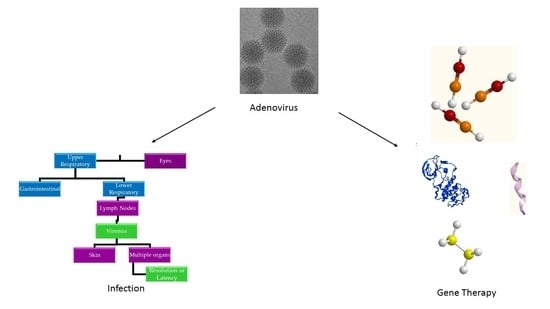
Perspective on Adenoviruses: Epidemiology, Pathogenicity, and Gene Therapy
Abstract
:1. Introduction
2. Epidemiology
3. Pathogenesis
4. Diagnosis, Treatment, and Prevention of Adenoviral Infections
5. Ad Vectors in Gene Therapy
6. Role of Extracellular Vesicles (EVs) in Ad Infection
7. Role of EVs and Ad in Therapeutic Applications
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Ryu, W.-S. Molecular Virology of Human Pathogenic Viruses; Academic Press: Cambridge, MA, USA, 2016; p. 440. [Google Scholar]
- Hoeben, R.C.; Uil, T.G. Adenovirus DNA replication. In Cold Spring Harbor Perspectives in Biology; Cold Spring Harbor Laboratory Press, Cold Spring Harbor: New York, NY, USA, 2013; Volume 5, p. a013003. [Google Scholar]
- Family-adenoviridae. In Virus Taxonomy; King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. (Eds.) Elsevier: San Diego, CA, USA, 2012; pp. 125–141. [Google Scholar]
- Ginsberg, H.S. The life and times of adenoviruses. In Advances in Virus Research; Maramorosch, K., Murphy, F.A., Shatkin, A.J., Eds.; Academic Press: Cambridge, MA, USA, 1999; Volume 54, pp. 1–13. [Google Scholar]
- Rowe, P.W.; Huebner, R.J.; Gilmore, L.K.; Parrott, R.H.; Ward, T.G. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc. Soc. Exp. Biol. Med. 1954, 84, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Ghebremedhin, B. Human adenovirus: Viral pathogen with increasing importance. Eur. J. Microbiol. Immunol. 2014, 4, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lion, T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 2014, 27, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Ampuero, J.S.; Ocaña, V.; Gómez, J.; Gamero, M.E.; Garcia, J.; Halsey, E.S.; Laguna-Torres, V.A. Adenovirus respiratory tract infections in peru. PLoS ONE 2012, 7, e46898. [Google Scholar] [CrossRef] [PubMed]
- Belsy, A.; Odalys, V.; Alexander, P.; Clara, S.; Angel, G.; Grehete, G.; Guelsys, G.; Luis, S.; Pedro, M.; Guadalupe, G.M.; et al. Molecular characterization of adenoviral infections in cuba: Report of an unusual association of species d adenoviruses with different clinical syndromes. Arch. Virol. 2009, 154, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Gompf, S. Adenovirus. Available online: https://emedicine.medscape.com/article/211738-overview (accessed on 13 June 2019).
- Pabbaraju, K.; Wong, S.; Fox, J.D. Detection of adenoviruses. In Diagnostic Virology Protocols; Stephenson, J.R., Warnes, A., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 1–15. [Google Scholar]
- Westerberg, S.; Hagbom, M.; Rajan, A.; Loitto, V.; Persson, B.D.; Allard, A.; Nordgren, J.; Sharma, S.; Magnusson, K.-E.; Arnberg, N.; et al. Interaction of human enterochromaffin cells with human enteric adenovirus 41 leads to serotonin release and subsequent activation of enteric glia cells. J. Virol. 2018, 92, e00026-18. [Google Scholar] [CrossRef]
- Tsoumakas, K.; Giamaiou, K.; Goussetis, E.; Graphakos, S.; Kossyvakis, A.; Horefti, E.; Mentis, A.; Elefsiniotis, I.; Pavlopoulou, I.D. Epidemiology of viral infections among children undergoing hematopoietic stem cell transplant: A prospective single-center study. Transpl. Infect. Dis 2019, e13095. [Google Scholar] [CrossRef] [PubMed]
- Kajon, A.E.; Lamson, D.M.; Bair, C.R.; Lu, X.; Landry, M.L.; Menegus, M.; Erdman, D.D.; St George, K. Adenovirus type 4 respiratory infections among civilian adults, northeastern united states, 2011–2015(1). Emerg. Infect. Dis. 2018, 24, 201–209. [Google Scholar] [CrossRef]
- Kandel, R.; Srinivasan, A.; D’Agata, E.M.; Lu, X.; Erdman, D.; Jhung, M. Outbreak of adenovirus type 4 infection in a long-term care facility for the elderly. Infect. Control. Hosp. Epidemiol. 2010, 31, 755–757. [Google Scholar] [CrossRef]
- Fang, X.; Xu, M.; Fang, Q.; Tan, H.; Zhou, J.; Li, Z.; Li, F.; Yang, S. Real-time utilization of metagenomic sequencing in the diagnosis and treatment monitoring of an invasive adenovirus b55 infection and subsequent herpes simplex virus encephalitis in an immunocompetent young adult. Open Forum Infect. Dis. 2018, 5, ofy114. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Oladosu, T.O.; Abdulkarim, A.A.; Okoh, A.I. Prevalence of adenovirus respiratory tract and hiv co-infections in patients attending the university of ilorin, teaching hospital, ilorin, nigeria. BMC Res. Notes 2014, 7, 870. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.G.; Barouch, D.H. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Elsevier Inc: Amsterdam, The Netherlands, 2014; Volume 2, pp. 1787–1793. [Google Scholar]
