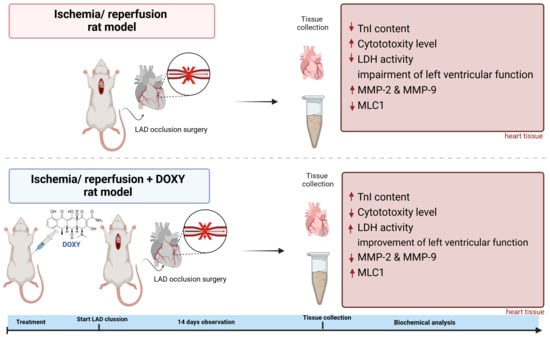
In Vivo Study on Doxycycline Protective Mechanisms during Myocardial Ischemia Injury in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Ethical Statement
2.2. Drug Preparation
2.3. Myocardial Infarction Surgery
2.4. Transthoracic Echocardiography
2.5. Evans Blue Followed by 2,3,5-Triphenyltetrazolium Chloride Staining of Hearts
2.6. Hematoxylin/Eosin Staining of Hearts
2.7. Preparation of Heart Tissue Homogenate
2.8. Quantitative Measurement of Cardiac Troponin I Protein
2.9. Measurement of LDH Activity
2.10. Assessment of Cytotoxicity Level in Rat Hearts
2.11. Matrix Metalloproteinase Activity Assay
2.12. Measurement of Total Matrix Metalloproteinase 2 and 9 Concentration in Heart Homogenates
2.13. Measurement of MLC1 Concentration
2.14. Statistical Analysis
3. Results
3.1. Procedures and Mortality
3.2. Doxycycline Protects from Heart Tissue Damage
3.3. Doxycycline Protects from I/R Associated Impairment of Left Ventricular Function
3.4. Effect of Doxy on MMPs Activity and Concentration
3.5. Administration of Doxy Protects from Cardiac Contractile Protein’s Degradation Due to I/R Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jobin, P.G.; Butler, G.S.; Overall, C.M. New Intracellular Activities of Matrix Metalloproteinases Shine in the Moonlight. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Krzywonos-Zawadzka, A.; Franczak, A.; Olejnik, A.; Radomski, M.; Gilmer, J.F.; Sawicki, G.; Woźniak, M.; Bil-Lula, I. Cardioprotective Effect of MMP-2-Inhibitor-NO-Donor Hybrid against Ischaemia/Reperfusion Injury. J. Cell. Mol. Med. 2019, 23, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, A.D.; Chow, A.K.; Ali, M.A.M.; Schulz, R. Matrix Metalloproteinase-2 and Myocardial Oxidative Stress Injury: Beyond the Matrix. Cardiovasc. Res. 2010, 85, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Srivastava, S.K.; Chaudhuri, T.K.; Upadhyay, G. Multifaceted Role of Matrix Metalloproteinases (MMPs). Front. Mol. Biosci. 2015, 2, 19. [Google Scholar] [CrossRef]
- Patrichi, G.; Patrichi, A.; Satala, C.-B.; Sin, A.I. Matrix Metalloproteinases and Heart Transplantation—A Pathophysiological and Clinical View. Medicina 2023, 59, 1295. [Google Scholar] [CrossRef] [PubMed]
- Krzywonos-Zawadzka, A.; Franczak, A.; Sawicki, G.; Woźniak, M.; Bil-Lula, I. Multidrug Prevention or Therapy of Ischemia-Reperfusion Injury of the Heart—Mini-Review. Environ. Toxicol. Pharmacol. 2017, 55, 55–59. [Google Scholar] [CrossRef]
- Jacob-Ferreira, A.L.; Kondo, M.Y.; Baral, P.K.; James, M.N.G.; Holt, A.; Fan, X.; Schulz, R. Phosphorylation Status of 72 kDa MMP-2 Determines Its Structure and Activity in Response to Peroxynitrite. PLoS ONE 2013, 8, e71794. [Google Scholar] [CrossRef]
