokin, H.; Srimark, N.; Chotivanich, K.; Pattanapanyasat, K. Differential Effect of Extracellular Vesicles Derived from Plasmodium falciparum-Infected Red Blood Cells on Monocyte Polarization. Int. J. Mol. Sci. 2023, 24, 2631. [Google Scholar] [CrossRef] [PubMed]
- Charoensappakit, A.; Puapatanakul, P.; Praditpornsilpa, K.; Palasuwan, A.; Noulsri, E.; Palasuwan, D. Urinary red blood cell-derived microparticles and phosphatidylserine-exposing red blood cells in glomerular and non-glomerular hematuria patients. Cytometry B Clin. Cytom. 2022, 102, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Manakeng, K.; Prasertphol, P.; Phongpao, K.; Chuncharunee, S.; Tanyong, D.; Worawichawong, S.; Svasti, S.; Chaichompoo, P. Elevated levels of platelet- and red cell-derived extracellular vesicles in transfusion-dependent beta-thalassemia/HbE patients with pulmonary arterial hypertension. Ann. Hematol. 2019, 98, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Gkaliagkousi, E.; Nikolaidou, B.; Gavriilaki, E.; Lazaridis, A.; Yiannaki, E.; Anyfanti, P.; Zografou, I.; Markala, D.; Douma, S. Increased erythrocyte- and platelet-derived microvesicles in newly diagnosed type 2 diabetes mellitus. Diab Vasc. Dis. Res. 2019, 16, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Hasse, S.; Julien, A.S.; Duchez, A.C.; Zhao, C.; Boilard, E.; Fortin, P.R.; Bourgoin, S.G. Red blood cell-derived phosphatidylserine positive extracellular vesicles are associated with past thrombotic events in patients with systemic erythematous lupus. Lupus Sci. Med. 2022, 9, e000605. [Google Scholar] [CrossRef]
- Rehni, A.K.; Shukla, V.; Navarro Quero, H.; Bidot, C., Jr.; Haase, C.R.; Crane, E.A.A.; Patel, S.G.; Koch, S.; Ahn, Y.S.; Jy, W.; et al. Preclinical Evaluation of Safety and Biodistribution of Red Cell Microparticles: A Novel Hemostatic Agent. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 474–483. [Google Scholar] [CrossRef]
- Yu, Z.L.; Zhao, Y.; Miao, F.; Wu, M.; Xia, H.F.; Chen, Z.K.; Liu, H.M.; Zhao, Y.F.; Chen, G. In Situ Membrane Biotinylation Enables the Direct Labeling and Accurate Kinetic Analysis of Small Extracellular Vesicles in Circulation. Anal. Chem. 2021, 93, 10862–10870. [Google Scholar] [CrossRef]
- Tang, J.C.; Lee, C.H.; Lu, T.; Vankayala, R.; Hanley, T.; Azubuogu, C.; Li, J.; Nair, M.G.; Jia, W.; Anvari, B. Membrane Cholesterol Enrichment of Red Blood Cell-Derived Microparticles Results in Prolonged Circulation. ACS Appl. Bio Mater. 2022, 5, 650–660. [Google Scholar] [CrossRef]
- Peng, B.; Nguyen, T.M.; Jayasinghe, M.K.; Gao, C.; Pham, T.T.; Vu, L.T.; Yeo, E.Y.M.; Yap, G.; Wang, L.; Goh, B.C.; et al. Robust delivery of RIG-I agonists using extracellular vesicles for anti-cancer immunotherapy. J. Extracell. Vesicles 2022, 11, e12187. [Google Scholar] [CrossRef]
- Borgheti-Cardoso, L.N.; Kooijmans, S.A.A.; Chamorro, L.G.; Biosca, A.; Lantero, E.; Ramirez, M.; Avalos-Padilla, Y.; Crespo, I.; Fernandez, I.; Fernandez-Becerra, C.; et al. Extracellular vesicles derived from Plasmodium-infected and non-infected red blood cells as targeted drug delivery vehicles. Int. J. Pharm. 2020, 587, 119627. [Google Scholar] [CrossRef]
- Xu, R.; Yu, Z.L.; Liu, X.C.; Xie, Q.H.; Wu, M.; Chen, G. Aptamer-Assisted Traceless Isolation of PD-L1-Positive Small Extracellular Vesicles for Dissecting Their Subpopulation Signature and Function. Anal. Chem. 2023, 95, 1016–1026. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.-R.; Xia, H.-F.; Gong, P.; Yu, Z.-L. Red Blood Cell-Derived Extracellular Vesicles: An Overview of Current Research Progress, Challenges, and Opportunities. Biomedicines 2023, 11, 2798. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines11102798
Ma S-R, Xia H-F, Gong P, Yu Z-L. Red Blood Cell-Derived Extracellular Vesicles: An Overview of Current Research Progress, Challenges, and Opportunities. Biomedicines. 2023; 11(10):2798. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines11102798
Chicago/Turabian StyleMa, Si-Rui, Hou-Fu Xia, Ping Gong, and Zi-Li Yu. 2023. "Red Blood Cell-Derived Extracellular Vesicles: An Overview of Current Research Progress, Challenges, and Opportunities" Biomedicines 11, no. 10: 2798. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines11102798