- Wan, G.H.; Huang, C.G.; Huang, Y.C.; Huang, J.P.; Yang, S.L.; Lin, T.Y.; Tsao, K.C. Surveillance of airborne adenovirus and mycoplasma pneumoniae in a hospital pediatric department. PLoS ONE 2012, 7, e33974. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Shah, D.; Breuer, J. Viral gastrointestinal infections and norovirus genotypes in a paediatric uk hospital, 2014–2015. J. Clin. Virol. 2016, 84, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kajon, A.E.; Moseley, J.M.; Metzgar, D.; Huong, H.S.; Wadleigh, A.; Ryan, M.A.; Russell, K.L. Molecular epidemiology of adenovirus type 4 infections in us military recruits in the postvaccination era (1997–2003). J. Infect. Dis. 2007, 196, 67–75. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, B.J.; Lee, E.J.; Park, K.S.; Park, H.S.; Jung, S.S.; Kim, J.O. Clinical features and courses of adenovirus pneumonia in healthy young adults during an outbreak among korean military personnel. PLoS ONE 2017, 12, e0170592. [Google Scholar] [CrossRef]
- Payne, S.B.; Grilli, E.A.; Smith, A.J.; Hoskins, T.W. Investigation of an outbreak of adenovirus type 3 infection in a boys’ boarding school. Epidemiol. Infect. 1984, 93, 277–283. [Google Scholar] [CrossRef]
- Bautista-Gogel, J.; Madsen, C.M.; Lu, X.; Sakthivel, S.K.; Froh, I.; Kamau, E.; Gerber, S.I.; Watson, J.T.; Cooper, S.S.; Schneider, E. Outbreak of respiratory illness associated with human adenovirus type 7 among persons attending officer candidates school, quantico, virginia, 2017. J. Infect. Dis. 2019. [Google Scholar] [CrossRef]
- Osborne, C.M.; Montano, A.C.; Robinson, C.C.; Schultz-Cherry, S.; Dominguez, S.R. Viral gastroenteritis in children in colorado 2006–2009. J. Med. Virol. 2015, 87, 931–939. [Google Scholar] [CrossRef]
- D’Angelo, L.J.; Hierholzer, J.C.; Keenlyside, R.A.; Anderson, L.J.; Martone, W.J. Pharyngoconjunctival fever caused by adenovirus type 4: Report of a swimming pool-related outbreak with recovery of virus from pool water. J. Infect. Dis. 1979, 140, 42–47. [Google Scholar] [CrossRef]
- Yoo, H.; Gu, S.H.; Jung, J.; Song, D.H.; Yoon, C.; Hong, D.J.; Lee, E.Y.; Seog, W.; Hwang, I.U.; Lee, D.; et al. Febrile respiratory illness associated with human adenovirus type 55 in south korea military, 2014–2016. Emerg. Infect. Dis. 2017, 23, 1016–1020. [Google Scholar] [CrossRef]
- Fedaoui, N.; Ayed, N.B.; Yahia, A.B.; Hammami, W.; Touzi, H.; Triki, H. Genetic variability of human adenovirus type 8 causing epidemic and sporadic cases of keratoconjunctivitis. Arch. Virol. 2016, 161, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P., 3rd; Kajon, A.E. Adenovirus: Epidemiology, global spread of novel serotypes, and advances in treatment and prevention. Semin. Respir. Crit. Care Med. 2016, 37, 586–602. [Google Scholar] [PubMed]
- Qiu, F.Z.; Shen, X.X.; Zhao, M.C.; Zhao, L.; Duan, S.X.; Chen, C.; Qi, J.J.; Li, G.X.; Wang, L.; Feng, Z.S.; et al. A triplex quantitative real-time pcr assay for differential detection of human adenovirus serotypes 2, 3 and 7. Virol. J. 2018, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.C.; Adriana, E.K.; Xiaoyan, L.; Linda, D.; Paul, O.R.; Paul, H.; Cillian, F.D.G.; Suzie, C.; Jeff, C.; Dean, D.E.; et al. Deaths associated with human adenovirus-14p1 infections, europe, 2009–2010. Emerg. Infect. Dis. J. 2011, 17, 1402. [Google Scholar]
- Magdalena Kendall, S.; Christina, C.; Lu, X.; Dianna, A.; LaDonna, G.; Eileen, S.; Susan, I.G.; Dean, D.E.; Ann, T. Human adenovirus associated with severe respiratory infection, oregon, USA, 2013–2014. Emerg. Infect. Dis. J. 2016, 22, 1044. [Google Scholar]
- Jérémy, L.; Audrey, M.; Maud, S.; Alexandre, L.; Christine, A.; Amélie, B.; Christel, R.; Martine, C.; Séverine, M.-D.; Jérôme Le, G.; et al. Severe pneumonia associated with adenovirus type 55 infection, france, 2014. Emerg. Infect. Dis. J. 2016, 22, 2012. [Google Scholar]
- Khanal, S.; Ghimire, P.; Dhamoon, A.S. The repertoire of adenovirus in human disease: The innocuous to the deadly. Biomedicines 2018, 6, 30. [Google Scholar] [CrossRef]
- Lai, C.-Y.; Lee, C.-J.; Lu, C.-Y.; Lee, P.-I.; Shao, P.-L.; Wu, E.-T.; Wang, C.-C.; Tan, B.-F.; Chang, H.-Y.; Hsia, S.-H.; et al. Adenovirus serotype 3 and 7 infection with acute respiratory failure in children in taiwan, 2010–2011. PLoS ONE 2013, 8, e53614. [Google Scholar] [CrossRef]
- New Jersey Department of Health. Available online: https://www.nj.gov/health/ (accessed on 26 June 2019).
- Centers for Disease Control and Prevention. Adenovirus Outbreaks; 2018. Available online: https://www.cdc.gov/adenovirus/outbreaks.html (accessed on 26 June 2019).
- Glor, J. Adenovirus Outbreak Kills 6 Children at N.J. Health Facility, Sickens 12 others; CBS Interactive Inc: Haskell, NJ, USA, 2018; Available online: https://www.cbsnews.com/news/adenovirus-wanaque-center-for-nursing-and-rehabilitation-new-jersey-outbreak-children/ (accessed on 26 June 2019).
- Foxx, M. University of Maryland Student Dies in Adenovirus Outbreak; National Broadcasting Co: New York, NY, USA, 2018; Available online: https://www.cbsnews.com/news/adenovirus-death-university-of-maryland-student-olivia-paregol-dies-of-adenovirus-related-illness/ (accessed on 26 June 2019).
