Plasma hsa-miR-22-3p Might Serve as an Early Predictor of Ventricular Function Recovery after ST-Elevation Acute Myocardial Infarction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
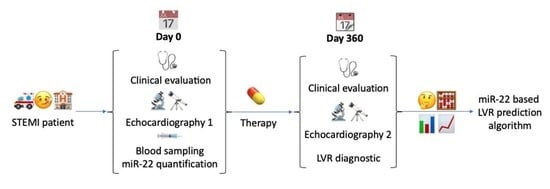
2.2. The Follow-Up Group
2.3. Specimen Collection
2.4. RNA Purification
2.5. PCR Detection
2.6. Statistical Analysis
3. Results
3.1. Baseline Clinical Data of Patients
3.2. miR in STEMI Patients vs. Control
3.3. miR and Clinical Parameters in STEMI Patients
3.4. miR in the Follow-Up Group
3.5. miR in Diabetic vs. Non-Diabetic Patients
4. Discussion
4.1. miR-22 and the Stressed Heart
4.2. MicroRNAs and DM
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anoop, S.V.; Shah, K.K.L.; Rodriguez, J.A.; Campbell, D.; Astengo, F.; Logue, J.; Gallacher, P.J.; Katikireddi, S.V.; Bing, R.; Alam, S.R.; et al. Clinical burden, risk factor impact and outcomes following myocardial infarction and stroke: A 25-year individual patient level linkage study. Lancet Reg. Health-Eur. 2021, 7, 100141. [Google Scholar] [CrossRef]
- Sulo, G.I.J.; Vollset, S.E.; Nygård, O.; Ebbing, M.; Sulo, E.; Egeland, G.M.; Tell, G.S. Heart Failure Complicating Acute Myocardial Infarction; Burden and Timing of Occurrence: A Nation-wide Analysis Including 86 771 Patients from the Cardiovascular Disease in Norway (CVDNOR) Project. J. Am. Heart Assoc. 2016, 5, e002667. [Google Scholar] [CrossRef]
- Minicucci, M.F.; Azevedo, P.S.; Polegato, B.F.; Paiva, S.A.R.; Zornoff, L.A.M. Heart Failure After Myocardial Infarction: Clinical Implications and Treatment. Clin. Cardiol. 2011, 34, 410–414. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Fonarow, G.C. Management of Post-Myocardial Infarction Patients with Left Ventricular Systolic Dysfunction. Am. J. Med. 2007, 120, 109–120. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Roger, V.L.; Weston, S.A.; Jiang, R.; Redfield, M.M. Longitudinal Changes in Ejection Fraction in Heart Failure Patients with Preserved and Reduced Ejection Fraction. Circ. Heart Fail. 2012, 5, 720–726. [Google Scholar] [CrossRef]
- Chew, D.S.; Heikki, H.; Schmidt, G.; Kavanagh, K.M.; Dommasch, M.; Thomsen, P.E.B.; Sinnecker, D.; Raatikainen, P.; Exner, D.V. Change in Left Ventricular Ejection Fraction Following First Myocardial Infarction and Outcome. JACC Clin. Electrophysiol. 2018, 4, 672–682. [Google Scholar] [CrossRef]
- Parodi, G.; Memisha, G.; Carrabba, N.; Signorini, U.; Migliorini, A.; Cerisano, G.; Antoniucci, D. Prevalence, Predictors, Time Course, and Long-Term Clinical Implications of Left Ventricular Functional Recovery After Mechanical Reperfusion for Acute Myocardial Infarction. Am. J. Cardiol. 2007, 100, 1718–1722. [Google Scholar] [CrossRef]
- Brooks, G.C.; Lee, B.K.; Rao, R.; Lin, F.; Morin, D.P.; Zweibel, S.L.; Buxton, A.E.; Pletcher, M.J.; Vittinghoff, E.; Olgin, J.E.; et al. Predicting Persistent Left Ventricular Dysfunction Following Myocardial Infarction: The PREDICTS Study. J. Am. Coll. Cardiol. 2016, 67, 1186–1196. [Google Scholar] [CrossRef]
