European Medicinal Leeches—New Roles in Modern Medicine
Abstract
:1. The Biology of Medicinal Leeches
2. The Pharmacological Potential of Medicinal Leeches
3. Salivary Proteins: Natural Drugs from Medicinal Leeches
| Salivary Protein | Mechanism of Action | Biological Significance | Reference |
|---|---|---|---|
| Hirustasin (Mass: 5.866 kDa) | Tissue kallikrein inhibitor and inhibitor of trypsin, chymotrypsin and neutrophil cathepsin G | Anti-inflammatory | [55] |
| Apyrase (Mass: 45 kDa) | Cleavage of adenosine 5′-diphosphate | Inhibitor of platelet aggregation | [67] |
| Bdellin B-3 (Mass: 6.141 kDa) | Inhibitor of plasmin, trypsin and sperm acrosin | Anti-inflammatory | [68] |
| Calin (Mass: 65 kDa) | Prevents the binding of von Willebrand factor to collagen | Inhibitor of platelet aggregation | [50,69] |
| Collagenase (Mass: 50 kDa) | Cleavage of collagen | Collagen digestion | [70] |
| Destabilase (Mass: 12.6–12.9 kDa) | Cleavage of fibrin clots, cleavage of peptidoglycans in bacterial walls | Anticoagulant/antimicrobial | [71,72,73] |
| Eglin C (Mass: 8.1 kDa) | Neutrophil elastase inhibitor, cathepsin G inhibitor | Anti-inflammatory | [74,75] |
| Hirudin (Mass: 7.1 kDa) | Thrombin inhibitor | Anticoagulant | [48,76] |
| Hirudin-like factors (Mass: 4.27–6.67; isoforms HLF1-HLF3) | Unknown for the three European species | [77,78] | |
| Hyaluronidase (Mass: 27.5 kDa) | Cleavage of hyaluronic acid | Extracellular matrix digestion | [79] |
| Leech-derived tryptase inhibitor (Mass: 4.7 kDa) | Mast cell tryptase inhibitor | Anti-inflammatory | [56,57] |
| Leech carboxypeptidase inhibitor (Mass: 7.2 kDa) | Carboxypeptidase B inhibitor | Unclear | [80] |
| Saratin (Mass: 12 kDa) | Inhibits the binding of von Willebrand factor to collagen | Inhibitor of platelet aggregation | [51,81] |
| Yagin (Mass: 15.4 kDa) | Factor Xa inhibitor | Anticoagulant | [82] |
4. Antistasins as a Representative Leech Salivary Protein Family
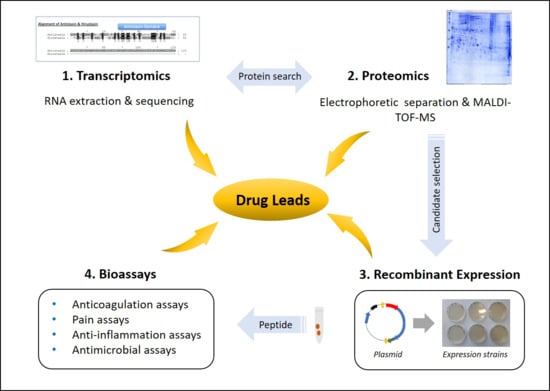
5. Leech Salivary Proteins as Drug Leads
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dickinson, M.H.; Lent, C.M. Feeding behavior of the medicinal leech, Hirudo medicinalis L. J. Comp. Physiol. A 1984, 154, 449–455. [Google Scholar] [CrossRef]
- Elliott, J.M.; Tullett, P.A. The effects of temperature, atmospheric pressure and season on the swimming activity of the medicinal leech, Hirudo medicinalis (Hirudinea; Hirudinidae), in a Lake District tarn. Freshwater Biol. 1986, 16, 405–415. [Google Scholar] [CrossRef]
- Hammersen, F. The muscle structure in the pharyngeal wall of Hirudo medicinalis and Haemopsis sanguisuga. Z. Zellforsch. Mikrosk. Anat. 1963, 60, 797–814. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.G.; Lent, C.M. Excitability and secretory activity in the salivary gland cells of jawed leeches (Hirudinea: Gnathobdellida). J. Exp. Biol. 1988, 137, 89–105. [Google Scholar] [PubMed]
- Lent, C.M.; Fliegner, K.H.; Freedman, E.; Dickinson, M.H. Ingestive behaviour and physiology of the medicinal leech. J. Exp. Biol. 1988, 137, 513–527. [Google Scholar] [PubMed]
- Zerbst-Boroffka, I. Ion transport mechanism in basal and diuretic nephridia of the leech, Hirudo medicinalis L. Comp. Biochem. Physiol. 1973, 86, 151–154. [Google Scholar] [CrossRef]
- Roters, F.J.; Zebe, E. Protease inhibitors in the alimentary tract of the medicinal leech Hirudo medicinalis: In vivo and in vitro studies. J. Comp. Physiol. B 1992, 162, 85–92. [Google Scholar] [CrossRef]
