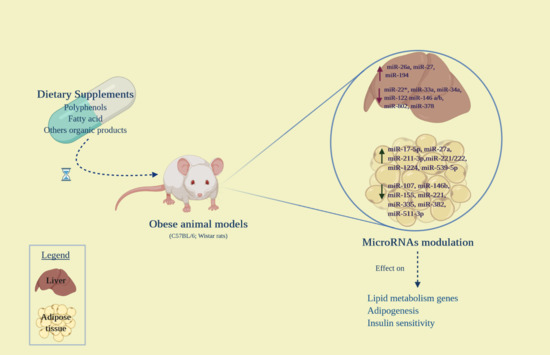
MicroRNA Modulation by Dietary Supplements in Obesity
Abstract
:1. Introduction
2. General Aspects of MicroRNAs
3. Effects of Dietary Supplements on MicroRNA Expression in Obesity
3.1. Polyphenols
3.2. Fatty Acids
3.3. Other Compounds
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Software
References
- World Health Organization. Obesity and Overweight: Fact Sheets 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 July 2020).
- Jayedi, A.; Soltani, S.; Zargar, M.S.; Khan, T.A.; Shab-Bidar, S. Central fatness and risk of all cause mortality: Systematic review and dose-response meta-analysis of 72 prospective cohort studies. BMJ 2020, 370, m3324. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, E.; Filardi, T.; Tartaglione, S.; Lenzi, A.; Angeloni, A.; Morano, S. Linking type 2 diabetes and gynecological cancer: An introductory overview. Clin. Chem Lab. Med. 2018, 56, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Pan, X.F.; Chen, J.; Cao, A.; Xia, L.; Zhang, Y.; Wang, J.; Li, H.; Liu, G.; Pan, A. Combined lifestyle factors, all-cause mortality and cardiovascular disease: A systematic review and meta-analysis of prospective cohort studies. J. Epidemiol. Community Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.H.; Still, C.D.; Endocrine, S. Pharmacological management of obesity: An endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.; Sekhon-Loodu, S.; Mantso, T.; Panayiotidis, M.I. Phytochemicals in regulating fatty acid beta-oxidation: Potential underlying mechanisms and their involvement in obesity and weight loss. Pharmacol. Ther. 2016, 165, 153–163. [Google Scholar] [CrossRef]
- Kantor, E.D.; Rehm, C.D.; Du, M.; White, E.; Giovannucci, E.L. Trends in Dietary Supplement Use Among US Adults From 1999–2012. JAMA 2016, 316, 1464–1474. [Google Scholar] [CrossRef]
- Wong, M.K.; Darvishzadeh, A.; Maler, N.A.; Bota, R.G. Dietary Supplement Nomenclature. Prim. Care Companion CNS Disord 2016, 18. [Google Scholar] [CrossRef] [Green Version]
- Food & Drug Administration. Dietary Supplement Profucts & Ingredients 2020. Available online: https://www.fda.gov/food/dietary-supplements/dietary-supplement-products-ingredients (accessed on 5 October 2020).