- Bil-Lula, I.; Lin, H.-B.; Biały, D.; Wawrzyńska, M.; Diebel, L.; Sawicka, J.; Woźniak, M.; Sawicki, G. Subthreshold Nitric Oxide Synthase Inhibition Improves Synergistic Effects of Subthreshold MMP-2/MLCK-Mediated Cardiomyocyte Protection from Hypoxic Injury. J. Cell. Mol. Med. 2016, 20, 1086–1094. [Google Scholar] [CrossRef]
- Cheung, P.Y.; Sawicki, G.; Wozniak, M.; Wang, W.; Radomski, M.W.; Schulz, R. Matrix Metalloproteinase-2 Contributes to Ischemia-Reperfusion Injury in the Heart. Circulation 2000, 101, 1833–1839. [Google Scholar] [CrossRef]
- Nip, L.H.; Uitto, V.-J.; Golub, L.M. Inhibition of Epithelial Cell Matrix Metalloproteinases by Tetracyclines. J. Periodontal Res. 1993, 28, 379–385. [Google Scholar] [CrossRef]
- Munzar, P.; Li, H.; Nicholson, K.L.; Wiley, J.L.; Balster, R.L. Enhancement of the Discriminative Stimulus Effects of Phencyclidine by the Tetracycline Antibiotics Doxycycline and Minocycline in Rats. Psychopharmacology 2002, 160, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Roach, D.M.; Fitridge, R.A.; Laws, P.E.; Millard, S.H.; Varelias, A.; Cowled, P.A. Up-Regulation of MMP-2 and MMP-9 Leads to Degradation of Type IV Collagen during Skeletal Muscle Reperfusion Injury; Protection by the MMP Inhibitor, Doxycycline. Eur. J. Vasc. Endovasc. Surg. 2002, 23, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Bil-Lula, I.; Krzywonos-Zawadzka, A.; Sawicka, J.; Bialy, D.; Wawrzynska, M.; Wozniak, M.; Sawicki, G. L-NAME Improves Doxycycline and ML-7 Cardioprotection from Oxidative Stress. Front. Biosci. 2018, 23, 298–309. [Google Scholar]
- Mayer, P. Hematoxylin and Eosin (H&E) Staining Protocol. Mitt. Zool Stn. Neapel. 1896, 12, 303. [Google Scholar]
- Olejnik, A.; Krzywonos-Zawadzka, A.; Banaszkiewicz, M.; Bil-Lula, I. Ameliorating Effect of Klotho Protein on Rat Heart during I/R Injury. Oxid. Med. Cell. Longev. 2020, 2020, 6427284. [Google Scholar] [CrossRef]
- Heussen, C.; Dowdle, E.B. Electrophoretic Analysis of Plasminogen Activators in Polyacrylamide Gels Containing Sodium Dodecyl Sulfate and Copolymerized Substrates. Anal. Biochem. 1980, 102, 196–202. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- DeCoux, A.; Lindsey, M.L.; Villarreal, F.; Garcia, R.A.; Schulz, R. Myocardial Matrix Metalloproteinase-2: Inside out and Upside Down. J. Mol. Cell. Cardiol. 2014, 77, 64–72. [Google Scholar] [CrossRef]
- Hori, M.; Nishida, K. Oxidative Stress and Left Ventricular Remodelling after Myocardial Infarction. Cardiovasc. Res. 2009, 81, 457–464. [Google Scholar] [CrossRef]
- Bräuninger, H.; Krüger, S.; Bacmeister, L.; Nyström, A.; Eyerich, K.; Westermann, D.; Lindner, D. Matrix Metalloproteinases in Coronary Artery Disease and Myocardial Infarction. Basic Res. Cardiol. 2023, 118, 18. [Google Scholar] [CrossRef]
- Yabluchanskiy, A.; Li, Y.; Chilton, R.J.; Lindsey, M.L. Matrix Metalloproteinases: Drug Targets for Myocardial Infarction. Curr. Drug Targets 2013, 14, 276–286. [Google Scholar]