- Roters, F.J. Untersuchungen über Die Verdauungsphysiologie des Blutegels Hirudo medicinalis. Ph.D. Thesis, University of Münster, Münster, Germany, 1985. [Google Scholar]
- Indergand, S.; Graf, J. Ingested blood contributes to the specificity of the symbiosis of Aeromonas veronii biovar sobria and Hirudo medicinalis, the medicinal leech. Appl. Environ. Microbiol. 2000, 66, 4735–4741. [Google Scholar] [CrossRef] [Green Version]
- Maltz, M.A.; Bomar, L.; Lapierre, P.; Morrison, H.G.; McClure, E.A.; Sogin, M.L.; Graf, J. Metagenomic analysis of the medicinal leech gut microbiota. Front. Microbiol. 2014, 5, 151. [Google Scholar] [CrossRef] [Green Version]
- Siddall, M.E.; Min, G.S.; Fontanella, F.M.; Phillips, A.J.; Watson, S.C. Bacterial symbiont and salivary peptide evolution in the context of leech phylogeny. Parasitology 2011, 138, 1815–1827. [Google Scholar] [CrossRef]
- Bomar, L.; Maltz, M.; Colston, S.; Graf, J. Directed culturing of microorganisms using metatranscriptomics. Mbio 2011, 2, e00012-11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maltz, M.A.; Graf, J. The Type II Secretion System Is Essential for Erythrocyte Lysis and Gut Colonization by the Leech Digestive Tract Symbiont Aeromonas veronii. Appl. Environ. Microbiol. 2011, 77, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziekońska-Rynko, J.; Bielecki, A.; Palińska, K. Activity of selected hydrolytic enzymes from leeches (Clitellata: Hirudinida) with different feeding strategies. Biologia 2009, 64, 370–376. [Google Scholar] [CrossRef]
- Abdualkader, A.M.; Ghawi, A.M.; Alaama, M.; Awang, M.; Merzouk, A. Leech therapeutic applications. Indian J. Pharm. Sci. 2013, 75, 127–137. [Google Scholar] [PubMed]
- Deganc, M.; Zdravic, F. Venous congestion of flaps treated by application of leeches. Br. J. Plast. Surg. 1960, 13, 187–192. [Google Scholar] [CrossRef]
- Ansari, S.; Fasihuzzaman, N.; Jabeen, A.; Sultana, A.; Khan, A.Q. Post-auricular leech therapy reduced headache & migraine days in chronic migraine. J. Drug Deliv. Ther. 2019, 9, 75–80. [Google Scholar]
- Bakhshi, M.; Jalalian, B.; Valian, M.; Shariati, S.; Saeidi, T.; Ranjbar, H. Can leech therapy be used as an alternative treatment for controlling migraine headache? A Pilot Study. Acta Fac. Med. Naissensis 2015, 32, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Andereya, S.; Stanzel, S.; Maus, U.; Mueller-Rath, R.; Mumme, T.; Siebert, C.H.; Stock, F.; Schneider, U. Assessment of leech therapy for knee osteoarthritis: A randomized study. Acta Orthop. 2008, 79, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Michalsen, A.; Moebus, S.; Spahn, G.; Esch, T.; Langhorst, J.; Dobos, G.J. Leech therapy for symptomatic treatment of knee osteoarthritis: Results and implications of a pilot study. Leech 2002, 84, 88. [Google Scholar]
- Michalsen, A.; Klotz, S.; Lüdtke, R.; Moebus, S.; Spahn, G.; Dobos, G.J. Effectiveness of leech therapy in osteoarthritis of the knee: A randomized, controlled trial. Ann. Intern. Med. 2003, 139, 724–730. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, A.K.; Singh, O.P.; Rai, N.P.; Dwivedi, A.K. Efficacy of leech therapy in the management of osteoarthritis (Sandhivata). Ayu 2011, 32, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Shiffa, M.; Siddiquib, M.A.; Sultana, A.; Zaman, F.; Fahamiya, N.; Akhtarc, M.U. Comparative clinical evaluation of leech therapy in the treatment of knee osteoarthritis. Eur. J. Integr. 2013, 5, 261–269. [Google Scholar] [CrossRef]