- Catanzaro, G.; Besharat, Z.M.; Chiacchiarini, M.; Abballe, L.; Sabato, C.; Vacca, A.; Borgiani, P.; Dotta, F.; Tesauro, M.; Po, A.; et al. Circulating MicroRNAs in Elderly Type 2 Diabetic Patients. Int. J. Endocrinol. 2018, 2018, 6872635. [Google Scholar] [CrossRef]
- Catanzaro, G.; Filardi, T.; Sabato, C.; Vacca, A.; Migliaccio, S.; Morano, S.; Ferretti, E. Tissue and circulating microRNAs as biomarkers of response to obesity treatment strategies. J. Endocrinol. Investig. 2020. [Google Scholar] [CrossRef] [PubMed]
- Filardi, T.; Catanzaro, G.; Mardente, S.; Zicari, A.; Santangelo, C.; Lenzi, A.; Morano, S.; Ferretti, E. Non-Coding RNA: Role in Gestational Diabetes Pathophysiology and Complications. Int. J. Mol. Sci 2020, 21, 4020. [Google Scholar] [CrossRef] [PubMed]
- Goni, L.; Cuervo, M.; Milagro, F.I.; Martinez, J.A. Future Perspectives of Personalized Weight Loss Interventions Based on Nutrigenetic, Epigenetic, and Metagenomic Data. J. Nutr. 2015, 146, 905S–912S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Guo, H.; Ingolia, N.T.; Weissman, J.S.; Bartel, D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010, 466, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Ni, W.J.; Leng, X.M. Dynamic miRNA-mRNA paradigms: New faces of miRNAs. Biochem. Biophys. Rep. 2015, 4, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Ameres, S.L.; Zamore, P.D. Diversifying microRNA sequence and function. Nat. Rev. Mol. Cell Biol. 2013, 14, 475–488. [Google Scholar] [CrossRef]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From Mechanism to Organism. Front. Cell Dev. Biol. 2020, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.M.; Abdelmohsen, K.; Mustapic, M.; Kapogiannis, D.; Gorospe, M. RNA in extracellular vesicles. Wiley Interdiscip. Rev. RNA 2017, 8. [Google Scholar] [CrossRef]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandao, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Cortez, M.A.; Bueso-Ramos, C.; Ferdin, J.; Lopez-Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in body fluids--the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Bandiera, S.; Pfeffer, S.; Baumert, T.F.; Zeisel, M.B. miR-122--a key factor and therapeutic target in liver disease. J. Hepatol. 2015, 62, 448–457. [Google Scholar] [CrossRef] [Green Version]
- Gerin, I.; Clerbaux, L.A.; Haumont, O.; Lanthier, N.; Das, A.K.; Burant, C.F.; Leclercq, I.A.; MacDougald, O.A.; Bommer, G.T. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J. Biol. Chem. 2010, 285, 33652–33661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rottiers, V.; Najafi-Shoushtari, S.H.; Kristo, F.; Gurumurthy, S.; Zhong, L.; Li, Y.; Cohen, D.E.; Gerszten, R.E.; Bardeesy, N.; Mostoslavsky, R.; et al. MicroRNAs in metabolism and metabolic diseases. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 225–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, N.L.; Singh, A.K.; Rotllan, N.; Goedeke, L.; Wing, A.; Canfran-Duque, A.; Diaz-Ruiz, A.; Araldi, E.; Baldan, A.; Camporez, J.P.; et al. Genetic Ablation of miR-33 Increases Food Intake, Enhances Adipose Tissue Expansion, and Promotes Obesity and Insulin Resistance. Cell Rep. 2018, 22, 2133–2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trajkovski, M.; Hausser, J.; Soutschek, J.; Bhat, B.; Akin, A.; Zavolan, M.; Heim, M.H.; Stoffel, M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 2011, 474, 649–653. [Google Scholar] [CrossRef] [Green Version]