- Radesich, C.; Cappelletto, C.; Indennidate, C.; Perotto, M.; Di Lenarda, A. Predicting left ventricular functional recovery in ischaemic cardiomyopathy: Needs and challenges. Eur. Heart J. Suppl. 2023, 25 (Suppl. B), B69–B74. [Google Scholar] [CrossRef]
- Lei, Z.; Li, B.; Peng, W. Predictors and prognostic impact of left ventricular ejection fraction trajectories in patients with ST-segment elevation myocardial infarction. Aging Clin Exp Res. 2022, 34, 1429–1438. [Google Scholar] [CrossRef]
- Khan, J.N.; McCann, G.P. Cardiovascular magnetic resonance imaging assessment of outcomes in acute myocardial infarction. World J. Cardiol. 2017, 9, 109–133. [Google Scholar] [CrossRef]
- Rischpler, C.; Langwieser, N.; Souvatzoglou, M.; Batrice, A.; van Marwick, S.; Snajberk, J.; Ibrahim, T.; Laugwitz, K.-L.; Nekolla, S.G.; Schwaiger, M. PET/MRI early after myocardial infarction: Evaluation of viability with late gadolinium enhancement transmurality vs. 18F-FDG uptake. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 661–669. [Google Scholar] [CrossRef]
- Węgiel, M.; Rakowski, T. Circulating biomarkers as predictors of left ventricular remodeling after myocardial infarction. Adv. Interv. Cardiol./Postępy W Kardiol. Interwencyjnej 2021, 17, 21–32. [Google Scholar] [CrossRef]
- Shrivastava, A.H.T.; Zeller, T.; Schulte, C. Biomarkers for Heart Failure Prognosis: Proteins, Genetic Scores and Non-coding RNAs. Front. Cardiovasc. Med. 2020, 7, 601364. [Google Scholar] [CrossRef]
- Konstam, M.A.; Kramer, D.G.; Patel, A.R.; Maron, M.S.; Udelson, J.E. Left Ventricular Remodeling in Heart Failure: Current Concepts in Clinical Significance and Assessment. JACC: Cardiovasc. Imaging 2011, 4, 98–108. [Google Scholar] [CrossRef]
- Divakaran, V.; Mann, D.L. The Emerging Role of MicroRNAs in Cardiac Remodeling and Heart Failure. Circ. Res. 2008, 103, 1072–1083. [Google Scholar] [CrossRef]
- Chen, C.; Ponnusamy, M.; Liu, C.; Gao, J.; Wang, K.; Li, P. MicroRNA as a Therapeutic Target in Cardiac Remodeling. BioMed Res. Int. 2017, 2017, 1278436. [Google Scholar] [CrossRef]
- Maries, L.; Marian, C.; Sosdean, R.; Goanta, F.; Sirbu, I.O.; Anghel, A. MicroRNAs-The Heart of Post-Myocardial Infarction Remodeling. Diagnostics 2021, 11, 1675. [Google Scholar] [CrossRef]
- Moscoso, I.; Cebro-Márquez, M.; Martínez-Gómez, Á.; Abou-Jokh, C.; Martinez-Monzonis, M.A.; Martines-Sande, J.L.; Gonzalez-Melchor, L.; Garcia-Seara, J.; Fernandez-Lopez, X.A.; Morana-Fernandez, S.; et al. Circulating miR-499a and miR-125b as Potential Predictors of Left Ventricular Ejection Fraction Improvement after Cardiac Resynchronization Therapy. Cells 2022, 11, 271. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, Y.; Wang, X.; Li, S.; Yu, T.; Duan, W.; Wu, J.; Wen, Z.; Iiao, Y.; Sun, Z.; et al. Circulating miR-1 as a potential predictor of left ventricular remodeling following acute ST-segment myocardial infarction using cardiac magnetic resonance. Quant. Imaging Med. Surg. 2020, 10, 1490–1503. [Google Scholar] [CrossRef]
- Devaux, Y.; Vausort, M.; McCann, G.P.; Kelly, D.; Collignon, O.; Ng, L.N.; Wagner, D.R.; Squire, I.B. A Panel of 4 microRNAs Facilitates the Prediction of Left Ventricular Contractility after Acute Myocardial Infarction. PLoS ONE 2013, 8, e70644. [Google Scholar] [CrossRef]
