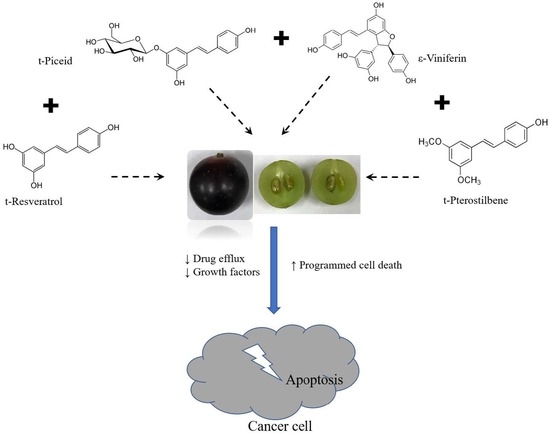
Synergistic Action of Stilbenes in Muscadine Grape Berry Extract Shows Better Cytotoxic Potential Against Cancer Cells Than Resveratrol Alone
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Berry Samples
2.3. Extraction of Stilbenoids
HPLC Analysis of Stilbenoid Content
2.4. Cell Culture and Reagents
2.4.1. Cytotoxicity Assay
2.4.2. Total RNA Extraction and cDNA Synthesis
2.4.3. Semi-Quantitative PCR
2.5. Statistical Analysis
3. Results
3.1. Extraction and Quantification of Stilbenes
3.2. Stilbene-Rich Muscadine Berry Extracts Induce Cytotoxicity in Cancer Cells
3.3. Resveratrol Alone is Less Cytotoxic Than the Whole Berry Extract
3.4. Synergistic Action of Stilbenes Induces Better Cellular Response Than Resveratrol Alone
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, Z.; Luo, J.; Huang, Y.; Guo, W.; Zhang, Y.; Guan, H.; Xu, C.; Lu, J. Profile of Polyphenol Compounds of Five Muscadine Grapes Cultivated in the United States and in Newly Adapted Locations in China. Int. J. Mol. Sci. 2017, 18, E631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, K.; Sims, C.; Odabasi, A.; Bartoshuk, L.; Conner, P.; Gray, D. Consumer Acceptability of Fresh-Market Muscadine Grapes. J. Food Sci. 2016, 81, S2808–S2816. [Google Scholar] [CrossRef] [PubMed]
- Burton, L.J.; Smith, B.A.; Smith, B.N.; Loyd, Q.; Nagappan, P.; McKeithen, D.; Wilder, C.L.; Platt, M.O.; Hudson, T.; Odero-Marah, V.A. Muscadine grape skin extract can antagonize Snail-cathepsin L-mediated invasion, migration and osteoclastogenesis in prostate and breast cancer cells. Carcinogenesis 2015, 36, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.; Ananga, A.; Dincheva, I.; Badjakov, I.; Gochev, V.; Tsolova, V. Chemical Composition, In Vitro Antioxidant Potential, and Antimicrobial Activities of Essential Oils and Hydrosols from Native American Muscadine Grapes. Molecules 2019, 24, E3355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basha, S.M.; Vasanthaiah, H.K.N.; Kambiranda, D.M.; Easwaran, K.; Queeley, G. Genetic variation in sugar composition among muscadine, Florida hybrid bunch and bunch grape genotypes. Int. J. Wine Res. 2012, 4, 15–23. [Google Scholar]
- Mendonca, P.; Darwish, A.G.; Tsolova, V.; El-Sharkawy, I.; Soliman, K.F.A. The Anticancer and Antioxidant Effects of Muscadine Grape Extracts on Racially Different Triple-negative Breast Cancer Cells. Anticancer Res. 2019, 39, 4043–4053. [Google Scholar] [CrossRef] [Green Version]
- You, Q.; Chen, F.; Wang, X.; Luo, P.G.; Jiang, Y. Inhibitory effects of muscadine anthocyanins on α-glucosidase and pancreatic lipase activities. J. Agric. Food Chem. 2011, 59, 9506–9511. [Google Scholar] [CrossRef]
- Li, R.; Kim, M.H.; Sandhu, A.K.; Gao, C.; Gu, L. Muscadine Grape (Vitis rotundifolia) or Wine Phytochemicals Reduce Intestinal Inflammation in Mice with Dextran Sulfate Sodium-Induced Colitis. J. Agric. Food Chem. 2017, 65, 769–776. [Google Scholar] [CrossRef]
- Percival, S.S.; Sims, C.A. Wine modifies the effects of alcohol on immune cells of mice. J. Nutr. 2000, 130, 1091–1094. [Google Scholar] [CrossRef]
- Mellen, P.B.; Daniel, K.R.; Brosnihan, K.B.; Hansen, K.J.; Herrington, D.M. Effect of muscadine grape seed supplementation on vascular function in subjects with or at risk for cardiovascular disease: A randomized crossover trial. J. Am. Coll. Nutr. 2010, 29, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Gourineni, V.; Shay, N.F.; Chung, S.; Sandhu, A.K.; Gu, L. Muscadine grape (Vitis rotundifolia) and wine phytochemicals prevented obesity-associated metabolic complications in C57BL/6J mice. J. Agric. Food Chem. 2012, 60, 7674–7681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okla, M.; Kang, I.; Kim, D.M.; Gourineni, V.; Shay, N.; Gu, L.; Chung, S. Ellagic acid modulates lipid accumulation in primary human adipocytes and human hepatoma Huh7 cells via discrete mechanisms. J. Nutr. Biochem. 