- Hedgpeth, D. Three new cases of adenovirus reported at u-md.’ S college park campus. The Washington Post, 2018. [Google Scholar]
- Kesson, A.M. Respiratory virus infections. Paediatr. Respir. Rev. 2007, 8, 240–248. [Google Scholar] [CrossRef]
- Jones, M.S., 2nd; Harrach, B.; Ganac, R.D.; Gozum, M.M.; Dela Cruz, W.P.; Riedel, B.; Pan, C.; Delwart, E.L.; Schnurr, D.P. New adenovirus species found in a patient presenting with gastroenteritis. J. Virol. 2007, 81, 5978–5984. [Google Scholar] [CrossRef]
- Tosh, A.K.; Broy-Aschenbrenner, A.; El Khatib, J.; Ge, B. Adenovirus-36 antibody status & bmi comparison among obese missouri adolescents. Mo. Med. 2012, 109, 402–403. [Google Scholar]
- Frange, P.; Peffault de Latour, R.; Arnaud, C.; Boddaert, N.; Oualha, M.; Avettand-Fenoel, V.; Bernaudin, F.; Aguilar, C.; Barnerias, C.; Leruez-Ville, M.; et al. Adenoviral infection presenting as an isolated central nervous system disease without detectable viremia in two children after stem cell transplantation. J. Clin. Microbiol. 2011, 49, 2361–2364. [Google Scholar] [CrossRef]
- Aoki, K.; Tagawa, Y. A twenty-one year surveillance of adenoviral conjunctivitis in sapporo, japan. Int. Ophthalmol. Clin. 2002, 42, 49–54. [Google Scholar] [CrossRef]
- Louie, J.K.; Kajon, A.E.; Holodniy, M.; Guardia-LaBar, L.; Lee, B.; Petru, A.M.; Hacker, J.K.; Schnurr, D.P. Severe pneumonia due to adenovirus serotype 14: A new respiratory threat? Clin. Infect. Dis. 2008, 46, 421–425. [Google Scholar] [CrossRef]
- Clark, T.W.; Fleet, D.H.; Wiselka, M.J. Severe community-acquired adenovirus pneumonia in an immunocompetent 44-year-old woman: A case report and review of the literature. J. Med. Case Rep. 2011, 5, 259. [Google Scholar] [CrossRef]
- Dubberke, E.R.; Tu, B.; Rivet, D.J.; Storch, G.A.; Apisarnthanarak, A.; Schmidt, R.E.; Weiss, S.; Polish, L.B. Acute meningoencephalitis caused by adenovirus serotype 26. J. Neurovirol. 2006, 12, 235–240. [Google Scholar] [CrossRef]
- Liu, L.; Qian, Y.; Zhang, Y.; Deng, J.; Jia, L.; Dong, H. Adenoviruses associated with acute diarrhea in children in beijing, china. PLoS ONE 2014, 9, e88791. [Google Scholar] [CrossRef]
- Centers for Disease Control (CDC). Outbreak of Pharyngoconjunctival Fever at a Summer Camp—North Carolina, 1991. MMWR Morb. Mortal. Wkly. Rep. 1992, 41, 342–344. [Google Scholar]
- Centers for Disease Control and Prevention. Adenovirus transmission; National Center for Immunization and Respiratory Diseases, Division of Viral Diseases: Atlanta, GA, USA, 2018. Available online: https://www.cdc.gov/adenovirus/about/transmission.html (accessed on 26 June 2019).
- Furuse, Y.; Ornelles, D.A.; Cullen, B.R. Persistently adenovirus-infected lymphoid cells express micrornas derived from the viral vai and especially vaii rna. Virology 2013, 447, 140–145. [Google Scholar] [CrossRef]
- Hofmayer, S.; Madisch, I.; Darr, S.; Rehren, F.; Heim, A. Unique sequence features of the human adenovirus 31 complete genomic sequence are conserved in clinical isolates. BMC Genom. 2009, 10, 557. [Google Scholar] [CrossRef]
- Lutz, P.; Kedinger, C. Properties of the adenovirus iva2 gene product, an effector of late-phase-dependent activation of the major late promoter. J. Virol. 1996, 70, 1396–1405. [Google Scholar]
- Robinson, C.M.; Seto, D.; Jones, M.S.; Dyer, D.W.; Chodosh, J. Molecular evolution of human species d adenoviruses. Infect. Genet. Evol. 2011, 11, 1208–1217. [Google Scholar] [CrossRef]
- Kamel, W.; Segerman, B.; Oberg, D.; Punga, T.; Akusjarvi, G. The adenovirus va rna-derived mirnas are not essential for lytic virus growth in tissue culture cells. Nucleic Acids Res. 2013, 41, 4802–4812. [Google Scholar] [CrossRef]
- Zhang, Y.; Bergelson, J.M. Adenovirus receptors. J. Virol. 2005, 79, 12125–12131. [Google Scholar] [CrossRef]
- Huang, G.H.; Xu, W.B. recent advance in new types of human adenovirus. Bing du xue bao = Chin. J. Virol. 2013, 29, 342–348. [Google Scholar]
- Philipson, L.; Lonberg-Holm, K.; Pettersson, U. Virus-receptor interaction in an adenovirus system. J. Virol. 1968, 2, 1064–1075. [Google Scholar]
- Cohen, C.J.; Shieh, J.T.; Pickles, R.J.; Okegawa, T.; Hsieh, J.T.; Bergelson, J.M. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 2001, 98, 15191–15196. [Google Scholar] [CrossRef] [Green Version]
- Walters, R.W.; Freimuth, P.; Moninger, T.O.; Ganske, I.; Zabner, J.; Welsh, M.J. Adenovirus fiber disrupts car-mediated intercellular adhesion allowing virus escape. Cell 2002, 110, 789–799. [Google Scholar] [CrossRef]
- Roelvink, P.W.; Lizonova, A.; Lee, J.G.; Li, Y.; Bergelson, J.M.; Finberg, R.W.; Brough, D.E.; Kovesdi, I.; Wickham, T.J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups a, c, d, e, and f. J. Virol. 1998, 72, 7909–7915. [Google Scholar]
- Wickham, T.J.; Mathias, P.; Cheresh, D.A.; Nemerow, G.R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 1993, 73, 309–319. [Google Scholar] [CrossRef]
- Patterson, S.; Russell, W.C. Ultrastructural and immunofluorescence studies of early events in adenovirus-hela cell interactions. J. Gen. Virol. 1983, 64, 1091–1099. [Google Scholar] [CrossRef]
- Li, E.; Stupack, D.; Bokoch, G.M.; Nemerow, G.R. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by rho family gtpases. J. Virol. 1998, 72, 8806–8812. [Google Scholar]
- Seth, P.; Pastan, I.; Willingham, M.C. Adenovirus-dependent changes in cell membrane permeability: Role of na+, k+-atpase. J. Virol. 1987, 61, 883–888. [Google Scholar]
- Gaggar, A.; Shayakhmetov, D.M.; Lieber, A. Cd46 is a cellular receptor for group b adenoviruses. Nat. Med. 2003, 9, 1408–1412. [Google Scholar] [CrossRef]
- Wolfrum, N.; Greber, U.F. Adenovirus signalling in entry. Cell. Microbiol. 2013, 15, 53–62. [Google Scholar] [CrossRef]
- Trinh, H.V.; Lesage, G.; Chennamparampil, V.; Vollenweider, B.; Burckhardt, C.J.; Schauer, S.; Havenga, M.; Greber, U.F.; Hemmi, S. Avidity binding of human adenovirus serotypes 3 and 7 to the membrane cofactor cd46 triggers infection. J. Virol. 2012, 86, 1623–1637. [Google Scholar] [CrossRef]
- Riley-Vargas, R.C.; Gill, D.B.; Kemper, C.; Liszewski, M.K.; Atkinson, J.P. Cd46: Expanding beyond complement regulation. Trends Immunol. 2004, 25, 496–503. [Google Scholar] [CrossRef]
- Nilsson, E.C.; Storm, R.J.; Bauer, J.; Johansson, S.M.; Lookene, A.; Angstrom, J.; Hedenstrom, M.; Eriksson, T.L.; Frangsmyr, L.; Rinaldi, S.; et al. The gd1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat. Med. 2011, 17, 105–109. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.Y.; Liu, Y.; Persson, J.; Beyer, I.; Moller, T.; Koyuncu, D.; Drescher, M.R.; Strauss, R.; Zhang, X.B.; et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat. Med. 2011, 17, 96–104. [Google Scholar] [CrossRef]
- Murali, V.K.; Ornelles, D.A.; Gooding, L.R.; Wilms, H.T.; Huang, W.; Tollefson, A.E.; Wold, W.S.; Garnett-Benson, C. Adenovirus death protein (adp) is required for lytic infection of human lymphocytes. J. Virol. 2014, 88, 903–912. [Google Scholar] [CrossRef]
- Lynch, J.P., 3rd.; Fishbein, M.; Echavarría, M. Adenoviruses. Semin Respir. Crit. Care. Med. 2011, 32, 494–511. [Google Scholar] [CrossRef]
- Centers for disease control and prevention. Adenovirus Clinical Diagnosis; National Center for Immunization and Respiratory Diseases, Division of Viral Diseases: Atlanta, GA, USA, 2018. Available online: https://www.cdc.gov/adenovirus/hcp/diagnosis.html (accessed on 26 June 2019).