- Hromádka, M.; Černá, V.; Pešta, M.; Kučerová, A.; Jarkovský, J.; Rajdl, D.; Rokyta, R.; Moťovská, Z. Prognostic Value of MicroRNAs in Patients after Myocardial Infarction: A Substudy of PRAGUE-18. Dis. Markers 2019, 2019, 2925019. [Google Scholar] [CrossRef]
- Satake, A.; Minatoguchi, S.; Heishima, K.; Yasuda, S.; Murase, H.; Yoshizumi, R.; Komaki, H.; Baba, S.; Ojio, S.; Tanaka, T.; et al. An Increase in Plasma MicroRNA-143 Levels in the Acute Phase Is Positively Correlated with Recovery of Cardiac Function in the Chronic Phase in Patients with Acute Myocardial Infarction. Circ. J. 2023, 87, 824–833. [Google Scholar] [CrossRef]
- Maciejak, A.; Kiliszek, M.; Opolski, G.; Segiet, A.; Matlak, K.; Dobrzycki, S.; Tulacz, D.; Sygitowicz, G.; Burzynska, B.; Gora, M. miR-22-5p revealed as a potential biomarker involved in the acute phase of myocardial infarction via profiling of circulating microRNAs. Mol. Med. Rep. 2016, 14, 2867–2875. [Google Scholar] [CrossRef]
- Li, H.; Zhang, P.; Li, F.; Yuan, G.; Wang, X.; Zhang, A.; Li, F. Plasma miR-22-5p, miR-132-5p, and miR-150-3p Are Associated with Acute Myocardial Infarction. BioMed Res. Int. 2019, 2019, 5012648. [Google Scholar] [CrossRef]
- Du, J.K.; Cong, B.H.; Yu, Q.; Wang, H.; Wang, L.; Wang, C.-N.; Tang, X.-L.; Lu, J.-Q.; Zhu, X.-Y.; Ni, X. Upregulation of microRNA-22 contributes to myocardial ischemia-reperfusion injury by interfering with the mitochondrial function. Free. Radic. Biol. Med. 2016, 96, 406–417. [Google Scholar] [CrossRef]
- Yang, J.; Chen, L.; Yang, J.; Ding, J.; Li, S.; Wu, H.; Zhang, J.; Fan, Z.; Dong, W.; Li, X. MicroRNA-22 targeting CBP protects against myocardial ischemia–reperfusion injury through anti-apoptosis in rats. Mol. Biol. Rep. 2014, 41, 555–561. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, W.; Zhang, Y.; Zhang, L.; Ding, H.; Qi, H.; Xue, S.; Yu, H.; Hu, L.; Liu, D. Circulating miR-22-5p and miR-122-5p are promising novel biomarkers for diagnosis of acute myocardial infarction. J. Cell. Physiol. 2019, 234, 4778–4786. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Wu, W.Y.; Biery, D.W.; Singh, A.; Divakaran, S.; Berman, A.N.; Ayuba, G.; DeFilippis, E.M.; Nasir, K.; Januzzi, J.L.; Di Carli, M.F. Recovery of Left Ventricular Systolic Function and Clinical Outcomes in Young Adults with Myocardial Infarction. J. Am. Coll. Cardiol. 2020, 75, 2804–2815. [Google Scholar] [CrossRef]
- Dauw, J.; Martens, P.; Deferm, S.; Bertrand, P.; Nijst, P.; Hermans, L.; Van den Bergh, M.; Housen, I.; Hijit, A.; Warnants, M. Left ventricular function recovery after ST-elevation myocardial infarction: Correlates and outcomes. Clin. Res. Cardiol. 2021, 110, 1504–1515. [Google Scholar] [CrossRef]
- Liang, Y.; Ridzon, D.; Wong, L.; Chen, C. Characterization of microRNA expression profiles in normal human tissues. BMC Genom. 2007, 8, 166. [Google Scholar] [CrossRef]
- Huang, Z.P.; Chen, J.; Seok, H.Y.; Zhang, Z.; Kataoka, M.; Hu, X.; Wang, D.-Z. MicroRNA-22 Regulates Cardiac Hypertrophy and Remodeling in Response to Stress. Circ. Res. 2013, 112, 1234–1243. [Google Scholar] [CrossRef]