- Jordan, S.D.; Kruger, M.; Willmes, D.M.; Redemann, N.; Wunderlich, F.T.; Bronneke, H.S.; Merkwirth, C.; Kashkar, H.; Olkkonen, V.M.; Bottger, T.; et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat. Cell Biol. 2011, 13, 434–446. [Google Scholar] [CrossRef]
- Zhu, H.; Shyh-Chang, N.; Segre, A.V.; Shinoda, G.; Shah, S.P.; Einhorn, W.S.; Takeuchi, A.; Engreitz, J.M.; Hagan, J.P.; Kharas, M.G.; et al. The Lin28/let-7 axis regulates glucose metabolism. Cell 2011, 147, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Hung, Y.H.; Kanke, M.; Kurtz, C.L.; Cubitt, R.; Bunaciu, R.P.; Miao, J.; Zhou, L.; Graham, J.L.; Hussain, M.M.; Havel, P.; et al. Acute suppression of insulin resistance-associated hepatic miR-29 in vivo improves glycemic control in adult mice. Physiol. Genom. 2019, 51, 379–389. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, D.; Xia, Z.; Chen, C.; Cheng, P.; Xie, H.; Luo, X. The role of microRNAs in adipocyte differentiation. Front. Med. 2013, 7, 223–230. [Google Scholar] [CrossRef]
- Price, N.L.; Fernandez-Hernando, C. miRNA regulation of white and brown adipose tissue differentiation and function. Biochim. Biophys. Acta 2016, 1861, 2104–2110. [Google Scholar] [CrossRef] [Green Version]
- Qin, N.; Chen, Y.; Jin, M.N.; Zhang, C.; Qiao, W.; Yue, X.L.; Duan, H.Q.; Niu, W.Y. Anti-obesity and anti-diabetic effects of flavonoid derivative (Fla-CN) via microRNA in high fat diet induced obesity mice. Eur. J. Pharm. Sci. 2016, 82, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Jeon, T.I.; Park, J.W.; Ahn, J.; Jung, C.H.; Ha, T.Y. Fisetin protects against hepatosteatosis in mice by inhibiting miR-378. Mol. Nutr. Food Res. 2013, 57, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Baselga-Escudero, L.; Pascual-Serrano, A.; Ribas-Latre, A.; Casanova, E.; Salvado, M.J.; Arola, L.; Arola-Arnal, A.; Blade, C. Long-term supplementation with a low dose of proanthocyanidins normalized liver miR-33a and miR-122 levels in high-fat diet-induced obese rats. Nutr. Res. 2015, 35, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Gracia, A.; Miranda, J.; Fernandez-Quintela, A.; Eseberri, I.; Garcia-Lacarte, M.; Milagro, F.I.; Martinez, J.A.; Aguirre, L.; Portillo, M.P. Involvement of miR-539-5p in the inhibition of de novo lipogenesis induced by resveratrol in white adipose tissue. Food Funct. 2016, 7, 1680–1688. [Google Scholar] [CrossRef]
- Tian, L.; Song, Z.; Shao, W.; Du, W.W.; Zhao, L.R.; Zeng, K.; Yang, B.B.; Jin, T. Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death Dis. 2017, 8, e2559. [Google Scholar] [CrossRef] [Green Version]
- Otton, R.; Bolin, A.P.; Ferreira, L.T.; Marinovic, M.P.; Rocha, A.L.S.; Mori, M.A. Polyphenol-rich green tea extract improves adipose tissue metabolism by down-regulating miR-335 expression and mitigating insulin resistance and inflammation. J. Nutr. Biochem. 2018, 57, 170–179. [Google Scholar] [CrossRef]
- Torres, L.F.; Cogliati, B.; Otton, R. Green Tea Prevents NAFLD by Modulation of miR-34a and miR-194 Expression in a High-Fat Diet Mouse Model. Oxid. Med. Cell Longev. 2019, 2019, 4168380. [Google Scholar] [CrossRef] [Green Version]
- Nazari, M.; Saberi, A.; Karandish, M.; Jalali, M.T. Adipose tissue miRNA level variation through conjugated linoleic acid supplementation in diet-induced obese rats. Adv. Clin. Exp. Med. 2018, 27, 1477–1482. [Google Scholar] [CrossRef]
- Parra, P.; Serra, F.; Palou, A. Expression of adipose microRNAs is sensitive to dietary conjugated linoleic acid treatment in mice. PLoS ONE 2010, 5, e13005. [Google Scholar] [CrossRef]