- Stange, R.; Moser, C.; Hopfenmueller, W.; Mansmann, U.; Buehring, M.; Uehleke, B. Randomised controlled trial with medical leeches for osteoarthritis of the knee. Complement. Ther. Med. 2012, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.M.; Abbas Jamil, S.S.; Sultana, A.; Zaman, F.; Fuzail, M. Safety and efficacy of leeching therapy for symptomatic knee osteoarthritis using Indian medicinal leech. Indian J. Tradit. Knowl. 2009, 8, 437–442. [Google Scholar]
- Hanif, H.; Nouri, M.; Amirjamshidi, A. Medicinal leech therapy in neurosurgical practice. J. Inj. Violence Res. 2012, 4, 72. [Google Scholar]
- Kusnetsova, L.P.; Lusov, V.A.; Volov, N.A.; Smirnova, N.A.; Bogdanova, L.S. Hirudotherapy in complex treatment of chronic heart failure. Russ. J. Cardiol. 2008, 2, 28–30. [Google Scholar]
- Nargiza, E.; Mirdjuraev, E.; Ergasheva, N. Leech therapy to prevent ischemic stroke: p1231. Eur. J. Neurol. 2010, 17, 170. [Google Scholar]
- Shankar, K.P.; Rao, S.D.; Umar, S.N.; Gopalakrishnaiah, V. A clinical trial for evaluation of leech application in the management of Vicarcikā (Eczema). Anc. Sci. Life 2014, 33, 236–241. [Google Scholar] [CrossRef]
- Amarprakash, P.D. Case study of leech application in diabetic foot ulcer. Int. J. Res. Ayurveda Pharm. 2012, 3, 748–751. [Google Scholar]
- Na, H.J. The Effects of live leech (Hirudo Medicinalis) therapy on diabetic foot: A clinical case report. Korean J. Orient. Med. 2003, 24, 136–138. [Google Scholar]
- Zaidi, S.A. Unani treatment and leech therapy saved the diabetic foot of a patient from amputation. Int. Wound J. 2016, 13, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S.A.; Rostami, S.; Teimoori, M. Leech therapy for treating priapism: Case report. Iran. J. Public Health 2017, 46, 985–988. [Google Scholar] [PubMed]
- Bumpous, J.M.; Byrne, P.J.; Bernstein, P.E. The use of medicinal leeches to treat macroglossia secondary to blunt trauma. Otolaryngol. Head Neck Surg. 2001, 125, 649–650. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, M.; Droog, W.; Sleeswijk Visser, S.; van Roessel, E.W.; Meynaar, I.A. Leech got your tongue? Haematoma of the tongue treated with medicinal leeches: A case report. Neth. J. Crit. Care 2010, 14, 268–270. [Google Scholar]
- Kalender, M.E.; Comez, G.; Sevinc, A.; Dirier, A.; Camci, C. Leech therapy for symptomatic relief of cancer pain. Pain Med. 2010, 11, 443–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philip, J.; Armitage, D.W.; Phillips, K.R.; Parr, N.J. Leech therapy for penoscrotal oedema in patients with hormone-refractory prostate carcinoma. BJU Int. 2003, 91, 579–580. [Google Scholar] [CrossRef] [Green Version]
- Darestani, K.D.; Mirghazanfari, S.M.; Moghaddam, K.G.; Hejazi, S. Leech therapy for linear incisional skin-wound healing in rats. J. Acupunct. Meridian Stud. 2014, 7, 194–201. [Google Scholar] [CrossRef]
- Ghods, R.; Abdi, M.; Pourrahimi, M.; Dabaghian, F.H. Leech therapy indications: A scoping review. Tradit. Med. Res. 2019, 4, 118–130. [Google Scholar]
- Gunawan, F.; Wibowo, Y.R.; Bunawan, N.C.; Turner, J.H. Controversy: Hirudotherapy (leech therapy) as an alternative treatment for osteoarthritis. Acta Med. Indones. 2015, 47, 176–180. [Google Scholar]
- Pilcher, H. Medicinal leeches: Stuck on you. Nature 2004, 432, 10–11. [Google Scholar] [CrossRef]