- Gupta, S.K.; Foinquinos, A.; Thum, S.; Remke, J.; Zimmer, K.; Bauters, C.; de Groote, P.; Boon, R.A.; de Windt, L.J.; Preissl, S.; et al. Preclinical Development of a MicroRNA-Based Therapy for Elderly Patients with Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 68, 1557–1571. [Google Scholar] [CrossRef]
- Van Rooij, E.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Hill, J.; Olson, E.N. Control of Stress-Dependent Cardiac Growth and Gene Expression by a MicroRNA. Science 2007, 316, 575–579. [Google Scholar] [CrossRef]
- Xu, X.D.; Song, X.W.; Li, Q.; Wang, G.-K.; Jing, Q.; Qin, Y.-W. Attenuation of MicroRNA-22 derepressed PTEN to effectively protect rat cardiomyocytes from hypertrophy. J. Cell. Physiol. 2012, 227, 1391–1398. [Google Scholar] [CrossRef]
- Tu, Y.; Wan, L.; Bu, L.; Zhao, D.; Dong, D.; Huang, T.; Cheng, Z.; Shen, B. MicroRNA-22 Downregulation by Atorvastatin in a Mouse Model of Cardiac Hypertrophy: A new Mechanism for Antihypertrophic Intervention. Cell. Physiol. Biochem. 2013, 31, 997–1008. [Google Scholar] [CrossRef]
- Gurha, P.; Abreu-Goodger, C.; Wang, T.; Ramirez, M.O.; Drumond, A.L.; van Dongen, S.; Chen, Y.; Bartonicek, N.; Enright, A.J.; Lee, B.; et al. Targeted Deletion of MicroRNA-22 Promotes Stress-Induced Cardiac Dilation and Contractile Dysfunction. Circulation 2012, 125, 2751–2761. [Google Scholar] [CrossRef]
- Gurha, P.; Wang, T.; Larimore, A.H.; Sassi, Y.; Abreu-Goodger, C.; Ramirez, M.O.; Reddy, A.K.; Engelhardt, S.; Taffet, G.E.; Wehrens, X.H.T.; et al. microRNA-22 Promotes Heart Failure through Coordinate Suppression of PPAR/ERR-Nuclear Hormone Receptor Transcription. PLoS ONE 2013, 8, e75882. [Google Scholar] [CrossRef]
- Chen, Z.; Qi, Y.; Gao, C. Cardiac myocyte-protective effect of microRNA-22 during ischemia and reperfusion through disrupting the caveolin-3/eNOS signaling. Int. J. Clin. Exp. Pathol. 2015, 8, 4614–4626. [Google Scholar]
- Gidlöf, O.; van der Brug, M.; Öhman, J.; Gilje, P.; Olde, B.; Wahlestedt, C.; Erlinge, D. Platelets activated during myocardial infarction release functional miRNA, which can be taken up by endothelial cells and regulate ICAM1 expression. Blood 2013, 121, 3908–3917. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, W.; Wani, M.; Yu, X.; Ashraf, M. Ischemic Preconditioning Potentiates the Protective Effect of Stem Cells through Secretion of Exosomes by Targeting Mecp2 via miR-22. PLoS ONE 2014, 9, e88685. [Google Scholar] [CrossRef]
- Schütte, J.P.; Manke, M.C.; Hemmen, K.; Münzer, P.; Schörg, B.F.; Ramos, G.C.; Pogoda, M.; Dicenta, V.; Hoffmann, S.H.L.; Pinnecker, J.; et al. Platelet-Derived MicroRNAs Regulate Cardiac Remodeling after Myocardial Ischemia. Circ. Res. 2023, 132, e96–e113. [Google Scholar] [CrossRef]
- Yap, J.; Tay, W.T.; Teng, T.H.K.; Anand, I.; Richards, A.M.; Ling, L.H.; MacDonald, M.R.; Chandramouli, C.; Tromp, J.; Siswanto, B.B.; et al. Association of Diabetes Mellitus on Cardiac Remodeling, Quality of Life, and Clinical Outcomes in Heart Failure with Reduced and Preserved Ejection Fraction. J. Am. Heart Assoc. 2019, 8, e013114. [Google Scholar] [CrossRef]