- Terletskaia-Ladwig, E.; Leinmüller, M.; Schneider, F.; Meier, S.; Enders, M. Laboratory approaches to the diagnosis of adenovirus infection depending on clinical manifestations. Infection 2007, 35, 438–443. [Google Scholar] [CrossRef]
- Song, E.; Wang, H.; Kajon, A.E.; Salamon, D.; Dong, S.; Ramilo, O.; Leber, A.; Jaggi, P. Diagnosis of pediatric acute adenovirus infections: Is a positive pcr sufficient? Pediatric Infect. Dis. J. 2016, 35, 827–834. [Google Scholar] [CrossRef]
- Meurman, O.; Ruuskanen, O.; Sarkkinen, H. Immunoassay diagnosis of adenovirus infections in children. J. Clin. Microbiol. 1983, 18, 1190–1195. [Google Scholar] [Green Version]
- Mayindou, G.; Ngokana, B.; Sidibe, A.; Moundele, V.; Koukouikila-Koussounda, F.; Christevy Vouvoungui, J.; Kwedi Nolna, S.; Velavan, T.P.; Ntoumi, F. Molecular epidemiology and surveillance of circulating rotavirus and adenovirus in congolese children with gastroenteritis. J. Med. Virol. 2016, 88, 596–605. [Google Scholar] [CrossRef]
- Timoshicheva, T.A.; Zabrodskaya, Y.A.; Ramsay, E.; Amosova, I.V. Use of hexon as an antigen for the production of monoclonal antibodies capable of detecting multiple adenovirus types. Biologicals 2019, 58, 44–49. [Google Scholar] [CrossRef]
- Harmon, M.W.; Drake, S.; Kasel, J.A. Detection of adenovirus by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1979, 9, 342–346. [Google Scholar]
- Grandien, M.; Pettersson, C.A.; Svensson, L.; Uhnoo, I. Latex agglutination test for adenovirus diagnosis in diarrheal disease. J. Med. Virol. 1987, 23, 311–316. [Google Scholar] [CrossRef]
- Trabelsi, A.; Pozzetto, B.; Mbida, A.D.; Grattard, F.; Ros, A.; Gaudin, O.G. Evaluation of four methods for rapid detection of adenovirus. Eur. J. Clin. Microbiol. Infect. Dis. 1992, 11, 535. [Google Scholar] [CrossRef]
- Lengyel, A.; Adám, E.; nász, I. Latex agglutination and adenoviruses. Ii. Detection of antigens. Acta Microbiol. Hung. 1993, 40, 85–90. [Google Scholar]
- Kalaiselvi, G.; Parthiban, M.; Narayanan, M.S.; Kumar, S.S.; Kathaperumal, K. Rapid latex agglutination test for serodiagnosis of fowl adenovirus serotype 4 using recombinant antigen. Vet. Arh. 2010, 80, 743–752. [Google Scholar]
- O’Neill, H.J.; McCaughey, C.; Coyle, P.V.; Wyatt, D.E.; Mitchell, F. Clinical utility of nested multiplex rt-pcr for group f adenovirus, rotavirus and norwalk-like viruses in acute viral gastroenteritis in children and adults. J. Clin. Virol. 2002, 25, 335–343. [Google Scholar] [CrossRef]
- Bennett, S.; Gunson, R.N. The development of a multiplex real-time rt-pcr for the detection of adenovirus, astrovirus, rotavirus and sapovirus from stool samples. J. Virol. Methods 2017, 242, 30–34. [Google Scholar] [CrossRef]
- Cortes-Hinojosa, G.; Gulland, F.M.; Goldstein, T.; Venn-Watson, S.; Rivera, R.; Archer, L.L.; Waltzek, T.B.; Gray, G.C.; Wellehan, J.F., Jr. Development and validation of a quantitative pcr for rapid and specific detection of california sea lion adenovirus 1 and prevalence in wild and managed populations. J. Vet. Diagn. Investig. 2017, 29, 193–197. [Google Scholar] [CrossRef]
- Lu, X.; Erdman, D.D. Quantitative real-time pcr assays for detection and type-specific identification of the endemic species c human adenoviruses. J. Virol. Methods 2016, 237, 174–178. [Google Scholar] [CrossRef]
- Lu, X.; Trujillo-Lopez, E.; Lott, L.; Erdman, D.D. Quantitative real-time pcr assay panel for detection and type-specific identification of epidemic respiratory human adenoviruses. J. Clin. Microbiol. 2013, 51, 1089–1093. [Google Scholar] [CrossRef]
- Echavarría, M. Adenoviruses in immunocompromised hosts. Clin. Microbiol. Rev. 2008, 21, 704–715. [Google Scholar] [CrossRef]
- Hara, M.; Takao, S.; Fukuda, S.; Shimazu, Y.; Kuwayama, M.; Miyazaki, K. Comparison of four rapid diagnostic kits using immunochromatography to detect influenza b viruses. Kansenshogaku zasshi. J. Jpn. Assoc. Infect. Dis. 2005, 79, 803–811. [Google Scholar] [CrossRef]
- Zhao. Comparison of four rapid diagnostic kits of immunochromatography for detection of influenza a and influenza b viruses. J. Microbiol. Biotechnol. 2017, 79, 803–811. [Google Scholar]
- Centers for Disease Control and Prevention. Adenovirus prevention and treatment; National Center for Immunization and Respiratory Diseases, Division of Viral Diseases: Atlanta, GA, USA, 2018. Available online: https://www.cdc.gov/adenovirus/about/prevention-treatment.html (accessed on 26 June 2019).