- Talbot, B.; Balvín, O.; Vonhof, M.J.; Broders, H.G.; Fenton, B.; Keyghobadi, N. Host association and selection on salivary protein genes in bed bugs and related blood-feeding ectoparasites. R. Soc. Open Sci. 2017, 4, 170446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Valen, L. A new evolutionary law. In Evolutionary Theory; Band 1; University of Chicago Press: Chicago, IL, USA, 1973; pp. 1–30. [Google Scholar]
- Babenko, V.V.; Podgorny, O.V.; Manuvera, V.A.; Kasianov, A.S.; Manolov, A.I.; Grafskaia, E.N.; Shirokov, D.A.; Kurdyumov, A.S.; Vinogradov, D.V.; Nikitina, A.S.; et al. Draft genome sequences of Hirudo medicinalis and salivary transcriptome of three closely related medicinal leeches. BioRxiv 2018. [Google Scholar] [CrossRef]
- Baskova, I.P.; Zavalova, L.L. Proteinase inhibitors from the medicinal leech Hirudo medicinalis. Biochemistry 2001, 66, 703–714. [Google Scholar] [PubMed]
- Baskova, I.P.; Zavalova, L.L.; Basanova, A.V.; Moshkovskii, S.A.; Zgoda, V.G. Protein profiling of the medicinal leech salivary gland secretion by proteomic analytical methods. Biochemistry 2004, 69, 770–775. [Google Scholar] [CrossRef]
- Hildebrandt, J.-P.; Lemke, S. Small bite, large impact—Saliva and salivary molecules in the medical leech, Hirudo medicinalis. Naturwissenschaften 2011, 98, 995–1008. [Google Scholar] [CrossRef]
- Ascenzi, P.; Amiconi, G.; Bode, W.; Bolognesi, M.; Coletta, M.; Menegatti, E. Proteinase inhibitors from the European medicinal leech Hirudo medicinalis: Structural, functional and biomedical aspects. Mol. Asp. Med. 1995, 16, 215–313. [Google Scholar] [CrossRef]
- Baskova, I.P.; Khalil, S.; Nartikova, V.F.; Paskhina, T.S. Inhibition of plasma kallikrein. Kininase and kinin-like activities of preparations from the medicinal leeches. Thromb. Res. 1992, 67, 721–730. [Google Scholar] [CrossRef]
- Deckmyn, H.; Stassen, J.M.; Vreys, I.; Van Houtte, E.; Sawyer, R.T.; Vermylen, J. Calin from Hirudo medicinalis, an inhibitor of platelet adhesion to collagen, prevents platelet-rich thrombosis in hamsters. Blood 1995, 85, 712–719. [Google Scholar] [CrossRef] [Green Version]
- Gronwald, W.; Bomke, J.; Maurer, T.; Domogalla, B.; Huber, F.; Schumann, F.; Kremer, W.; Fink, F.; Rysiok, T.; Frech, M.; et al. Structure of the leech protein saratin and characterization of its binding to collagen. J. Mol. Biol. 2008, 381, 913–927. [Google Scholar] [CrossRef]
- Haycraft, J.B. On the action of a secretion obtained from the medicinal leech on the coagulation of the blood. Proc. R. Soc. Lond. B 1884, 36, 478–487. [Google Scholar]
- Linker, A.; Meyer, K.; Hoffman, P. The production of hyaluronate oligosaccharides by leech hyaluronidase and alkali. J. Biol. Chem. 1960, 235, 924–927. [Google Scholar] [PubMed]
- Mittl, P.R.; Di Marco, S.; Fendrich, G.; Pohlig, G.; Heim, J.; Sommerhoff, C.; Fritz, H.; Priestle, J.P.; Grütter, M.G. A new structural class of serine protease inhibitors revealed by the structure of the hirustasin-kallikrein complex. Structure 1997, 5, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Söllner, C.; Mentele, R.; Eckerskorn, C.; Fritz, H.; Sommerhoff, C.P. Isolation and characterization of hirustasin, an antistasin-type serine-proteinase inhibitor from the medical leech Hirudo medicinalis. Eur. J. Biochem. 1994, 219, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Sommerhoff, C.P.; Söllner, C.; Mentele, R.; Piechottka, G.P.; Auerswald, E.A.; Fritz, H. A Kazal-type inhibitor of human mast cell tryptase: Isolation from the medical leech Hirudo medicinalis, characterization, and sequence analysis. Biol. Chem. Hoppe Seyler 1994, 375, 685–694. [Google Scholar] [CrossRef]