- Akashi, N.; Tsukui, T.; Yamamoto, K.; Seguchi, M.; Taniguchi, Y.; Sakakura, K.; Wada, H.; Momomura, S.-I.; Fujita, H. Comparison of clinical outcomes and left ventricular remodeling after ST-elevation myocardial infarction between patients with and without diabetes mellitus. Heart Vessel. 2021, 36, 1445–1456. [Google Scholar] [CrossRef]
- Traub, J.; Schürmann, P.; Schmitt, D.; Gassenmaier, T.; Fette, G.; Frantz, S.; Störk, S.; Beyersdorf, N.; Boivin-Jahns, V.; Jahns, R.; et al. Features of metabolic syndrome and inflammation independently affect left ventricular function early after first myocardial infarction. Int. J. Cardiol. 2023, 370, 43–50. [Google Scholar] [CrossRef]
- Pan, W.; Lu, H.; Lian, B.; Liao, P.; Guo, L.; Zhang, M. Prognostic value of HbA1c for in-hospital and short-term mortality in patients with acute coronary syndrome: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2019, 18, 169. [Google Scholar] [CrossRef]
- Karayiannides, S.; Djupsjö, C.; Kuhl, J.; Hofman-Bang, C.; Norhammar, A.; Holzmann, M.J.; Lundman, P. Long-term prognosis in patients with acute myocardial infarction and newly detected glucose abnormalities: Predictive value of oral glucose tolerance test and HbA1c. Cardiovasc. Diabetol. 2021, 20, 122. [Google Scholar] [CrossRef]
- Senese, R.; Cioffi, F.; Petito, G.; de Lange, P.; Russo, A.; Goglia, F.; Lanni, A.; Potenza, N. miR-22-3p is involved in gluconeogenic pathway modulated by 3,5-diiodo-L-thyronine (T2). Sci. Rep. 2019, 9, 16645. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, H.X.; Jena, P.K.; Sheng, L.; Ali, M.R.; Wan, Y.-J.Y. miR-22 inhibition reduces hepatic steatosis via FGF21 and FGFR1 induction. JHEP Rep. 2020, 2, 100093. [Google Scholar] [CrossRef]
- Kaudewitz, D.; Zampetaki, A.; Mayr, M. MicroRNA Biomarkers for Coronary Artery Disease? Curr. Atheroscler. Rep. 2015, 17, 70. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Hao, X.; Yang, J.; Han, X.; Li, H.; Li, T.; Wang, D.; Teng, Y.; Ma, L.; et al. Comparison of the Clinical Value of miRNAs and Conventional Biomarkers in AMI: A Systematic Review. Front. Genet. 2021, 12, 668324. [Google Scholar] [CrossRef]
- Leancă, S.A.; Crișu, D.; Petriș, A.O.; Afrăsânie, I.; Genes, A.; Costache, A.D.; Tesloianu, D.N.; Costache, I.I. Left Ventricular Remodeling after Myocardial Infarction: From Physiopathology to Treatment. Life 2022, 12, 1111. [Google Scholar] [CrossRef]
- Gronda, E.; Sacchi, S.; Benincasa, G.; Vanoli, E.; Napoli, C. Unresolved issues in left ventricular postischemic remodeling and progression to heart failure. J. Cardiovasc. Med. 2019, 20, 640–649. [Google Scholar] [CrossRef]




| Variable | All (n = 105) | Follow Up Group (n = 43) | p |
|---|---|---|---|
| Age (years) mean ± SD | 60.83 ± 12.9 | 57.81 ± 11.73 | 0.172 * |
| Female/Male n/n | 29/76 | 11/32 | 0.253 |
| Risk factors, n (%) | |||
| Hypertension | 75 (71.43) | 33 (76.74) | 0.509 |
| Hypercholesterolemia | 23 (11.90) | 11 (25.58) | 0.631 |
| Current smoker | 53 (50.48) | 24 (55.81) | 0.555 |
| Obesity (BMI > 30 kg/m2) | 31 (29.52) | 16 (37.21) | 0.363 |