- Chen, X.; Xu, H.; Ding, L.; Lou, G.; Liu, Y.; Yao, Y.; Chen, L.; Huang, W.; Fu, X. Identification of miR-26a as a target gene of bile acid receptor GPBAR-1/TGR5. PLoS ONE 2015, 10, e0131294. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Jung, C.H.; Choi, W.H.; Ha, T.Y. Zerumbone ameliorates high-fat diet-induced adiposity by restoring AMPK-regulated lipogenesis and microRNA-146b/SIRT1-mediated adipogenesis. Oncotarget 2017, 8, 36984–36995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.H.; Ahn, J.; Jung, C.H.; Jang, Y.J.; Ha, T.Y. beta-Lapachone Prevents Diet-Induced Obesity by Increasing Energy Expenditure and Stimulating the Browning of White Adipose Tissue via Downregulation of miR-382 Expression. Diabetes 2016, 65, 2490–2501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, R.F.M.; Pogacnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Canape, C.; Catanzaro, G.; Terreno, E.; Karlsson, M.; Lerche, M.H.; Jensen, P.R. Probing treatment response of glutaminolytic prostate cancer cells to natural drugs with hyperpolarized [5-(13) C]glutamine. Magn. Reson. Med. 2015, 73, 2296–2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santangelo, C.; Zicari, A.; Mandosi, E.; Scazzocchio, B.; Mari, E.; Morano, S.; Masella, R. Could gestational diabetes mellitus be managed through dietary bioactive compounds? Current knowledge and future perspectives. Br. J. Nutr. 2016, 115, 1129–1144. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkanen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Cai, E.P.; Lin, J.K. Epigallocatechin gallate (EGCG) and rutin suppress the glucotoxicity through activating IRS2 and AMPK signaling in rat pancreatic beta cells. J. Agric. Food Chem 2009, 57, 9817–9827. [Google Scholar] [CrossRef]
- Choi, M.S.; Jung, U.J.; Yeo, J.; Kim, M.J.; Lee, M.K. Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes Metab Res. Rev. 2008, 24, 74–81. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, D. Long-term exposure to genistein improves insulin secretory function of pancreatic beta-cells. Eur. J. Pharm. 2009, 616, 321–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llaneza, P.; Gonzalez, C.; Fernandez-Inarrea, J.; Alonso, A.; Diaz, F.; Arnott, I.; Ferrer-Barriendos, J. Soy isoflavones, diet and physical exercise modify serum cytokines in healthy obese postmenopausal women. Phytomedicine 2011, 18, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S.; Jun, H.S. Role of bioactive food components in diabetes prevention: Effects on Beta-cell function and preservation. Nutr. Metab Insights 2014, 7, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Landberg, R.; Naidoo, N.; van Dam, R.M. Diet and endothelial function: From individual components to dietary patterns. Curr. Opin. Lipidol. 2012, 23, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Liu, M.; Liu, X.; Dong, L.Q.; Glickman, R.D.; Slaga, T.J.; Zhou, Z.; Liu, F. Up-regulation of adiponectin by resveratrol: The essential roles of the Akt/FOXO1 and AMP-activated protein kinase signaling pathways and DsbA-L. J. Biol. Chem. 2011, 286, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Eseberri, I.; Lasa, A.; Churruca, I.; Portillo, M.P. Resveratrol metabolites modify adipokine expression and secretion in 3T3-L1 pre-adipocytes and mature adipocytes. PLoS ONE 2013, 8, e63918. [Google Scholar] [CrossRef] [Green Version]
- Kang, L.; Heng, W.; Yuan, A.; Baolin, L.; Fang, H. Resveratrol modulates adipokine expression and improves insulin sensitivity in adipocytes: Relative to inhibition of inflammatory responses. Biochimie 2010, 92, 789–796. [Google Scholar] [CrossRef]