- Stubbs, M.T.; Morenweiser, R.; Stürzebecher, J.; Bauer, M.; Bode, W.; Huber, R.; Piechottka, G.P.; Matschiner, G.; Sommerhoff, C.P.; Fritz, H.; et al. The three-dimensional structure of recombinant leech-derived tryptase inhibitor in complex with trypsin. Implications for the structure of human mast cell tryptase and its inhibition. J. Biol. Chem. 1997, 272, 19931–19937. [Google Scholar] [CrossRef] [Green Version]
- Vilahur, G.; Duran, X.; Juan-Babot, O.; Casani, L.; Badimon, L. Antithrombotic effects of saratin on human atherosclerotic plaques. Thromb. Haemost. 2004, 92, 191–226. [Google Scholar] [CrossRef]
- Min, G.-S.; Sarkar, I.N.; Siddall, M.E. Salivary Transcriptome of the North American Medicinal Leech, Macrobdella decora. J. Parasitol. 2010, 96, 1211–1221. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Shi, P.; You, H.; Liu, Y.; Chen, S. Transcriptomic analysis of the salivary gland of medicinal leech Hirudo nipponia. PLoS ONE 2018, 13, e0205875. [Google Scholar] [CrossRef]
- Kvist, S.; Min, G.-S.; Siddall, M.E. Diversity and selective pressures of anticoagulants in three medicinal leeches (Hirudinida: Hirudinidae, Macrobdellidae). Ecol. Evol. 2013, 3, 918–933. [Google Scholar] [CrossRef]
- Khan, M.S.; Guan, D.-L.; Kvist, S.; Ma, L.B.; Xie, J.X.; Xu, S.Q. Transcriptomics and differential gene expression in Whitmania pigra (Annelida: Clitellata: Hirudinida: Hirudinidae): Contrasting feeding and fasting modes. Ecol. Evol. 2019, 9, 4706–4719. [Google Scholar] [CrossRef] [Green Version]
- Lemke, S.; Müller, C.; Hildebrandt, J.-P. Be ready at any time: Postprandial synthesis of salivary proteins in salivary gland cells of the haematophagous leech Hirudo verbana. J. Exp. Biol. 2016, 219, 1139–1145. [Google Scholar] [CrossRef] [Green Version]
- Franta, Z.; Vogel, H.; Lehmann, R.; Rupp, O.; Goesmann, A.; Vilcinskas, A. Next generation sequencing identifies five major classes of potentially therapeutic enzymes secreted by Lucilia sericata medical maggots. BioMed Res. Int. 2016, 2016, 8285428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves-Moreira, D.; Matsubara, F.; Schemczssen-Graeff, Z.; De Bona, E.; Heidemann, V.; Guerra-Duarte, C.; Gremski, L.; Chávez-Olórtegui, C.; Senff-Ribeiro, A.; Chaim, O.; et al. Brown Spider (Loxosceles) venom toxins as potential biotools for the development of novel therapeutics. Toxins 2019, 11, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feygina, E.; Katrukha, G.; Semenov, G. Neutral Endopeptidase (Neprilysin) in Therapy and Diagnostics: Yin and Yang. Biochemistry 2019, 84, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Rigbi, M.; Orevi, M.; Eldor, A. Platelet aggregation and coagulation inhibitors in leech saliva and their roles in leech therapy. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: New York, NY, USA, 1996; Volume 22, pp. 273–278. [Google Scholar]
- Fink, E.; Rehm, H.; Gippner, C.; Bode, W.; Eulitz, M.; Machleidt, W.; Fritz, H. The primary structure of bdellin B-3 from the leech Hirudo medicinalis. Bdellin B-3 is a compact proteinase inhibitor of a “non-classical” Kazal type. It is present in the leech in a high molecular mass form. Biol. Chem. Hoppe Seyler 1986, 367, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Munro, R.; Jones, C.P.; Sawyer, R.T. Calin—A platelet adhesion inhibitor from the saliva of the medicinal leech. Blood Coagul. Fibrinolysis 1991, 2, 179–184. [Google Scholar] [CrossRef]