- Rutala, W.A.; Peacock, J.E.; Gergen, M.F.; Sobsey, M.D.; Weber, D.J. Efficacy of hospital germicides against adenovirus 8, a common cause of epidemic keratoconjunctivitis in health care facilities. Antimicrob. Agents Chemother. 2006, 50, 1419–1424. [Google Scholar] [CrossRef]
- Brennan, D. What are Adenovirus Infections? WebMD Health Services: Portland, OR, USA, 2018. [Google Scholar]
- Choudhry, A.; Mathena, J.; Albano, J.D.; Yacovone, M.; Collins, L. Safety evaluation of adenovirus type 4 and type 7 vaccine live, oral in military recruits. Vaccine 2016, 34, 4558–4564. [Google Scholar] [CrossRef] [Green Version]
- Russell, K.L.; Hawksworth, A.W.; Ryan, M.A.K.; Strickler, J.; Irvine, M.; Hansen, C.J.; Gray, G.C.; Gaydos, J.C. Vaccine-preventable adenoviral respiratory illness in us military recruits, 1999–2004. Vaccine 2006, 24, 2835–2842. [Google Scholar] [CrossRef]
- Barraza, E.M.; Ludwig, S.L.; Gaydos, J.C.; Brundage, J.F. Reemergence of adenovirus type 4 acute respiratory disease in military trainees: Report of an outbreak during a lapse in vaccination. J. Infect. Dis. 1999, 179, 1531–1533. [Google Scholar] [CrossRef]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zeng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef]
- Wold, W.S.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433. [Google Scholar] [CrossRef]
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018, 20, e3015. [Google Scholar] [CrossRef]
- Douglas, J.T. Adenovirus-Mediated Gene Delivery. In Gene Delivery to Mammalian Cells; Methods in Molecular Biology™; Heiser, W.C., Ed.; Humana Press: Totowa, NJ, USA, 2004; Volume 246, pp. 3–14. [Google Scholar]
- Chandler, R.J.; Venditti, C.P. Gene therapy for metabolic diseases. Transl. Sci. Rare Dis. 2016, 1, 73–89. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, H.A.; Danel, C.; Longenecker, G.; Metzger, M.; Setoguchi, Y.; Rosenfeld, M.A.; Gant, T.W.; Thorgeirsson, S.S.; Stratford-Perricaudet, L.D.; Perricaudet, M.; et al. Adenovirus–mediated in vivo gene transfer and expression in normal rat liver. Nat. Genet. 1992, 1, 372–378. [Google Scholar] [CrossRef]
- Gomez-Gutierrez, J.G.; Nitz, J.; Sharma, R.; Wechman, S.L.; Riedinger, E.; Martinez-Jaramillo, E.; Sam Zhou, H.; McMasters, K.M. Combined therapy of oncolytic adenovirus and temozolomide enhances lung cancer virotherapy in vitro and in vivo. Virology 2016, 487, 249–259. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, H.; Ugai, H.; Matthews, Q.L.; Curiel, D.T. Derivation of a triple mosaic adenovirus for cancer gene therapy. PLoS ONE 2009, 4, e8526. [Google Scholar] [CrossRef]
- Dmitriev, I.P.; Kashentseva, E.A.; Kim, K.H.; Matthews, Q.L.; Krieger, S.S.; Parry, J.J.; Nguyen, K.N.; Akers, W.J.; Achilefu, S.; Rogers, B.E.; et al. Monitoring of biodistribution and persistence of conditionally replicative adenovirus in a murine model of ovarian cancer using capsid-incorporated mcherry and expression of human somatostatin receptor subtype 2 gene. Mol. Imaging 2014, 13. [Google Scholar] [CrossRef]
- Sharma, A.; Tandon, M.; Bangari, D.S.; Mittal, S.K. Adenoviral vector-based strategies for cancer therapy. Curr. Drug Ther. 2009, 4, 117–138. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Nagasato, M.; Yoshida, T.; Aoki, K. Recent advances in genetic modification of adenovirus vectors for cancer treatment. Cancer Sci. 2017, 108, 831–837. [Google Scholar] [CrossRef]
- Wu, Q.; Xia, D.; Carlsen, S.; Xiang, J. Adenovirus-mediated transgene-engineered dendritic cell vaccine of cancer. Curr. Gene Ther. 2005, 5, 237–247. [Google Scholar] [CrossRef]
- Tagawa, M.; Kawamura, K.; Ueyama, T.; Nakamura, M.; Tada, Y.; Ma, G.; Li, Q.; Suzuki, N.; Shimada, H.; Ochiai, T. Cancer therapy with local oncolysis and topical cytokine secretion. Front. Biosci. 2008, 13, 2578–2587. [Google Scholar] [CrossRef] [Green Version]
- Matthews, K.S.; Alvarez, R.D.; Curiel, D.T. Advancements in adenoviral based virotherapy for ovarian cancer. Adv. Drug Deliv. Rev. 2009, 61, 836–841. [Google Scholar] [CrossRef]
- Fukazawa, T.; Matsuoka, J.; Yamatsuji, T.; Maeda, Y.; Durbin, M.L.; Naomoto, Y. Adenovirus-mediated cancer gene therapy and virotherapy (review). Int. J. Mol. Med. 2010, 25, 3–10. [Google Scholar] [CrossRef]
- Aguilar, L.K.; Guzik, B.W.; Aguilar-Cordova, E. Cytotoxic immunotherapy strategies for cancer: Mechanisms and clinical development. J. Cell. Biochem. 2011, 112, 1969–1977. [Google Scholar] [CrossRef]
- Aurisicchio, L.; Ciliberto, G. Genetic cancer vaccines: Current status and perspectives. Expert Opin. Biol. Ther. 2012, 12, 1043–1058. [Google Scholar] [CrossRef]
- Duarte, S.; Carle, G.; Faneca, H.; de Lima, M.C.; Pierrefite-Carle, V. Suicide gene therapy in cancer: Where do we stand now? Cancer Lett. 2012, 324, 160–170. [Google Scholar] [CrossRef]
- Deisseroth, A.; Tang, Y.; Zhang, L.; Akbulut, H.; Habib, N. Taa/ecdcd40l adenoviral prime-protein boost vaccine for cancer and infectious diseases. Cancer Gene Ther. 2013, 20, 65–69. [Google Scholar] [CrossRef]
- Crystal, R.G. Adenovirus: The first effective in vivo gene delivery vector. Hum. Gene Ther. 2014, 25, 3–11. [Google Scholar] [CrossRef]
- Singh, P.K.; Doley, J.; Kumar, G.R.; Sahoo, A.P.; Tiwari, A.K. Oncolytic viruses & their specific targeting to tumour cells. Indian J. Med. Res. 2012, 136, 571–584. [Google Scholar]