| Diabetes mellitus | 24 (22.86) | 8 (18.6) | 0.569 |
| Presentation | |||
| CK-MB (U/L) mean ± SD | 108.97± 130.3 | 120.31± 138.8 | 0.944 ** |
| Time from symptoms onset to reperfusion (hours) | 6.3 ± 3.4 | 6.7 ± 3.7 | 0.945 ** |
| eGFR, mean ± SD | 65.1 ± 23.7 | 72.6 ± 19.3 | 0.075 ** |
| Killip Class, n (%) | |||
| 1 | 77 (73.33) | 34 (79.07) | 0.465 |
| 2 | 26 (24.76) | 9 (20.93) | 0.617 |
| 3 | 1 (0.95) | 0 | 0.522 |
| Burden of CAD, n (%) | |||
| 0 | 1 (0.95) | 0 | 0.522 |
| 1 | 68 (64.76) | 27 (62.79) | 0.818 |
| 2 | 29 (27.62) | 13 (30.23) | 0.749 |
| 3 | 6 (5.71) | 3 (6.98) | 0.772 |
| Complete revascularization n(%) | |||
| 0 (incomplete) | 8 (7.62) | 6 (13.95) | 0.230 |
| 1 (complete) | 80 (76.19) | 32 (74.42) | 0.818 |
| 2 (surgical revascularization) | 7 (6.67) | 1 (2.33) | 0.289 |
| 3 (incomplete due to chronic occlusion or small vessels) | 9 (8.57) | 4 (9.30) | 0.889 |
| In-hospital death, n (%) | 7 (6.67) | - | 0.084 |
| Type of infarction, n (%) | |||
| Anterior | 49 (46.67) | 23 (53.49) | 0.453 |
| Inferior | 51 (48.57) | 19 (44.19) | 0.624 |
| Other | 5 (4.76) | 1 (2.33) | 0.496 |
| Characteristics | LVR (n = 14) | Non-LVR (n = 29) | p |
|---|---|---|---|
| Age (years) mean ± SD | 62.31 ± 11.32 | 56.6 ± 11.88 | 0.3545 * |
| Female, n (%) | 3 (21.43) | 9 (31.03) | 0.509 |
| CK-MB (U/L) mean ± SD | 153.9 ± 166.6 | 105.4 ± 125.2 | 0.379 * |
| Killip Class, n (%) | |||
| 1 | 10 (71.43) | 24 (82.76) | 0.390 |
| 2 | 4 (28.57) | 5 (17.24) | 0.389 |
| 3 | 0 | 0 | - |
| Burden of CAD, n (%) | |||
| 0 | 0 | 0 | |
| 1 | 7 (50.0) | 20 (68.97) | 0.226 |
| 2 | 5 (35.71) | 8 (27.59) | 0.589 |
| 3 | 2 (14.29) | 1 (3.45) | 0.190 |
| Complete revascularization n(%) | |||
| 0 (incomplete) | 4 (28.57) | 2 (6.90) | 0.055 |
| 1 (complete) | 9 (64.29) | 23 (79.31) | 0.289 |
| 2 (surgical revascularization) | 1 (7.14) | 0 | 0.144 |
| 3 (incomplete due to chronic occlusion or small vessels) | 0 | 4 (13.79) | 0.145 |
| Thrombolysis, n (%) | 2 (14.29) | 7 (24.14) | 0.459 |
| Cardiovascular history/risk factors, n (%) | |||
| Hypertension | 12 (85.71) | 21 (72.41) | 0.332 |
| Diabetes mellitus | 2 (14.29) | 6 (20.69) | 0.61 |
| Hypercholesterolemia | 2 (14.29) | 9 (31.03) | 0.238 |
| Current smoker | 5 (35.71) | 19 (65.52) | 0.066 |
| Obesity (BMI > 30 kg/m2) | 7 (50.0) | 9 (31.03) | 0.226 |
| Anemia: Hb < 13.5 g/dL (men), <12 g/dL (women) | 4 (28.57) | 3 (10.34) | 0.128 |
| Medication (n%) | |||
| Aspirin | 14 (100) | 29 (100) | - |
| Aldosterone receptor antagonist | 11 (78.57) | 25 (86.21) | 0.522 |
| Betablocker | 11 (78.57) | 26 (89.66) | 0.328 |
| ACEi/ARB | 9 (64.29) | 24 (82.76) | 0.180 |
| Clopidogrel | 7 (50) | 10 (34.48) | 0.327 |
| Nitrate | 4 (28.57) | 4 (13.79) | 0.242 |
| Statin | 14 (100) | 29 (100) | - |
| Ticagrelor | 7 (50) | 10 (34.48) | 0.327 |
| Type of infarction, n (%) | |||
| Anterior | 8 (57.14) | 15 (51.72) | 0.741 |
| Inferior | 6 (42.86) | 13 (44.83) | 0.904 |
| Other | 0 | 1 (3.45) | |
| Echo parameters | |||
| Average change EF (%) | −10.32 | 12.92 | 0.0003 * |
| Average change EDV (%) | 26.65 | −7.28 | <0.0001 * |
| Correlation Coefficient (p) | DM | Erythrocyte Number | Hematocrit | |