- Rigbi, M.; Levy, H.; Iraqi, F.; Teitelbaum, M.; Orevi, M.; Alajoutsijarvi, A.; Horovitz, A.; Galun, R. The saliva of the medicinal leech Hirudo medicinalis—I. Biochemical characterization of the high molecular weight fraction. Comp. Biochem. Physiol. B 1987, 87, 567–573. [Google Scholar] [CrossRef]
- Baskova, I.P.; Zavalova, L.L. Polyfunctionality of lysozyme destabilase from the medicinal leech. Russ. J. Bioorg. Chem. 2008, 34, 304–309. [Google Scholar] [CrossRef]
- Zavalova, L.L.; Baskova, I.P.; Lukyanov, S.A.; Sass, A.V.; Snezhkov, E.V.; Akopov, S.B.; Artamonova, I.I.; Archipova, V.S.; Nesmeyanov, V.A.; Kozlov, D.G.; et al. Destabilase from the medicinal leech is a representative of a novel family of lysozymes. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 2000, 1478, 69–77. [Google Scholar] [CrossRef]
- Zavalova, L.L.; Yudina, T.G.; Artamonova, I.I.; Baskova, I.P. Antibacterial non-glycosidase activity of invertebrate destabilase-lysozyme and of its helical amphipathic peptides. Chemotherapy 2006, 52, 158–160. [Google Scholar] [CrossRef] [Green Version]
- Braun, N.J.; Bodmer, J.L.; Virca, G.D.; Metz-Virca, G.; Maschler, R.; Bieth, J.G.; Schnebli, H.P. Kinetic studies on the interaction of eglin c with human leukocyte elastase and cathepsin G. Biol. Chem. Hoppe Seyler 1987, 368, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Junger, W.G.; Hallstrom, S.; Redl, H.; Schlag, G. Inhibition of human, ovine, and baboon neutrophil elastase with eglin c and secretory leukocyte proteinase inhibitor. Biol. Chem. Hoppe Seyler 1992, 373, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Markwardt, F. Untersuchungen über hirudin. Naturwiss 1955, 42, 537–538. [Google Scholar] [CrossRef]
- Müller, C.; Mescke, K.; Liebig, S.; Mahfoud, H.; Lemke, S.; Hildebrandt, J.-P. More than just one: Multiplicity of Hirudins and Hirudin-like Factors in the Medicinal Leech, Hirudo medicinalis. Mol. Genet. Genom. 2016, 291, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Haase, M.; Lemke, S.; Hildebrandt, J.-P. Hirudins and hirudin-like factors in Hirudinidae: Implications for function and phylogenetic relationships. Parasitol. Res. 2017, 116, 313–325. [Google Scholar] [CrossRef]
- Hovingh, P.; Linker, A. Hyaluronidase activity in leeches (Hirudinea). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1999, 124, 319–326. [Google Scholar] [CrossRef]
- Reverter, D.; Vendrell, J.; Canals, F.; Horstmann, J.; Aviles, F.X.; Fritz, H.; Sommerhoff, C.P. A carboxypeptidase inhibitor from the medical leech Hirudo medicinalis. Isolation, sequence analysis, cDNA cloning, recombinant expression, and characterization. J. Biol. Chem. 1998, 273, 32927–32933. [Google Scholar] [CrossRef] [Green Version]
- Domogalla, B. NMR-Lösungsstruktur des Proteins Saratin, Strukturelle Charakterisierung der Saratin-Kollagen-Interaktion und des Carausius Morosus-hyperthrehalosämischen Hormons (Cam-HrTH-I). Ph.D. Thesis, University of Regensburg, Regensburg, Germany, 2005. [Google Scholar]
- Kornowski, R.; Eldor, A.; Werber, M.M.; Ezov, N.; Zwang, E.; Nimrod, A.; Chernine, A.; Finkelstein, A.; Panet, A.; Laniado, S.; et al. Enhancement of recombinant tissue-type plasminogen activator thrombolysis with a selective factor Xa inhibitor derived from the leech Hirudo medicinalis: Comparison with heparin and hirudin in a rabbit thrombosis model. Coron. Artery Dis. 1996, 7, 903–909. [Google Scholar] [CrossRef]