2015, 26, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.U.; Percival, S.S.; Talcott, S.T. Extracts from red muscadine and cabernet sauvignon wines induce cell death in MOLT-4 human leukemia cells. Food Chem. 2008, 108, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.U.; Lee, J.H.; Percival, S.S.; Talcott, S.T. Induction of cell death in Caco-2 human colon carcinoma cells by ellagic acid rich fractions from muscadine grapes (Vitis rotundifolia). J. Agric. Food Chem. 2006, 54, 5336–5343. [Google Scholar] [CrossRef]
- Paller, C.J.; Zhou, X.C.; Heath, E.I.; Taplin, M.E.; Mayer, T.; Stein, M.N.; Bubley, G.J.; Pili, R.; Hudson, T.; Kakarla, R.; et al. Muscadine Grape Skin Extract (MPX) in Men with Biochemically Recurrent Prostate Cancer: A Randomized, Multicenter, Placebo-Controlled Clinical Trial. Clin. Cancer Res. 2018, 24, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Kambiranda, D.M.; Basha, S.M.; Stringer, S.J.; Obuya, J.O.; Snowden, J.J. Multi-year Quantitative Evaluation of Stilbenoids Levels Among Selected Muscadine Grape Cultivars. Molecules 2019, 24, E981. [Google Scholar] [CrossRef] [Green Version]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxidative Med. Cell. Longev. 2016. [Google Scholar] [CrossRef] [Green Version]
- Coombe, B.G. Growth stages of the grape vine: Adoption of a system for identifying grape vine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Liu, C.; Wang, L.; Wang, J.; Wu, B.; Liu, W.; Fan, P.; Liang, Z.; Li, S. Resveratrols in Vitis berry skins and leaves: Their extraction and analysis by HPLC. Food Chem. 2013, 136, 643–649. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRIreagent). Cold Spring Harb. Protoc. 2010, 6, 5439. [Google Scholar] [CrossRef] [PubMed]
- Marone, M.; Mozzetti, S.; De Ritis, D.; Pierelli, L.; Scambia, G. Semiquantitative RT-PCR analysis to assess the expression levels of multiple transcripts from the same sample. Biol. Proced. Online 2001, 3, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boichuk, S.; Galembikova, A.; Sitenkov, A.; Khusnutdinov, R.; Dunaev, P.; Valeeva, E.; Usolova, N. Establishment and characterization of a triple negative basal-like breast cancer cell line with multi-drug resistance. Oncol. Lett. 2017, 14, 5039–5045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volk-Draper, L.D.; Rajput, S.; Hall, K.L.; Wilber, A.; Ran, S. Novel model for basaloid triple-negative breast cancer: Behavior in vivo and response to therapy. Neoplasia 2012, 14, 926–942. [Google Scholar] [CrossRef] [Green Version]
- Khazaei, S.; Abdul Hamid, R.; Ramachandran, V.; Mohd Esa, N.; Pandurangan, A.K.; Danazadeh, F.; Ismail, P. Cytotoxicity and Proapoptotic Effects of Allium atroviolaceum Flower Extract by Modulating Cell Cycle Arrest and Caspase-Dependent and p53-Independent Pathway in Breast Cancer Cell Lines. Evid. Based Complement. Alternat. Med. 2017. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Wei, Z.; Zhang, S.; Peng, X.; Huang, Y.; Zhang, Y.; Lu, J. Phenolic Fractions from Muscadine Grape “Noble” Pomace can Inhibit Breast Cancer Cell MDA-MB-231 Better than those from European Grape “Cabernet Sauvignon” and Induce S-Phase Arrest and Apoptosis. J. Food Sci. 2017, 82, 1254–1263. [Google Scholar] [CrossRef]
- Burton, L.J.; Rivera, M.; Hawsawi, O.; Zou, J.; Hudson, T.; Wang, G.; Zhang, Q.; Cubano, L.; Boukli, N.; Odero-Marah, V. Muscadine Grape Skin Extract Induces an Unfolded Protein Response-Mediated Autophagy in Prostate Cancer Cells: A TMT-Based Quantitative Proteomic Analysis. PLoS ONE 2016, 11, e0164115. [Google Scholar] [CrossRef]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Larrosa, M.; González-Sarrías, A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Dudley, J.I.; Das, D.K. Dose-dependency of resveratrol in providing health benefits. Dose Response 2010, 8, 478–500. [Google Scholar] [CrossRef]