- Cheng, P.-H.; Wechman, S.L.; McMasters, K.M.; Zhou, H.S. Oncolytic replication of e1b-deleted adenoviruses. Viruses 2015, 7, 5767–5779. [Google Scholar] [CrossRef]
- Peng, Z. Current status of gendicine in china: Recombinant human ad-p53 agent for treatment of cancers. Hum. Gene Ther. 2005, 16, 1016–1027. [Google Scholar] [CrossRef]
- Zhang, W.W.; Roth, A.J. Anti-oncogene and tumor suppressor gene therapy-examples from a lung cancer animal model. In Vivo 1994, 8, 755–769. [Google Scholar]
- Han, J.; Li, N. Adenoviral vector-mediated delivery of p21waf1/cip1 prevents retinal neovascularization in an oxygen-induced retinopathy model. Curr. Eye Res. 2016, 41, 1113–1117. [Google Scholar] [CrossRef]
- Trang, P.; Wiggins, J.F.; Daige, C.L.; Cho, C.; Omotola, M.; Brown, D.; Weidhaas, J.B.; Bader, A.G.; Slack, F.J. Systemic delivery of tumor suppressor microrna mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol. Ther. 2011, 19, 1116–1122. [Google Scholar] [CrossRef]
- Sherr, C.; McCormick, F. The rb and p53 pathways in cancer. Cancer Cell 2002, 2, 103–112. [Google Scholar] [CrossRef]
- Zhang, W.W.; Li, L.; Li, D.; Liu, J.; Li, X.; Li, W.; Xu, X.; Zhang, M.J.; Chandler, L.A.; Lin, H.; et al. The first approved gene therapy product for cancer ad-p53 (gendicine): 12 years in the clinic. Hum. Gene Ther. 2018, 29, 160–179. [Google Scholar] [CrossRef]
- Pearson, S.; Jia, H.; Kandachi, K. China approves first gene therapy. Nat. Biotechnol. 2004, 22, 3–4. [Google Scholar] [CrossRef]
- Wirth, T.; Ylä-Herttuala, S. Gene therapy used in cancer treatment. Biomedicines 2014, 2, 149–162. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, D. Adenoviral vector-based strategies against infectious disease and cancer. Hum. Vaccines Immunother. 2016, 12, 2064–2074. [Google Scholar] [CrossRef] [Green Version]
- Wolf, J.K.; Bodurka, D.C.; Gano, J.B.; Deavers, M.; Ramondetta, L.; Ramirez, P.T.; Levenback, C.; Gershenson, D.M. A phase i study of adp53 (ingn 201; advexin) for patients with platinum-and paclitaxel-resistant epithelial ovarian cancer. Gynecol. Oncol. 2004, 94, 442–448. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, Y.; Xu, C.; Xu, H.; Zhou, X.; Yang, J.; Xie, Y.; Tao, M. Adenovirus-mediated p53 and ing4 gene co-transfer elicits synergistic antitumor effects through enhancement of p53 acetylation in breast cancer. Oncol. Rep. 2016, 35, 243–252. [Google Scholar] [CrossRef]
- Nemunaitis, J.; Nemunaitis, J. Head and neck cancer: Response to p53-based therapeutics. Head Neck 2011, 33, 131–134. [Google Scholar] [CrossRef]
- Nie, B.; Shen, Z.; Wen, J.B.; Wong, O.G.; Hsueh, W.D.; Huo, L.F.; Kung, H.F.; Jiang, B.; Lin, M.C. Aav-hgfk1 and ad-p53 cocktail therapy prolongs survival of mice with colon cancer. Mol. Cancer Ther. 2008, 7, 2855–2865. [Google Scholar] [CrossRef]
- Azab, B.M.; Dash, R.; Das, S.K.; Bhutia, S.K.; Sarkar, S.; Shen, X.N.; Quinn, B.A.; Dent, P.; Dmitriev, I.P.; Wang, X.Y.; et al. Enhanced prostate cancer gene transfer and therapy using a novel serotype chimera cancer terminator virus (ad.5/3-ctv). J. Cell. Physiol. 2014, 229, 34–43. [Google Scholar] [CrossRef]
- Gu, L.; Icyuz, M.; Krendelchtchikova, V.; Krendelchtchikov, A.; Johnston, A.E.; Matthews, Q.L. Development of an ad5h3 chimera using the “antigen capsid-incorporation” strategy for an alternative vaccination approach. Open Virol. J. 2016, 10, 10–20. [Google Scholar] [CrossRef]
- Vannucci, L.; Lai, M.; Chiuppesi, F.; Ceccherini-Nelli, L.; Pistello, M. Viral vectors: A look back and ahead on gene transfer technology. New Microbiol. 2013, 36, 1–22. [Google Scholar]
- Singh, S.; Kumar, R.; Agrawal, B. Adenoviral vector-based vaccines and gene therapies: Current status and future prospects. In Adenoviruses; Desheva, Y., Ed.; Intech Open Access: London, UK, 2018; pp. 1–41. [Google Scholar]
- Pereboev, A.V.; Nagle, J.M.; Shakhmatov, M.A.; Triozzi, P.L.; Matthews, Q.L.; Kawakami, Y.; Curiel, D.T.; Blackwell, J.L. Enhanced gene transfer to mouse dendritic cells using adenoviral vectors coated with a novel adapter molecule. Mol. Ther. 2004, 9, 712–720. [Google Scholar] [CrossRef]
- Stoff-Khalili, M.A.; Rivera, A.A.; Stoff, A.; Michael Mathis, J.; Rocconi, R.P.; Matthews, Q.L.; Numnum, M.T.; Herrmann, I.; Dall, P.; Eckhoff, D.E.; et al. Combining high selectivity of replication via cxcr4 promoter with fiber chimerism for effective adenoviral oncolysis in breast cancer. Int. J. Cancer 2007, 120, 935–941. [Google Scholar] [CrossRef]
- Matthews, Q.L.; Sibley, D.A.; Wu, H.; Li, J.; Stoff-Khalili, M.A.; Waehler, R.; Mathis, J.M.; Curiel, D.T. Genetic incorporation of a herpes simplex virus type 1 thymidine kinase and firefly luciferase fusion into the adenovirus protein ix for functional display on the virion. Mol. Imaging 2006, 5, 510–519. [Google Scholar] [CrossRef]
- Tang, Y.; Le, L.P.; Matthews, Q.L.; Han, T.; Wu, H.; Curiel, D.T. Derivation of a triple mosaic adenovirus based on modification of the minor capsid protein ix. Virology 2008, 377, 391–400. [Google Scholar] [CrossRef]
- National Institute of Health. Gene therapy clinical trials worldwide. National Institute of Health, Human Gene Transfer Protocol List. 2018. Available online: http://www.abedia.com/wiley/. (accessed on 3 June 2019).