- Schwarz, A.; Cabezas-Cruz, A.; Kopecký, J.; Valdés, J.J. Understanding the evolutionary structural variability and target specificity of tick salivary Kunitz peptides using next generation transcriptome data. BMC Evol. Biol. 2014, 14, 4. [Google Scholar] [CrossRef] [Green Version]
- Andersen, J.F. Structure and mechanism in salivary proteins from blood-feeding arthropods. Toxicon 2010, 56, 1120–1129. [Google Scholar] [CrossRef] [Green Version]
- Mans, B.J.; Neitz, A.W.H. Adaptation of ticks to a blood-feeding environment: Evolution from a functional perspective. Insect Biochem. Mol. Biol. 2004, 34, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.D.; Baker, R.J. Secretory gene recruitments in vampire bat salivary adaptation and potential convergences with sanguivorous leeches. Front. Ecol. Evol. 2015. [Google Scholar] [CrossRef] [Green Version]
- Champagne, D.E. Antihemostatic molecules from saliva of blood-feeding arthropods. Pathophysiol. Haemost. Thromb. 2005, 34, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.R.; Hong, S.J.; Ha, K.S.; Joe, C.O.; Kang, K.W. A cysteine-rich serine protease inhibitor (Guamerin II) from the non-blood sucking leech Whitmania edentula: Biochemical characterization and amino acid sequence analysis. J. Enzym. Inhib. 1996, 10, 81–91. [Google Scholar] [CrossRef]
- Kim, D.R.; Kang, K.W. Amino acid sequence of piguamerin, an antistasin-type protease inhibitor from the blood sucking leech Hirudo nipponia. Eur. J. Biochem. 1998, 254, 692–697. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Chu, T.T.; Kim, D.Y.; Kim, D.R.; Nguyen, C.M.; Choi, J.; Lee, J.R.; Hahn, M.J.; Kim, K.K. The crystal structure of guamerin in complex with chymotrypsin and the development of an elastase-specific inhibitor. J. Mol. Biol. 2008, 376, 184–192. [Google Scholar] [CrossRef]
- Nutt, E.M.; Jain, D.; Lenny, A.B.; Schaffer, L.; Siegl, P.K.; Dunwiddie, C.T. Purification and characterization of recombinant antistasin: A leech-derived inhibitor of coagulation factor Xa. Arch. Biochem. Biophys. 1991, 285, 37–44. [Google Scholar] [CrossRef]
- Rester, U.; Bode, W.; Moser, M.; Parry, M.A.; Huber, R.; Auerswald, E. Structure of the complex of the antistasin-type inhibitor bdellastasin with trypsin and modelling of the bdellastasin-microplasmin system. J. Mol. Biol. 1999, 293, 93–106. [Google Scholar] [CrossRef]
- Rester, U.; Bode, W.; Sampaio, C.A.M.; Auerswald, E.; Lopes, A.P.Y. Cloning, purification, crystallization and preliminary X-ray diffraction analysis of the antistasin-type inhibitor ghilanten (domain I) from Haementeria ghilianii in complex with porcine beta-trypsin. Acta Crystallogr. D Biol. Crystallogr. 2001, 57, 1038–1041. [Google Scholar] [CrossRef] [Green Version]
- Joo, S.S.; Won, T.J.; Kim, J.S.; Yoo, Y.M.; Tak, E.S.; Park, S.Y.; Park, H.Y.; Hwang, K.W.; Park, S.C.; Lee, D.I. Inhibition of Coagulation Activation and Inflammation by a Novel Factor Xa Inhibitor Synthesized from the Earthworm Eisenia andrei. Biol. Pharm. Bull. 2009, 32, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Holstein, T.W.; Mala, C.; Kurz, E.; Bauer, K.; Greber, M.; David, C.N. The primitive metazoan Hydra expresses antistasin, a serine protease inhibitor of vertebrate blood coagulation: cDNA cloning, cellular localization and developmental regulation. FEBS Lett. 1992, 309, 288–292. [Google Scholar] [CrossRef] [Green Version]