- Ali, M.A.M.; Chow, A.K.; Kandasamy, A.D.; Fan, X.; West, L.J.; Crawford, B.D.; Simmen, T.; Schulz, R. Mechanisms of Cytosolic Targeting of Matrix Metalloproteinase-2. J. Cell. Physiol. 2012, 227, 3397–3404. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.F.; Heindl, B.; Kupatt, C.; Zahler, S. Endothelial Function and Hemostasis. Z. Kardiol. 2000, 89, 160–167. [Google Scholar] [CrossRef]
- Schulz, R.; Nava, E.; Moncada, S. Induction and Potential Biological Relevance of a Ca2+-Independent Nitric Oxide Synthase in the Myocardium. Br. J. Pharmacol. 1992, 105, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R. Intracellular Targets of Matrix Metalloproteinase-2 in Cardiac Disease: Rationale and Therapeutic Approaches. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 211–242. [Google Scholar] [CrossRef]
- Ishida, H.; Ichimori, K.; Hirota, Y.; Fukahori, M.; Nakazawa, H. Peroxynitrite-Induced Cardiac Myocyte Injury. Free Radic. Biol. Med. 1996, 20, 343–350. [Google Scholar] [CrossRef]
- Wang, W.; Schulze, C.J.; Suarez-Pinzon, W.L.; Dyck, J.R.B.; Sawicki, G.; Schulz, R. Intracellular Action of Matrix Metalloproteinase-2 Accounts for Acute Myocardial Ischemia and Reperfusion Injury. Circulation 2002, 106, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Sato, H.; Takino, T.; Seiki, M. The C-Terminal Region of Membrane Type Matrix Metalloproteinase Is a Functional Transmembrane Domain Required for pro-Gelatinase A Activation. J. Biol. Chem. 1995, 270, 801–805. [Google Scholar] [CrossRef]
- Arpino, V.; Brock, M.; Gill, S.E. The Role of TIMPs in Regulation of Extracellular Matrix Proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef]
- Donnini, S.; Monti, M.; Roncone, R.; Morbidelli, L.; Rocchigiani, M.; Oliviero, S.; Casella, L.; Giachetti, A.; Schulz, R.; Ziche, M. Peroxynitrite Inactivates Human-Tissue Inhibitor of Metalloproteinase-4. FEBS Lett. 2008, 582, 1135–1140. [Google Scholar] [CrossRef]
- Ferdinandy, P. Peroxynitrite: Just an Oxidative/Nitrosative Stressor or a Physiological Regulator as Well? Br. J. Pharmacol. 2006, 148, 1–3. [Google Scholar] [CrossRef]
- Owens, M.W.; Milligan, S.A.; Jourd’heuil, D.; Grisham, M.B. Effects of Reactive Metabolites of Oxygen and Nitrogen on Gelatinase A Activity. Am. J. Physiol. 1997, 273, L445–L450. [Google Scholar] [CrossRef]
- Cerisano, G.; Buonamici, P.; Valenti, R.; Sciagrà, R.; Raspanti, S.; Santini, A.; Carrabba, N.; Dovellini, E.V.; Romito, R.; Pupi, A.; et al. Early Short-Term Doxycycline Therapy in Patients with Acute Myocardial Infarction and Left Ventricular Dysfunction to Prevent the Ominous Progression to Adverse Remodelling: The TIPTOP Trial. Eur. Heart J. 2014, 35, 184–191. [Google Scholar] [CrossRef]
- Yasmin, W.; Strynadka, K.D.; Schulz, R. Generation of Peroxynitrite Contributes to Ischemia-Reperfusion Injury in Isolated Rat Hearts. Cardiovasc. Res. 1997, 33, 422–432. [Google Scholar] [CrossRef]