- U.S. Department of Health and Human Services. Gene Therapy Using an Adenovirus Vector; U.S. National Library of Medicine: Bethesda, MD, USA, 2013. Available online: https://nlm.nih.gov. (accessed on 3 June 2019).
- Grove, J.; Marsh, M. The cell biology of receptor-mediated virus entry. J. Cell Biol. 2011, 195, 1071–1082. [Google Scholar] [CrossRef] [Green Version]
- Sims, B.; Gu, L.; Krendelchtchikov, A.; Matthews, Q.L. Neural stem cell-derived exosomes mediate viral entry. Int. J. Nanomed. 2014, 9, 4893–4897. [Google Scholar] [CrossRef] [Green Version]
- Nolte, E.; Cremer, T.; Gallo, R.C.; Margolis, L.B. Extracellular vesicles and viruses: Are they close relatives? Proc. Natl. Acad. Sci. USA 2016, 113, 9155–9161. [Google Scholar] [CrossRef] [Green Version]
- Gould, S.J.; Booth, A.M.; Hildreth, J.E. The trojan exosome hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 10592–10597. [Google Scholar] [CrossRef]
- Hildreth, J.E.K. Hiv as trojan exosome: Immunological paradox explained? Front. Immunol. 2017, 8, 1715. [Google Scholar] [CrossRef]
- Booth, A.M.; Fang, Y.; Fallon, J.K.; Yang, J.M.; Hildreth, J.E.; Gould, S.J. Exosomes and hiv gag bud from endosome-like domains of the t cell plasma membrane. J. Cell Biol. 2006, 172, 923–935. [Google Scholar] [CrossRef]
- Nguyen, D.G.; Booth, A.; Gould, S.J.; Hildreth, J.E. Evidence that hiv budding in primary macrophages occurs through the exosome release pathway. J. Biol. Chem. 2003, 278, 52347–52354. [Google Scholar] [CrossRef]
- Crenshaw, B.J.; Gu, L.; Sims, B.; Matthews, Q.L. Exosome biogenesis and biological function in response to viral infections. Open Virol. J. 2018, 12, 134–148. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Jones, L.B.; Bell, C.R.; Bibb, K.E.; Gu, L.; Coats, M.T.; Matthews, Q.L. Pathogens and their effect on exosome biogenesis and composition. Biomedicines 2018, 6, 79. [Google Scholar] [CrossRef]
- Thery, C.; Boussac, M.; Veron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic analysis of dendritic cell-derived exosomes: A secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef]
- Sims, B.; Farrow, A.L.; Williams, S.D.; Bansal, A.; Krendelchtchikov, A.; Matthews, Q.L. Tetraspanin blockage reduces exosome-mediated hiv-1 entry. Arch. Virol. 2018, 163, 1683–1689. [Google Scholar] [CrossRef]
- Sims, B.; Farrow, A.L.; Williams, S.D.; Bansal, A.; Krendelchtchikov, A.; Gu, L.; Matthews, Q.L. Role of tim-4 in exosome-dependent entry of hiv-1 into human immune cells. Int. J. Nanomed. 2017, 12, 4823–4833. [Google Scholar] [CrossRef]
- Matthews, Q.L. Capsid-incorporation of antigens into adenovirus capsid proteins for a vaccine approach. Mol. Pharm. 2011, 8, 3–11. [Google Scholar] [CrossRef]
- Wang, R.; Ding, Q.; Yaqoob, U.; de Assuncao, T.M.; Verma, V.; Hirsova, P.; Cao, S.; Mukhopadhyay, D.; Huebert, R.; Shah, V.H. Exosome adherence and internalization by hepatic stellate cells triggers sphingosine 1-phosphate-dependent migration. J. Boil. Chem. 2015, 290, 30684–30696. [Google Scholar] [CrossRef]
- Witek, R.P.; Yang, L.; Liu, R.; Jung, Y.; Omenetti, A.; Syn, W.-K.; Choi, S.S.; Cheong, Y.; Fearing, C.M.; Agboola, K.M.; et al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology 2009, 136, 320–330. [Google Scholar] [CrossRef]
- Rosa, G.; Fratini, M.; Libera, S.; Iaconelli, M.; Muscillo, M. Viral infections acquired indoors through airborne, droplet or contact transmission. Ann. Dell’istituto Super. Sanità 2013, 49, 124–132. [Google Scholar]
- Beach, A.; Zhang, H.G.; Ratajczak, M.Z.; Kakar, S.S. Exosomes: An overview of biogenesis, composition and role in ovarian cancer. J. Ovarian Res. 2014, 7, 14. [Google Scholar] [CrossRef]
- Crenshaw, B.J.; Sims, B.; Matthews, Q.L. Biological function of exosomes as diagnostic markers and therapeutic delivery vehicles in carcinogenesis and infectious diseases. In Nanomedicines; Farrukh, M.A., Ed.; IntechOpen: London, UK, 2018; pp. 1–33. [Google Scholar]
- Thery, C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011, 3, 15. [Google Scholar] [CrossRef]
- Taylor, D.D.; Gercel-Taylor, C. Microrna signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008, 110, 13–21. [Google Scholar] [CrossRef]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. Discovery of double-stranded genomic DNA in circulating exosomes. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2016; Volume 81, pp. 275–280. [Google Scholar]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Meckes, D.G., Jr.; Raab-Traub, N. Microvesicles and viral infection. J. Virol. 2011, 85, 12844–12854. [Google Scholar] [CrossRef]
- Sampey, G.C.; Meyering, S.S.; Zadeh, M.A.; Saifuddin, M.; Hakami, R.M.; Kashanchi, F. Exosomes and their role in cns viral infections. J. Neurovirology 2014, 20, 199–208. [Google Scholar] [CrossRef]
- Davey, N.E.; Trave, G.; Gibson, T.J. How viruses hijack cell regulation. Trends Biochem. Sci. 2011, 36, 159–169. [Google Scholar] [CrossRef]
- Greenhill, C. Hepatitis: New route of hcv transmission. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 504. [Google Scholar] [CrossRef]
- Hofer, U. Viral pathogenesis: Cloak and dagger. Nat. Rev. Microbiol. 2013, 11, 360. [Google Scholar]
- Kadiu, I.; Narayanasamy, P.; Dash, P.K.; Zhang, W.; Gendelman, H.E. Biochemical and biologic characterization of exosomes and microvesicles as facilitators of hiv-1 infection in macrophages. J. Immunol. 2012, 189, 744–754. [Google Scholar] [CrossRef]