- Jin, T.; Kim, O.Y.; Shin, M.J.; Choi, E.Y.; Lee, S.S.; Han, Y.S.; Chung, J.H. Fisetin up-regulates the expression of adiponectin in 3T3-L1 adipocytes via the activation of silent mating type information regulation 2 homologue 1 (SIRT1)-deacetylase and peroxisome proliferator-activated receptors (PPARs). J. Agric. Food Chem. 2014, 62, 10468–10474. [Google Scholar] [CrossRef]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxid. Redox. Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Vari, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Updated knowledge about polyphenols: Functions, bioavailability, metabolism, and health. Crit. Rev. Food Sci. Nutr. 2012, 52, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Costa, L.G.; Lean, M.E.; Crozier, A. Polyphenols and health: What compounds are involved? Nutr. Metab. Cardiovasc. Dis. 2010, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? Antioxidants 2020, 9, 982. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef]
- Adhami, V.M.; Syed, D.N.; Khan, N.; Mukhtar, H. Dietary flavonoid fisetin: A novel dual inhibitor of PI3K/Akt and mTOR for prostate cancer management. Biochem. Pharm. 2012, 84, 1277–1281. [Google Scholar] [CrossRef] [Green Version]
- Scarpulla, R.C.; Vega, R.B.; Kelly, D.P. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 2012, 23, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Rottiers, V.; Naar, A.M. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012, 13, 239–250. [Google Scholar] [CrossRef]
- Niu, Y.; Mo, D.; Qin, L.; Wang, C.; Li, A.; Zhao, X.; Wang, X.; Xiao, S.; Wang, Q.; Xie, Y.; et al. Lipopolysaccharide-induced miR-1224 negatively regulates tumour necrosis factor-alpha gene expression by modulating Sp1. Immunology 2011, 133, 8–20. [Google Scholar] [CrossRef]
- Magana, M.M.; Koo, S.H.; Towle, H.C.; Osborne, T.F. Different sterol regulatory element-binding protein-1 isoforms utilize distinct co-regulatory factors to activate the promoter for fatty acid synthase. J. Biol. Chem. 2000, 275, 4726–4733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filardi, T.; Vari, R.; Ferretti, E.; Zicari, A.; Morano, S.; Santangelo, C. Curcumin: Could This Compound Be Useful in Pregnancy and Pregnancy-Related Complications? Nutrients 2020, 12, 3179. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Altieri, B.; Polese, B.; De Conno, B.; Muscogiuri, G.; Colao, A.; Savastano, S.; Obesity Programs of Nutrition, E.R.; Assessment, G. Nutritionist and obesity: Brief overview on efficacy, safety, and drug interactions of the main weight-loss dietary supplements. Int. J. Obes. Suppl. 2019, 9, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Pols, T.W.; Nomura, M.; Maida, A.; Watanabe, M.; Auwerx, J.; Schoonjans, K. TGR5 potentiates GLP-1 secretion in response to anionic exchange resins. Sci. Rep. 2012, 2, 430. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Jin, L.; Wang, X.; Luo, A.; Hu, J.; Zheng, X.; Tsark, W.M.; Riggs, A.D.; Ku, H.T.; Huang, W. MicroRNA-26a targets ten eleven translocation enzymes and is regulated during pancreatic cell differentiation. Proc. Natl. Acad. Sci. USA 2013, 110, 17892–17897. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Dong, B.; Tian, Y.; Lefebvre, P.; Meng, Z.; Wang, X.; Pattou, F.; Han, W.; Wang, X.; Lou, F.; et al. MicroRNA-26a regulates insulin sensitivity and metabolism of glucose and lipids. J. Clin. Investig. 2015, 125, 2497–2509. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.H.; Kim, D.W.; Jo, E.J.; Kim, Y.K.; Jo, Y.S.; Park, J.H.; Yoo, S.K.; Park, M.K.; Kwak, T.H.; Kho, Y.L.; et al. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes 2009, 58, 965–974. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.H.; Koh, Y.C.; Lee, T.L.; Wang, B.; Chen, W.K.; Nagabhushanam, K.; Ho, C.T. Resveratrol and Oxyresveratrol Activate Thermogenesis via Different Transcriptional Coactivators in High-Fat Diet-Induced Obese Mice. J. Agric. Food Chem. 2019, 67, 13605–13616. [Google Scholar] [CrossRef]