- Han, J.H.; Law, S.W.; Keller, P.M.; Kniskern, P.J.; Silberklang, M.; Tung, J.-S.; Gasic, T.B.; Gasic, G.J.; Friedman, P.A.; Ellis, R.W. Cloning and expression of cDNA encoding antistasin, a leech-derived protein having anti-coagulant and anti-metastatic properties. Proc. Natl. Acad. Sci. USA 1989, 83, 1084–1088. [Google Scholar] [CrossRef]
- Jung, H.I.; Kim, S.I.; Ha, K.S.; Joe, C.O.; Kang, K.W. Isolation and characterization of guamerin, a new human leukocyte elastase inhibitor from Hirudo nipponia. J. Biol. Chem. 1995, 270, 13879–13884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, A.; Ren, C.; Chen, T.; Jiang, X.; Sun, H.; Hu, C. Identification and functional characterization of a novel antistasin/WAP-like serine protease inhibitor from the tropical sea cucumber, Stichopus monotuberculatus. Fish Shellfish Immunol. 2016, 59, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, M., Jr.; Kato, I. Protein inhibitors of proteinases. Annu. Rev. Biochem. 1980, 49, 593–626. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [Green Version]
- Kaas, Q.; Craik, D.J. Bioinformatics-Aided Venomics. Toxins 2015, 7, 2159–2187. [Google Scholar] [CrossRef] [Green Version]
- Verriere, B.; Sabatier, B.; Carbonnelle, E.; Mainardi, J.L.; Prognon, P.; Whitaker, I.; Lantieri, L.; Hivelin, M. Medicinal leech therapy and Aeromonas spp. infection. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1001–1006. [Google Scholar] [CrossRef]
- Strube, K.H.; Kröger, B.; Bialojan, S.; Otte, M.; Dodt, J. Isolation, sequence analysis, and cloning of haemadin. An anticoagulant peptide from the Indian leech. J. Biol. Chem. 1993, 268, 8590–8595. [Google Scholar]
- Marco, S.D.; Fendrich, G.; Knecht, R.; Strauss, A.; Pohlig, G.; Heim, J.; Priestle, J.-P.; Sommerhoff, C.P.; Grütter, M.G. Recombinant hirustasin: Production in yeast, crystallization, and interaction with serine proteases. Protein Sci. 1997, 6, 109–118. [Google Scholar] [CrossRef]
- Pohlig, G.; Fendrich, G.; Knecht, R.; Eder, B.; Piechottka, G.; Sommerhoff, C.P.; Heim, J. Purification, characterization and biological evaluation of recombinant leech-derived tryptase inhibitor (rLDTI) expressed at high level in the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 1996, 241, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, S.A.; Nadeau, D.; Tirado, J.; Hollis, G.F.; Knabb, R.M.; Jia, S. Production and purification of recombinant hirudin expressed in the methylotrophic yeast Pichia pastoris. Protein Expr. Purif. 1996, 8, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Kollewe, C.; Vilcinskas, A. Production of recombinant proteins in insect cells. Am. J. Bioch. Biotech. 2013, 9, 255–271. [Google Scholar] [CrossRef]
- Wu, S.-L.; Jiang, H.; Lu, Q.; Dai, S.; Hancock, W.S.; Karger, B.L. Mass Spectrometric Determination of Disulfide Linkages in Recombinant Therapeutic Proteins Using On-line LC-MS with Electron Transfer Dissociation (ETD). Anal. Chem. 2009, 81, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemke, S.; Vilcinskas, A. European Medicinal Leeches—New Roles in Modern Medicine. Biomedicines 2020, 8, 99. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8050099
Lemke S, Vilcinskas A. European Medicinal Leeches—New Roles in Modern Medicine. Biomedicines. 2020; 8(5):99. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8050099
Chicago/Turabian StyleLemke, Sarah, and Andreas Vilcinskas. 2020. "European Medicinal Leeches—New Roles in Modern Medicine" Biomedicines 8, no. 5: 99. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8050099