Characterization of Osteoarthritis in a Medial Meniscectomy-Induced Animal Model Using Contrast-Enhanced X-ray Microtomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
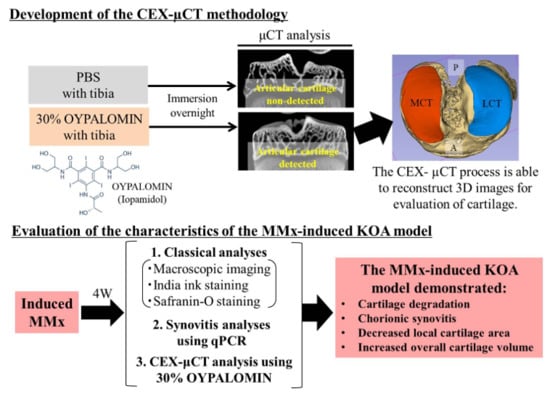
2.2. Establishing the Foundation of the CEX-μCT Method
- Source-to-image distance (SID): 200 mm
- Source-to-object distance (SOD): 40 mm
- Tube voltage: 47 kV
- Number of views: 1200
- Scaling coefficient: 1200
- Image sharp: 512
- Widthwise pixel spacing (mm/pixel): 0.021181
- Lengthwise pixel spacing (mm/pixel): 0.021181
- Saved image type: TIF 16-bit
2.3. Evaluation of Subchondral Bone in MMx-Induced KOA Using X-ray μCT
2.4. CEX-μCT Analysis of MMx-Induced KOA
2.5. Macroscopic Imaging of Articular Cartilage in MMx-Induced KOA
2.6. Histological Analyses
2.7. Evaluation of Synovitis by Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.8. Statistical Analyses
3. Results
3.1. Optimal Oypalomin Concentration for CEX-μCT Was 30%
3.2. Subchondral Bone Degradation Was Not Discovered by MMx-Induced KOA According to the X-ray μCT Analyses
3.3. MMx-Treated Knees Showed Articular Cartilage Degradation on Macroscopic Imaging
3.4. MMx-Treated Knees Showed Articular Cartilage Degradation Using Either Tissue Staining or CEX-μCT Analyses
3.5. MMx-Induced KOA Increased Total Cartilage Volumes
3.6. MMx-Treatment-Induced Gene Expression Associated with Knee Synovitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CEX-μCT | contrast enhanced X-ray micro computed tomography |
| PBS | phosphate-balanced saline |
| P | posterior |
| A | anterior |
| MCT | medial cartilage of tibial plateau |
| LCT | lateral cartilage of tibial plateau |
| MMx | medial meniscectomy |
| KOA | knee osteoarthritis |
| qPCR | real-time quantitative polymerase chain reaction |
References
- Lyn, M.; Marita, C. Epidemiology and Risk Factors for Osteoarthritis; David, H., Monica, R.C., Eds.; UpToDate. Inc.: Waltham, MA, USA, 2019; Available online: https://www.uptodate.com/contents/epidemiology-and-risk-factors-for-osteoarthritis (accessed on 29 August 2019).
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcome. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Japanese Orthopedic Association Knee Osteoarthritis. Available online: https://www.joa.or.jp/public/sick/condition/knee_osteoarthritis.html (accessed on 29 August 2019).
- Takayama, K.; Kawakami, Y.; Kobayashi, M.; Greco, N.; Cummins, J.H.; Matsushita, T.; Kuroda, R.; Kurosaka, M.; Fu, F.H.; Huard, J. Local intra-articular injection of rapamycin delays articular cartilage degeneration in a murine model of osteoarthritis. Arthritis Res. 2014, 16, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, H.; Qu, N.; Chen, X.; Zhou, Y.; Zheng, X.; Zhang, B.; Xia, C. The inhibition of PLCγ1 protects chondrocytes against osteoarthritis, implicating its binding to Akt. Oncotarget 2017, 9, 4461–4474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, C.; Huang, X.; Xiao, Y.; Song, M.; Ma, Y.; Yan, J.; You, H.; Wu, H. Schisandrin A inhibits the IL-1β-induced inflammation and cartilage degradation via suppression of MAPK and NF-κB signal pathways in rat chondrocytes. Front. Pharmacol. 2019, 10, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, J.; Ma, C.; Xu, K.; Xu, L.; He, Y.; Moqbel, S.A.A.; Hu, P.; Jiang, L.; Chen, W.; Bao, J.; et al. Schisandrin B ameliorated chondrocytes inflammation and osteoarthritis via suppression of NF-κB and MAPK signal pathways. Drug Des. Devel. Ther. 2018, 12, 1195–1204. [Google Scholar] [CrossRef] [Green Version]
- Desando, G.; Cavallo, C.; Sartoni, F.; Martini, L.; Parrilli, A.; Veronesi, F.; Fini, M.; Giardino, R.; Facchini, A.; Grigolo, B. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res. 2013, 15, R22. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wei, X.; Zhou, J.; Zhang, J.; Li, K.; Chen, Q.; Terek, R.; Fleming, B.C.; Goldring, M.B.; Ehrlich, M.G.; et al. Identification of α2-macroglobulin as a master inhibitor of cartilage-degrading factors that attenuates the progression of posttraumatic osteoarthritis. Arthritis Rheumatol. 2014, 66, 1843–1853. [Google Scholar] [CrossRef] [Green Version]