|---|---|---|---|---|
| STEMI cohort | r | 0.241 | −0.228 | −0.2014 |
| p value | 0.0133 * | 0.0193 | 0.0394 | |
| 95% confidence interval | - | −0.4079 to −0.0322 | −0.3833 to −0.0044 | |
| Follow up cohort | r | −0.2164 | −0.121 | |
| p value | 0.20772 | 0.1634 | 0.4397 | |
| 95% confidence interval | 0.18134 * | −0.4922 to −0.0988 | −0.4142 to −0.1950 |
| Correlation Test | EDVi | %ΔEDV | EFi | % ΔEF | |
|---|---|---|---|---|---|
| LVR cohort | r | −0.3417 * | 0.3784 * | −0.0669 | 0.1802 ** |
| p value | 0.2319 | 0.1432 | 0.8204 | 0.5376 | |
| 95% confidence interval | −0.7384 to 0.2308 | −0.1904 to 0.7570 | −0.5886 to 0.4940 | −0.3874 to 0.6487 | |
| Non-LVR cohort | r | 0.3081 ** | −0.03249 * | 0.2728 * | −0.5325 * |
| p value | 0.1039 | 0.8671 | 0.1523 | 0.0029 | |
| 95% confidence interval | −0.0772 to 0.6133 | −0.3943 to 0.3380 | −0.1042 to 0.5812 | −0.7522 to −0.2063 |
| Area under the ROC Curve | With miR-22 | Without miR-22 |
|---|---|---|
| Area | 0.9056 | 0.7389 |
| Std. Error | 0.06133 | 0.1007 |
| 95% confidence interval | 0.7854 to 1.000 | 0.5416 to 0.9362 |
| p value | 0.0006 | 0.0427 |
| Negative predictive power (%) | 80 | 66.67 |
| Positive predictive power (%) | 94.74 | 78.26 |
| Tjur’s R squared | 0.4592 | 0.1675 |
| Hosmer-Lemeshov test (p) | 13.04 (0.1105) | 12.5 (0.1445) |
| Characteristics | Diabetic (n = 24) | Non-Diabetic (n = 81) | p Value |
|---|---|---|---|
| Age, years | 63.63 ± 10.00 | 60.00 ± 13.59 | 0.159 * |
| Female, n (%) | 10 (41.67) | 19 (23.46) | 0.081 |
| Cardiovascular history/risk factors, n (%) | |||
| Hypertension | 21 (87.50) | 54 (66.67) | 0.048 |
| Hypercholesterolemia | 2 (8.33) | 21 (25.93) | 0.067 |
| Current smoker | 10 (41.67) | 43 (53.09) | 0.327 |
| Obesity | 9 (37.5) | 22 (27.16) | 0.327 |
| Presentation | |||
| CK-MB (U/L) | 88.29 | 114.68 | 0.394 * |
| Type of infarction | |||
| Anterior | 14 (58.33) | 35 (43.21) | 0.194 |
| Inferior | 10 (41.67) | 41 (50.62) | 0.441 |
| Other | 5 (6.17) | ||
| In-hospital death | 5 (20.83) | 2 (2.47) | 0.0015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maries, L.; Moatar, A.I.; Chis, A.R.; Marian, C.; Luca, C.T.; Sirbu, I.-O.; Gaiță, D. Plasma hsa-miR-22-3p Might Serve as an Early Predictor of Ventricular Function Recovery after ST-Elevation Acute Myocardial Infarction. Biomedicines 2023, 11, 2289. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines11082289
Maries L, Moatar AI, Chis AR, Marian C, Luca CT, Sirbu I-O, Gaiță D. Plasma hsa-miR-22-3p Might Serve as an Early Predictor of Ventricular Function Recovery after ST-Elevation Acute Myocardial Infarction. Biomedicines. 2023; 11(8):2289. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines11082289
Chicago/Turabian StyleMaries, Liana, Alexandra Ioana Moatar, Aimee Rodica Chis, Catalin Marian, Constantin Tudor Luca, Ioan-Ovidiu Sirbu, and Dan Gaiță. 2023. "Plasma hsa-miR-22-3p Might Serve as an Early Predictor of Ventricular Function Recovery after ST-Elevation Acute Myocardial Infarction" Biomedicines 11, no. 8: 2289. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines11082289