- Van Dongen, H.M.; Masoumi, N.; Witwer, K.W.; Pegtel, D.M. Extracellular vesicles exploit viral entry routes for cargo delivery. Microbiol. Mol. Biol. Rev. MMBR 2016, 80, 369–386. [Google Scholar] [CrossRef]
- Wurdinger, T.; Gatson, N.N.; Balaj, L.; Kaur, B.; Breakefield, X.O.; Pegtel, D.M. Extracellular vesicles and their convergence with viral pathways. Adv. Virol. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Martin, C.; Patel, M.; Williams, S.; Arora, H.; Sims, B. Human breast milk-derived exosomes attenuate cell death in intestinal epithelial cells. Innate Immun. 2018, 24, 278–284. [Google Scholar] [CrossRef]
- Zhu, L.; Song, H.; Zhang, X.; Xia, X.; Sun, H. Inhibition of porcine reproductive and respiratory syndrome virus infection by recombinant adenovirus- and/or exosome-delivered the artificial micrornas targeting sialoadhesin and cd163 receptors. Virol. J. 2014, 11, 225. [Google Scholar] [CrossRef]
- Ran, L.; Tan, X.; Li, Y.; Zhang, H.; Ma, R.; Ji, T.; Dong, W.; Tong, T.; Liu, Y.; Chen, D.; et al. Delivery of oncolytic adenovirus into the nucleus of tumorigenic cells by tumor microparticles for virotherapy. Biomaterials 2016, 89, 56–66. [Google Scholar] [CrossRef]
- Garofalo, M.; Saari, H.; Somersalo, P.; Crescenti, D.; Kuryk, L.; Aksela, L.; Capasso, C.; Madetoja, M.; Koskinen, K.; Oksanen, T.; et al. Antitumor effect of oncolytic virus and paclitaxel encapsulated in extracellular vesicles for lung cancer treatment. J. Control. Release 2018, 283, 223–234. [Google Scholar] [CrossRef]
- Garofalo, M.; Villa, A.; Rizzi, N.; Kuryk, L.; Mazzaferro, V.; Ciana, P. Systemic administration and targeted delivery of immunogenic oncolytic adenovirus encapsulated in extracellular vesicles for cancer therapies. Viruses 2018, 10, 558. [Google Scholar] [CrossRef]
- Kim, O.Y.; Lee, J.; Gho, Y.S. Extracellular vesicle mimetics: Novel alternatives to extracellular vesicle-based theranostics, drug delivery, and vaccines. Semin. Cell Dev. Biol. 2017, 67, 74–82. [Google Scholar] [CrossRef]
- Kooijmans, S.A.; Vader, P.; van Dommelen, S.M.; van Solinge, W.W.; Schiffelers, R.M. Exosome mimetics: A novel class of drug delivery systems. Int. J. Nanomed. 2012, 7, 1525–1541. [Google Scholar]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Ingato, D.; Lee, J.U.; Sim, S.J.; Kwon, Y.J. Good things come in small packages: Overcoming challenges to harness extracellular vesicles for therapeutic delivery. J. Control. Release 2016, 241, 174–185. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Badawi, M.; Pomeroy, S.; Sutaria, D.S.; Xie, Z.; Baek, A.; Jiang, J.; Elgamal, O.A.; Mo, X.; Perle, K.; et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from hek293t cells. J. Extracell. Vesicles 2017, 6, 1324730. [Google Scholar] [CrossRef]



| Group | Serotype | Associated Disease or Infections | References |
|---|---|---|---|
| A | 12, 18, 31, 61 | gastrointestinal, respiratory, urinary, cryptic enteric infection, linked to obesity, meningoencephalitis | [7,41,42,43,44] |
| B | 3, 7, 11, 14, 16, 21, 34, 35, 50, 55, 66 | conjunctivitis, gastrointestinal, respiratory, urinary, pneumonia, meningoencephalitis, cystitis | [7,41,42,44,45,46,47] |
| C | 1, 2, 5, 6, 57 | respiratory, gastrointestinal, obesity, pneumonia, hepatitis | [7,41,42,45] |
| D | 8–10, 13, 15, 17, 19, 20, 22–30, 32, 33, 36–39, 42–49, 51, 53, 54, 56, 58-60, 63-67 | conjunctivitis, gastrointestinal, linked to obesity, meningoencephalitis | [7,42,43,45,48] |
| E | 4 | conjunctivitis, respiratory, pneumonia | [7,41,47] |
| F | 40, 41 | gastrointestinal, infantile diarrhea | [7,42,49] |
| G | 52 | gastrointestinal | [7,42] |
| Adenoviral Vector | Phase | Transgene | Condition | Administration Route | Clinical Trial Identifier |
|---|---|---|---|---|---|
| Ad-CCL21-DC | I | Serotype 5/C-C motif chemokine ligand 21 (CCL21) cDNA | Dendritic cells Advanced non-small cell lung cancer | Intratumoral | US-1720 NCT03546361 |
| ETBX-071 | I | PSA/MUC1/brachyury | Prostatic neoplasms Prostate cancer | Subcutaneous | US-1738 NCT03481816 |
| AAV8-VRC07 (VRC-HIV AAV070-00-GT) | I | Anti-HIV-1 monoclonal antibody (VRC07) | HIV infection | Intramuscular | US-1495 NCT03374202 |
| Ad5-CB-CFTR | I | Cystic fibrosis transmembrane conductance regulator gene | Cystic fibrosis | Intranasal | NCT00004779 |
| LOAd703 | I/II | Vector-directed cell lysis TMZ-CD40L and 4-1BBl cDNAs | Pancreatic cancer Ovarian cancer Biliary carcinoma Colorectal cancer | Intratumoral | US-1483 NCT03225989 |
| Ad5-DNX-2401 | II | Vector-directed cell lysis | Glioblastoma, Gliosarcoma | Intratumoral | US-1487 NCT03896568 |
| Ad-p53 | II | Tumor suppressor | Squamous cell carcinoma of the head and neck | Intratumoral | US-1767 NCT03544723 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crenshaw, B.J.; Jones, L.B.; Bell, C.R.; Kumar, S.; Matthews, Q.L. Perspective on Adenoviruses: Epidemiology, Pathogenicity, and Gene Therapy. Biomedicines 2019, 7, 61. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines7030061
Crenshaw BJ, Jones LB, Bell CR, Kumar S, Matthews QL. Perspective on Adenoviruses: Epidemiology, Pathogenicity, and Gene Therapy. Biomedicines. 2019; 7(3):61. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines7030061
Chicago/Turabian StyleCrenshaw, Brennetta J., Leandra B. Jones, Courtnee’ R. Bell, Sanjay Kumar, and Qiana L. Matthews. 2019. "Perspective on Adenoviruses: Epidemiology, Pathogenicity, and Gene Therapy" Biomedicines 7, no. 3: 61. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines7030061