- Rork, T.H.; Hadzimichalis, N.M.; Kappil, M.A.; Merrill, G.F. Acetaminophen Attenuates Peroxynitrite-Activated Matrix Metalloproteinase-2-Mediated Troponin I Cleavage in the Isolated Guinea Pig Myocardium. J. Mol. Cell. Cardiol. 2006, 40, 553–561. [Google Scholar] [CrossRef]





| Echocardiographic Parameters | Heart Rate (bpm) | Cardiac Output (mL/min) | Stroke Volume (µL) | Fractional Shortening (%) | Ejection Fraction (%) | Enddiastolic Volume (µL) | Endsystolic Volume (µL) | |
|---|---|---|---|---|---|---|---|---|
| baseline | sham | 386.7 ± 20.2 | 64.7 ± 12 | 206 ± 27.4 | 48.2 ± 5.7 | 87.1 ± 2.5 | 176.6 ± 36 | 36.77 ± 8 |
| I/R | 349.3 ± 30.3 | 68 ± 12 | 204.4 ± 21.6 | 46.2 ± 10.9 | 79.4 ± 11.5 | 159.8 ± 46.7 | 26.77 ± 12.6 | |
| I/R Doxy | 384.2 ± 48.6 | 83.6 ± 31 | 211.1 ± 55.4 | 51.32 ± 3.7 | 83.3 ± 11.8 | 174.9 ± 42.3 | 32.49 ± 17.6 | |
| 7 days | sham | 408.7 ± 16.3 | 80.9 ± 9.7 | 212.5 ± 30 | 48.53 ± 5.5 | 95.11 ± 5.7 | 176.5 ± 17 | 38 ± 5.6 |
| I/R | 378.3 ± 35 | 51.73 ± 15.6 | 140 ± 25.1 | 27.56 ± 7.5 | 63.59 ± 12.9 | 290 ± 99.8 | 60.93 ± 13.77 | |
| I/R Doxy | 384.4 ± 23 | 89.24 ± 18.4 | 201.4 ± 45.2 | 49.02 ± 12.9 | 87.69 ± 6 | 219 ± 54.4 | 26.03 ± 9.8 | |
| 14 days | sham | 369.3 ±18 | 87.18 ± 17.5 | 220.9 ± 29.3 | 50.64 ± 2 | 93.21 ± 2 | 184.7 ± 15 | 28.78 ±7.7 |
| I/R | 378 ± 34.9 | 54.71 ± 13.1 | 111.6 ± 44 | 35.06 ± 8.8 | 64.99 ± 12.2 | 280.3 ± 78 | 55.7, ± 32.9 | |
| I/R Doxy | 391.2 ± 43.5 | 79.17 ± 11.4 | 194.6 ± 36.3 | 50.51 ± 7.6 | 86.38 ± 5 | 219.4 ± 54 | 31.93 ±11.5 | |
| p | sham vs. I/R | 0.328/0.313/>0.99 | 0.971/0.049 */0.009 * | 0.996/0.032 */0.006 * | 0.934/0.018 */0.012 * | 0.57/0.002 */0.002 * | 0.844/0.006 */0.016 * | 0.566/0.033 */0.002 * |
| sham vs. I/R Doxy | 0.995/0.502/0.201 | 0.446/0.756/0.49 | 0.983/0.9/0.636 | 0.864/0.997/0.1 | 0.882/0.582/0.573 | 0.999/0.39/0.827 | 0.907/0.36/0.924 | |
| I/R vs. I/R Doxy | 0.266/0.931/0.791 | 0.427/0.004 */0.037 * | 0.947/0.033 */0.012 * | 0.556/0.005 */0.004 * | 0.812/0.004 */0.004 * | 0.826/0.037 */0.020 * | 0.767/0.001 */0.001 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzywonos-Zawadzka, A.; Olejnik, A.; Sawicki, G.; Bil-Lula, I. In Vivo Study on Doxycycline Protective Mechanisms during Myocardial Ischemia Injury in Rats. Biomedicines 2024, 12, 634. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines12030634
Krzywonos-Zawadzka A, Olejnik A, Sawicki G, Bil-Lula I. In Vivo Study on Doxycycline Protective Mechanisms during Myocardial Ischemia Injury in Rats. Biomedicines. 2024; 12(3):634. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines12030634
Chicago/Turabian StyleKrzywonos-Zawadzka, Anna, Agnieszka Olejnik, Grzegorz Sawicki, and Iwona Bil-Lula. 2024. "In Vivo Study on Doxycycline Protective Mechanisms during Myocardial Ischemia Injury in Rats" Biomedicines 12, no. 3: 634. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines12030634