- Tippani, R.; Prakhya, L.J.; Porika, M.; Sirisha, K.; Abbagani, S.; Thammidala, C. Pterostilbene as a potential novel telomerase inhibitor: Molecular docking studies and its in vitro evaluation. Curr. Pharm. Biotechnol. 2014, 14, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Kala, R.; Shah, H.N.; Martin, S.L.; Tollefsbol, T.O. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent γ-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer 2015, 15, 672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storniolo, C.E.; Quifer-Rada, P.; Lamuela-Raventos, R.M.; Moreno, J.J. Piceid presents antiproliferative effects in intestinal epithelial Caco-2 cells, effects unrelated to resveratrol release. Food Funct. 2014, 5, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Nivelle, L.; Hubert, J.; Courot, E.; Jeandet, P.; Aziz, A.; Nuzillard, J.M.; Renault, J.H.; Clément, C.; Martiny, L.; Delmas, D.; et al. Anti-Cancer Activity of Resveratrol and Derivatives Produced by Grapevine Cell Suspensions in a 14 L Stirred Bioreactor. Molecules 2017, 22, 474. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Lin, F.; Yang, Y.; Chen, S.S. A Methodology for Cancer Therapeutics by Systems Pharmacology-Based Analysis: A Case Study on Breast Cancer-Related Traditional Chinese Medicines. PLoS ONE 2017, 12, e0169363. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lo-Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front Nutr. 2016, 3, 8. [Google Scholar] [CrossRef] [Green Version]








| Cultivar | Berry Color | Stilbene Content (µg/g) | |||
|---|---|---|---|---|---|
| t-Piceid | t-Resveratrol | Ɛ-Viniferin | t-Pterostilbene | ||
| Pineapple | Bronze | 279.75 ± 25 | 36.85 ± 1.15 | 24.35 ± 0.65 | ND * |
| Southern Home | Black | 480 ± 20 | 35 ± 1 | 1157.5 ± 22.5 | 4.5 ± 0.1 |
| Gene | Position | Primer Sequence (3′ to 5′) | Annealing Temperature | Amplicon Size (bp) | Reference |
|---|---|---|---|---|---|
| GAPDH | F | GACCACAGTCCATGCCATCA | 60 | 450 | [23] |
| R | TCCACCACCCTGTTGCTGTA | ||||
| Bcl-2 | F | ATGTCCAGCCAGCTGCACCTGAC | 60 | 319 | |
| R | GCAGAGTCTTCAGAGACAGCCAGG | ||||
| Fas | F | CAGGCTAACCCCACTCTATG | 61 | 450 | |
| R | TGGGGGTGCATTAGGCCATT | ||||
| Cas-8 | F | ACTTCAGACACCAGGCAGGGCT | 62 | 500 | |
| R | GCCCCTGCATCCAAGTGTGTTC | ||||
| ybx-1 | F | GACTGCCATAGAGAATAACCCCAG | 62 | 496 | |
| R | CTCTCTAGGCTGTTTTGGGCGAGGA | ||||
| EGFR | F | GGAGCCTCTTACACCCAGTG | 61 | 198 | [24] |
| R | GCTTTCGGAGATGTTGCTTC | ||||
| VEGF-A | F | CACATAGGAGAGATGAGCTTC | 60 | 100 | |
| R | CCGCCTCGGCTTGTCACAT | ||||
| cdk1 | F | GGGTCAGCTCGCTACTCAAC | 61 | 333 | [25] |
| R | AAGTTTTTGACGTGGGATGC | ||||
| p53 | F | TGTGGAGTATTTGGATGACA | 58 | 550 | |
| R | GAACATGAGTTTTTTATGGC | ||||
| β-Actin | F | TGGGTCAGAAGGATTCCTATGT | 60 | 276 | [24] |
| R | CAGCCTGGATAGCAACGTACA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balasubramani, S.P.; Rahman, M.A.; Basha, S.M. Synergistic Action of Stilbenes in Muscadine Grape Berry Extract Shows Better Cytotoxic Potential Against Cancer Cells Than Resveratrol Alone. Biomedicines 2019, 7, 96. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines7040096
Balasubramani SP, Rahman MA, Basha SM. Synergistic Action of Stilbenes in Muscadine Grape Berry Extract Shows Better Cytotoxic Potential Against Cancer Cells Than Resveratrol Alone. Biomedicines. 2019; 7(4):96. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines7040096
Chicago/Turabian StyleBalasubramani, Subramani Paranthaman, Mohammad Atikur Rahman, and Sheikh Mehboob Basha. 2019. "Synergistic Action of Stilbenes in Muscadine Grape Berry Extract Shows Better Cytotoxic Potential Against Cancer Cells Than Resveratrol Alone" Biomedicines 7, no. 4: 96. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines7040096