- Ozeki, N.; Muneta, T.; Koga, H.; Nakagawa, Y.; Mizuno, M.; Tsuji, K.; Mabuchi, Y.; Akazawa, C.; Kobayashi, E.; Matsumoto, K.; et al. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthr. Cartil. 2016, 24, 1061–1070. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Lin, A.S.; Levenston, M.E.; Guldberg, R.E. Quantitative assessment of articular cartilage morphology via EPIC-microCT. Osteoarthr. Cartil. 2009, 17, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Willett, N.J.; Thote, T.; Hart, M.; Moran, S.; Guldberg, R.E.; Kamath, R.V. Quantitative pre-clinical screening of therapeutics for joint diseases using contrast enhanced micro-computed tomography. Osteoarthr. Cartil. 2016, 24, 1604–1612. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, K.; Muneta, T.; Ozeki, N.; Nakagawa, Y.; Udo, M.; Saito, R.; Koga, H.; Tsuji, K.; Sekiya, I. Weekly injections of Hylan G-F 20 delay cartilage degeneration in partial meniscectomized rat knees. BMC Musculoskelet. Disord. 2016, 17, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; Van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthritis Cartilage 2006, 14, 13–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manabe, I. Inflammaging and age-associated diseases. Nippon. Ronen Igakkai Zasshi 2017, 54, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Pahwa, R.; Jialal, I. Chronic Inflammation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK493173/ (accessed on 6 March 2020).
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [Green Version]
- Suarez-Carmona, M.; Lesage, J.; Cataldo, D.; Gilles, C. EMT and inflammation: Inseparable actors of cancer progression. Mol. Oncol. 2017, 11, 805–823. [Google Scholar] [CrossRef]
- Rigoglou, S.; Papavassiliou, A.G. The NF-κB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef]
- Yuan, G.H.; Tanaka, M.; Masuko-Hongo, K.; Shibakawa, A.; Kato, T.; Nishioka, K.; Nakamura, H. Characterization of cells from pannus-like tissue over articular cartilage of advanced osteoarthritis. PLoS ONE 2013, 8, e79662. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.C.; Lin, C.Y.; Wang, H.S.; Lyu, S.R. Matrix metalloproteases and tissue inhibitors of metalloproteinases in medial plica and pannus-like tissue contribute to knee osteoarthritis progression. PLoS ONE 2013, 8, e79662. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.J.; Huang, J.F.; Du, W.X.; Tong, P.J. Expression and significance of MMP3 in synovium of knee joint at different stage in osteoarthritis patients. Asian Pac. J. Trop. Med. 2014, 4, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1β signaling in osteoarthritis—chondrocytes in focus. Cell Signal. 2019, 53, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Van der Kraan, P.M.; Van den Berg, W.B. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugasawa, T.; Kuji, T.; Aoki, K.; Yanazawa, K.; Takenouchi, A.; Watanabe, M.; Tome, Y.; Takeuchi, Y.; Aita, Y.; Yahagi, N.; et al. Characterization of Osteoarthritis in a Medial Meniscectomy-Induced Animal Model Using Contrast-Enhanced X-ray Microtomography. Biomedicines 2020, 8, 56. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8030056
Sugasawa T, Kuji T, Aoki K, Yanazawa K, Takenouchi A, Watanabe M, Tome Y, Takeuchi Y, Aita Y, Yahagi N, et al. Characterization of Osteoarthritis in a Medial Meniscectomy-Induced Animal Model Using Contrast-Enhanced X-ray Microtomography. Biomedicines. 2020; 8(3):56. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8030056
Chicago/Turabian StyleSugasawa, Takehito, Tomoaki Kuji, Kai Aoki, Koki Yanazawa, Akiko Takenouchi, Makoto Watanabe, Yoshiya Tome, Yoshinori Takeuchi, Yuichi Aita, Naoya Yahagi, and et al. 2020. "Characterization of Osteoarthritis in a Medial Meniscectomy-Induced Animal Model Using Contrast-Enhanced X-ray Microtomography" Biomedicines 8, no. 3: 56. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8030056