- Cheang, W.S.; Wong, W.T.; Wang, L.; Cheng, C.K.; Lau, C.W.; Ma, R.C.W.; Xu, A.; Wang, N.; Huang, Y.; Tian, X.Y. Resveratrol ameliorates endothelial dysfunction in diabetic and obese mice through sirtuin 1 and peroxisome proliferator-activated receptor delta. Pharm. Res. 2019, 139, 384–394. [Google Scholar] [CrossRef]
- Luongo, C.; Dentice, M.; Salvatore, D. Deiodinases and their intricate role in thyroid hormone homeostasis. Nat. Rev. Endocrinol. 2019, 15, 479–488. [Google Scholar] [CrossRef]

| Study | Model | Compound | Tissue/Cell Line | Regulated miRNAs | Target Gene/Pathway |
|---|---|---|---|---|---|
| Qin et al. [43] | Male C57BL/6J mouse | Fla-CN | Liver and adipose tissue | miR-27 (↑) | PPAR-γ, C/EBP α, SREBP-1c, FAS (↓ in adipose tissue) |
| Jeon et al. [44] | Male C57BL/6J mouse | Fisetin | Liver | miR-22*, miR-146a, miR-146b, miR-802, miR-378 (↓) | ↓ PGC-1β (miR-378) |
| Baselga-Escudero et al. [45] | Female Wistar rat | GPSE | Liver | miR-33a, miR-122 (↓) | Lipid metabolism |
| Gracia et al. [46] | Wistar rat | Resveratrol | VAT | miR-211-3p, miR-1224, miR-539-5p (↑); miR-511-3p (↓) | PPAR-γ, HSL, SP1 |
| Tian et al. [47] | Male C57BL/6J mouse | Curcumin | Epididymal adipose tissue | miR-17-5p (↑) | Adipogenic differentiation |
| Otton et al. [48] | C57BL/6 mouse | Green tea | Epididymal adipose tissue | miR-155, miR-221, miR-335 (↓) | Adipogenesis |
| Torres et al. [49] | C57BL/6 mouse | Green tea | Liver | miR-34a (↓), miR-194 (↑) | SIRT1, PPAR-α, Insig2 (miR-34a) Apoa5, Hmgcs2 (miR-194) |
| Study | Model | Compound | Tissue/Cell Line | Regulated miRNAs | Target Gene/Pathway |
|---|---|---|---|---|---|
| Nazari et al. [50] | Wistar rats | CLA | Adipose tissue | miR-27a (↑) | Adipogenesis |
| Parra et al. [51] | C57BL/6J mouse | CLA | Retroperitoneal adipose tissue | miR-107 (↓), miR-221 (↑), miR-222 (↑) | Adipocyte differentiation |
| Chen et al. [52] | C57BL/6 mouse | OA | Liver and Kupffer cells | miR-26a (↑) | Lipid and glucose metabolism |
| Ahn et al. [53] | C57BL/6N mouse | Zerumbone | Adipose tissue and differentiated 3T3-L1 cells | miR-146b (↓) | SIRT1 (↑) |
| Choi et al. [54] | Male C57BL/6 mouse | BLC | SAT | miR-382 (↓) | Dio2 (↑)/Adipose tissue browning |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filardi, T.; Sabato, C.; Lubrano, C.; Santangelo, C.; Morano, S.; Lenzi, A.; Migliaccio, S.; Ferretti, E.; Catanzaro, G. MicroRNA Modulation by Dietary Supplements in Obesity. Biomedicines 2020, 8, 545. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8120545
Filardi T, Sabato C, Lubrano C, Santangelo C, Morano S, Lenzi A, Migliaccio S, Ferretti E, Catanzaro G. MicroRNA Modulation by Dietary Supplements in Obesity. Biomedicines. 2020; 8(12):545. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8120545
Chicago/Turabian StyleFilardi, Tiziana, Claudia Sabato, Carla Lubrano, Carmela Santangelo, Susanna Morano, Andrea Lenzi, Silvia Migliaccio, Elisabetta Ferretti, and Giuseppina Catanzaro. 2020. "MicroRNA Modulation by Dietary Supplements in Obesity" Biomedicines 8, no. 12: 545. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8120545